Impact of Harvest Maturity and Controlled Atmosphere on Strawberry Quality Under Simulated Export Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Container Conditions
2.2. Quality Evaluation
2.3. Sample Preparation for Quantitation of Amino Acids and Metabolites
2.4. Analysis of Free Amino Acids
2.5. Gas Chromatography Mass Spectrometry (GC-MS) of Polar Phase Metabolites
2.6. Ultra-Performance Liquid Chromatography (UPLC)–Quadrupole Time-of-Flight (QTOF)–MS Analysis of Metabolites
2.7. Analysis of Volatile Organic Compounds
2.8. Evaluation of Lipid Peroxidation Using IVIS
2.9. Statistical Analysis
3. Results and Discussion
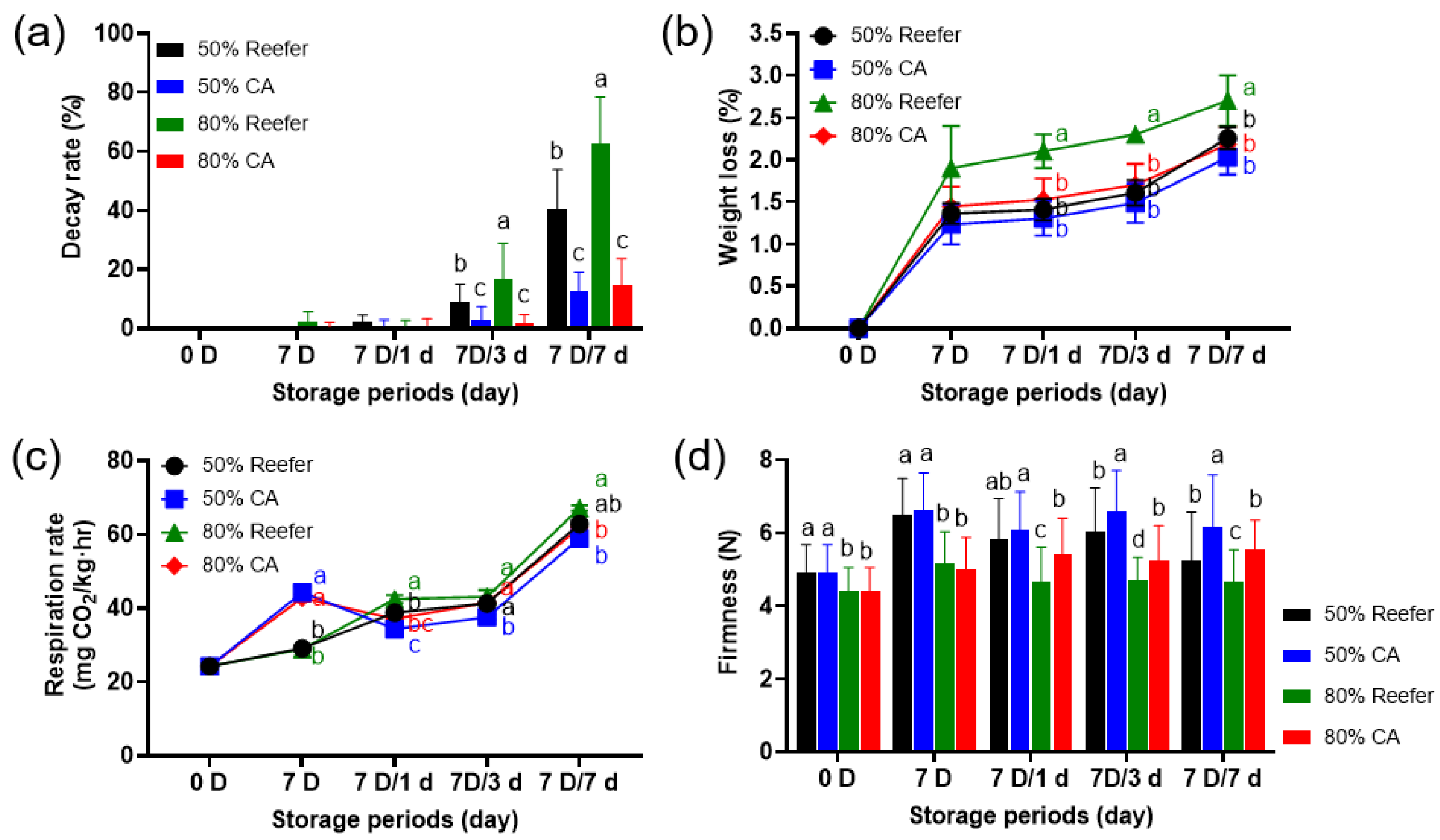
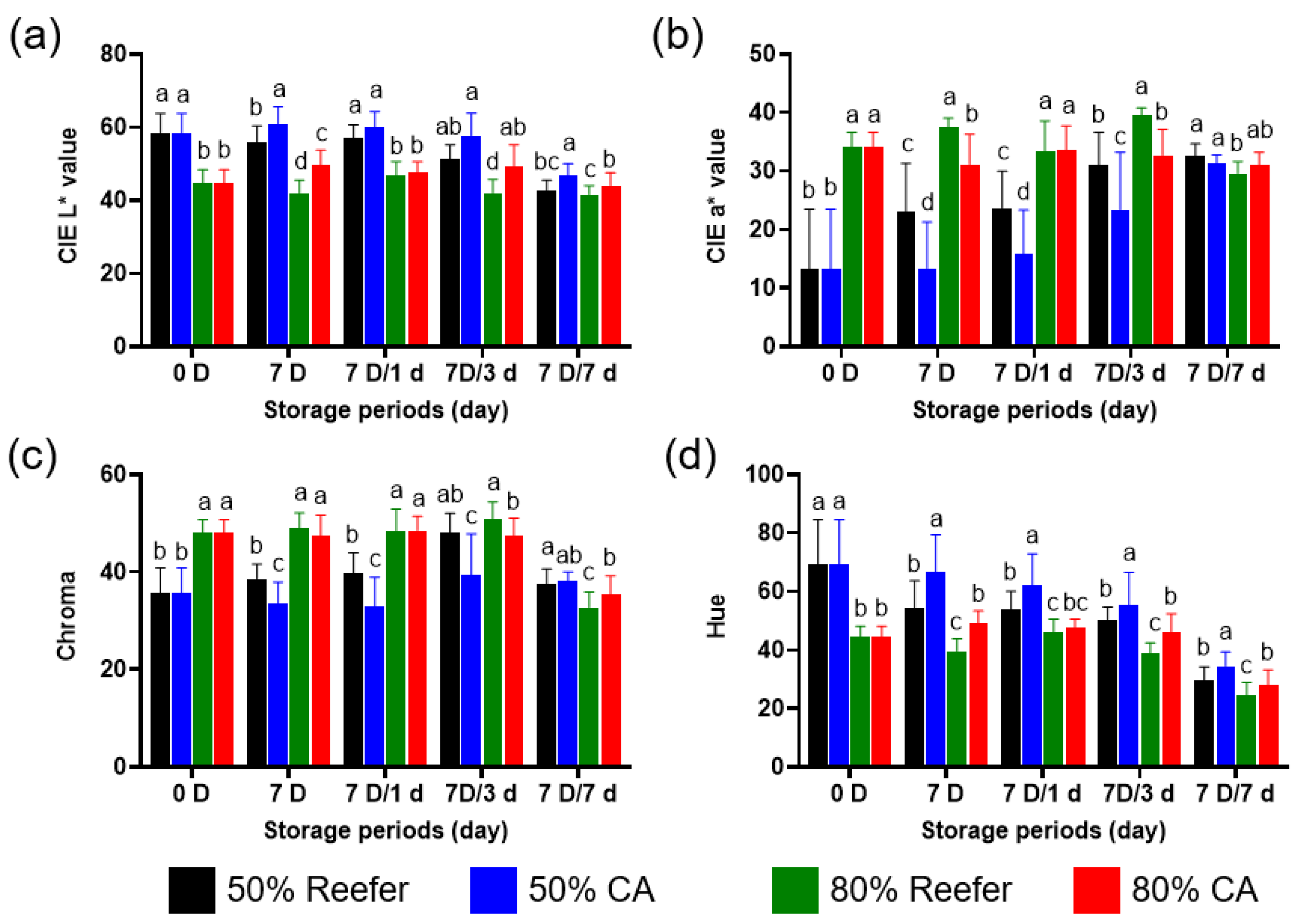
3.1. Quality Characteristics According to the Maturity Stage and Container Environment
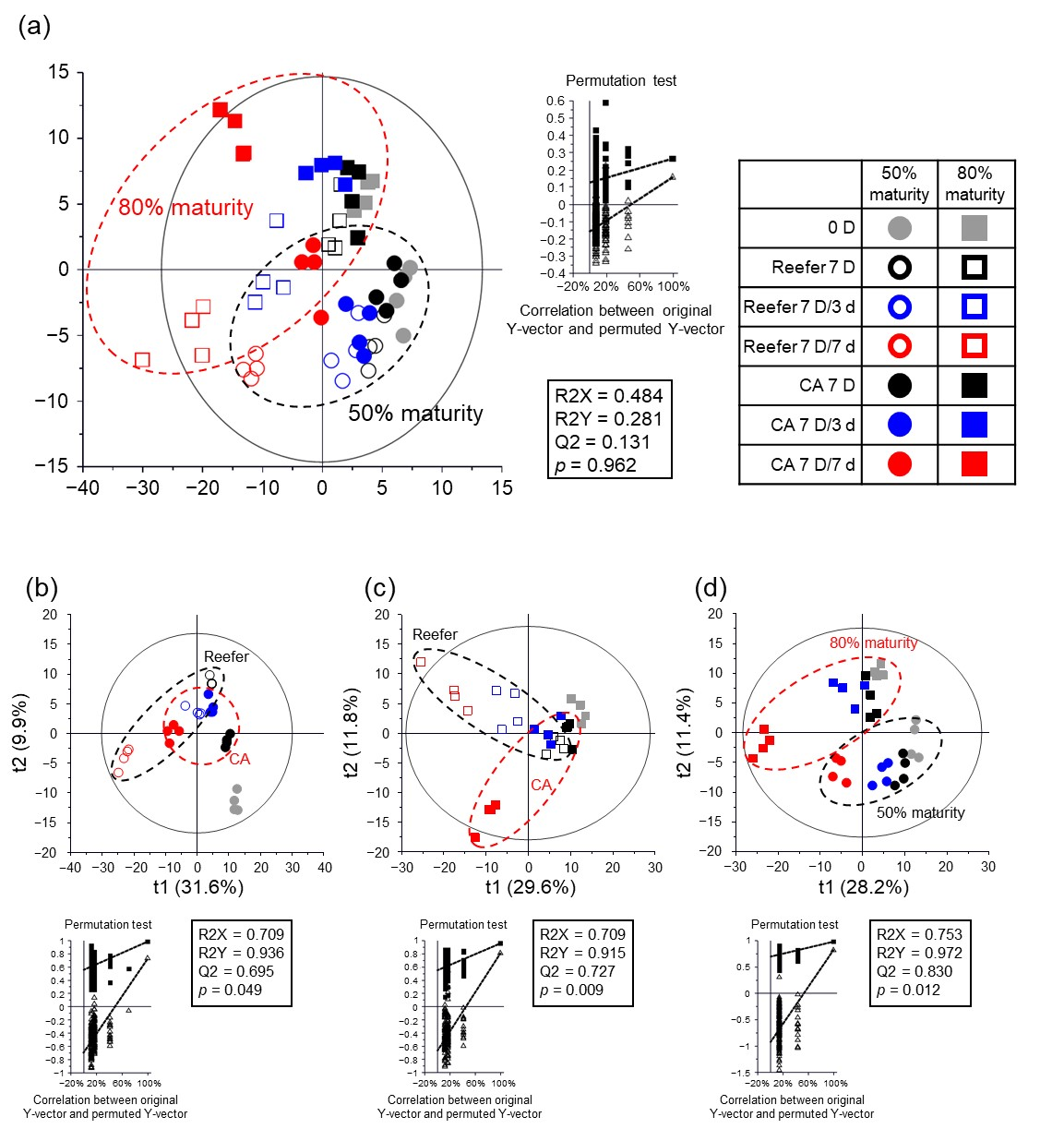
3.2. Multivariate Statistical Analysis of Metabolites According to the Maturity Stage and Container Environment
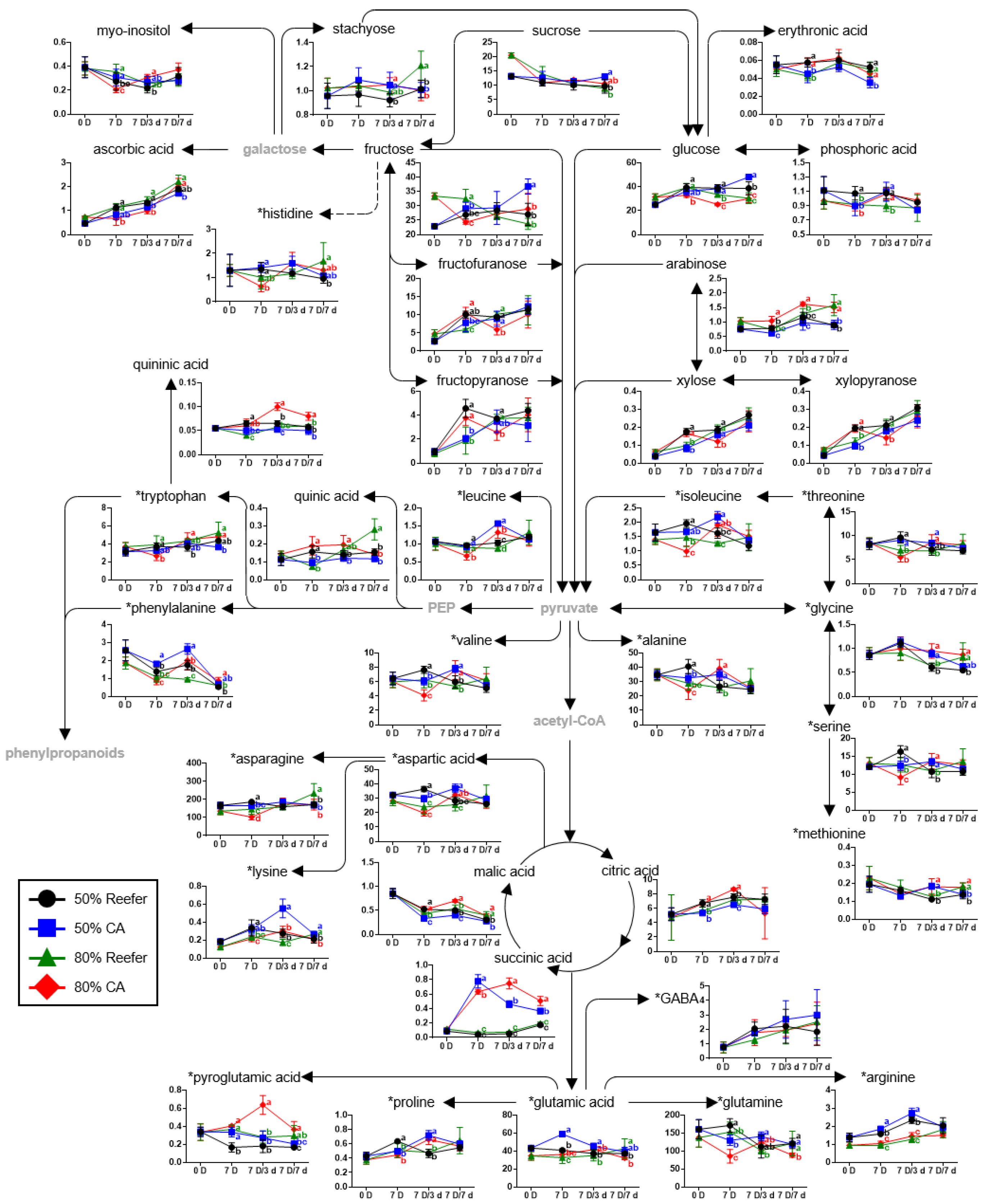
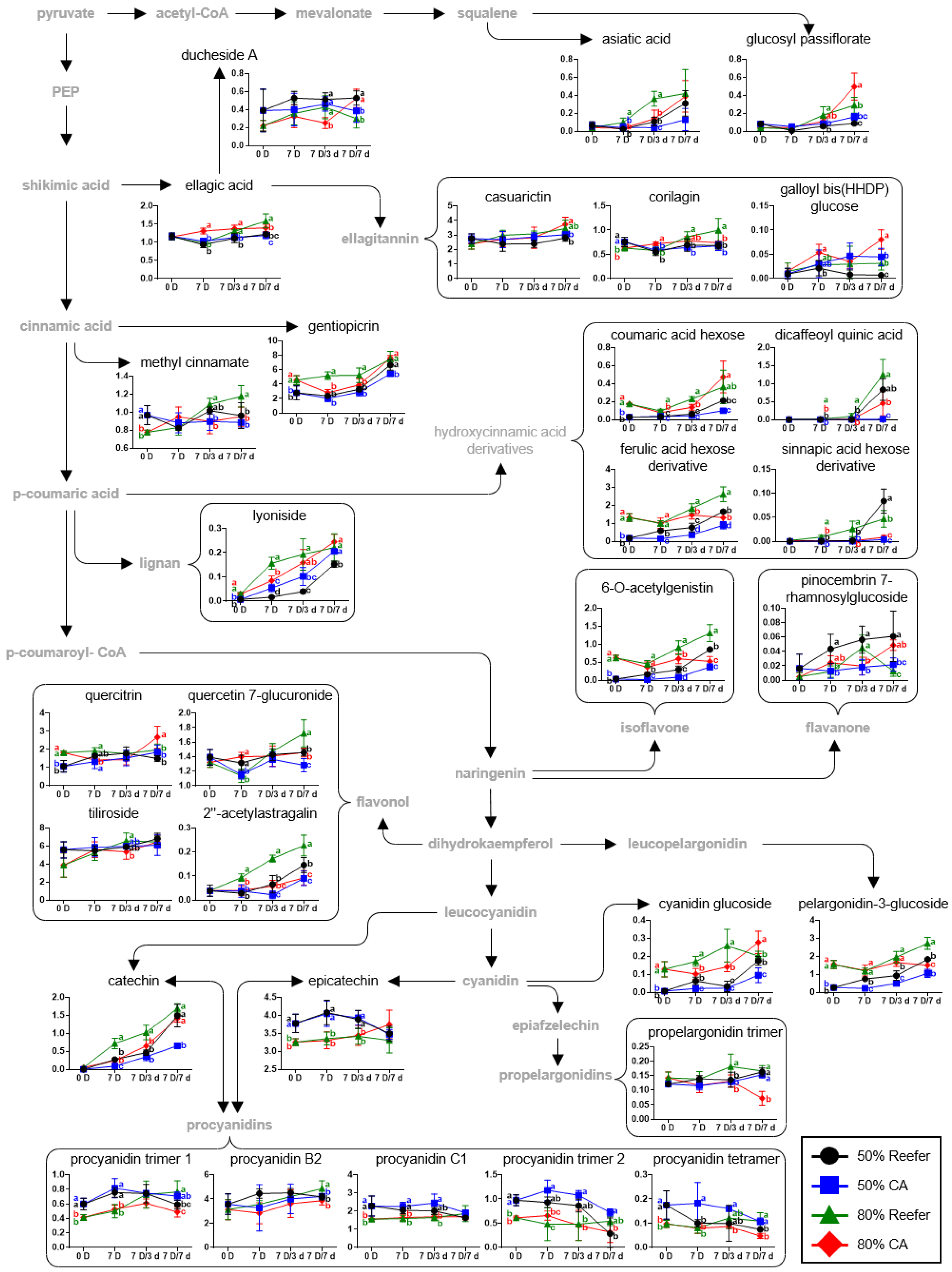
3.3. Primary and Secondary Metabolites According to the Maturity Stage and Container Environment
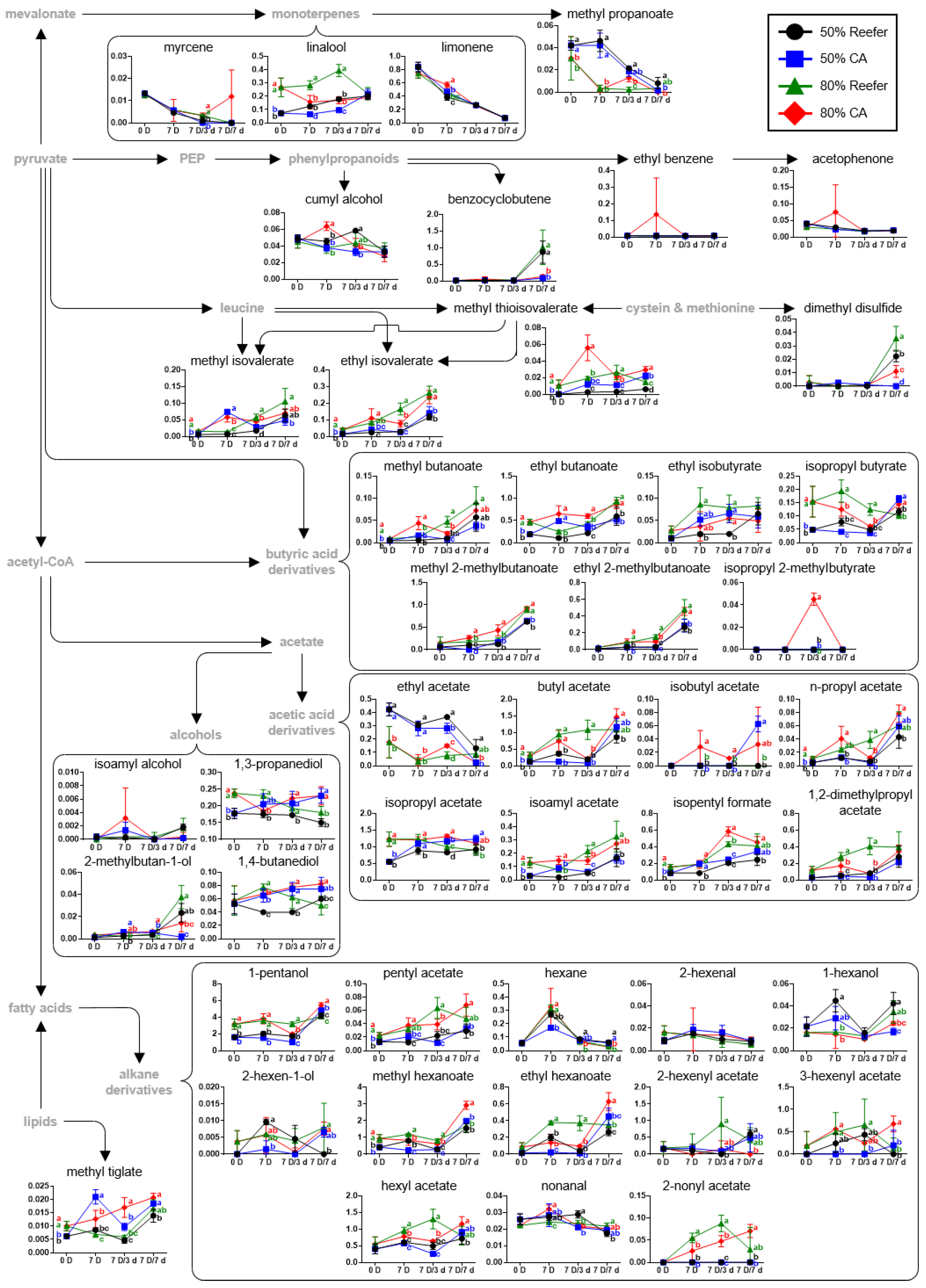
3.4. Analysis of VOCs According to Maturity Stage and Container Environment
3.5. Pathway Analysis According to Maturity and Container Environment
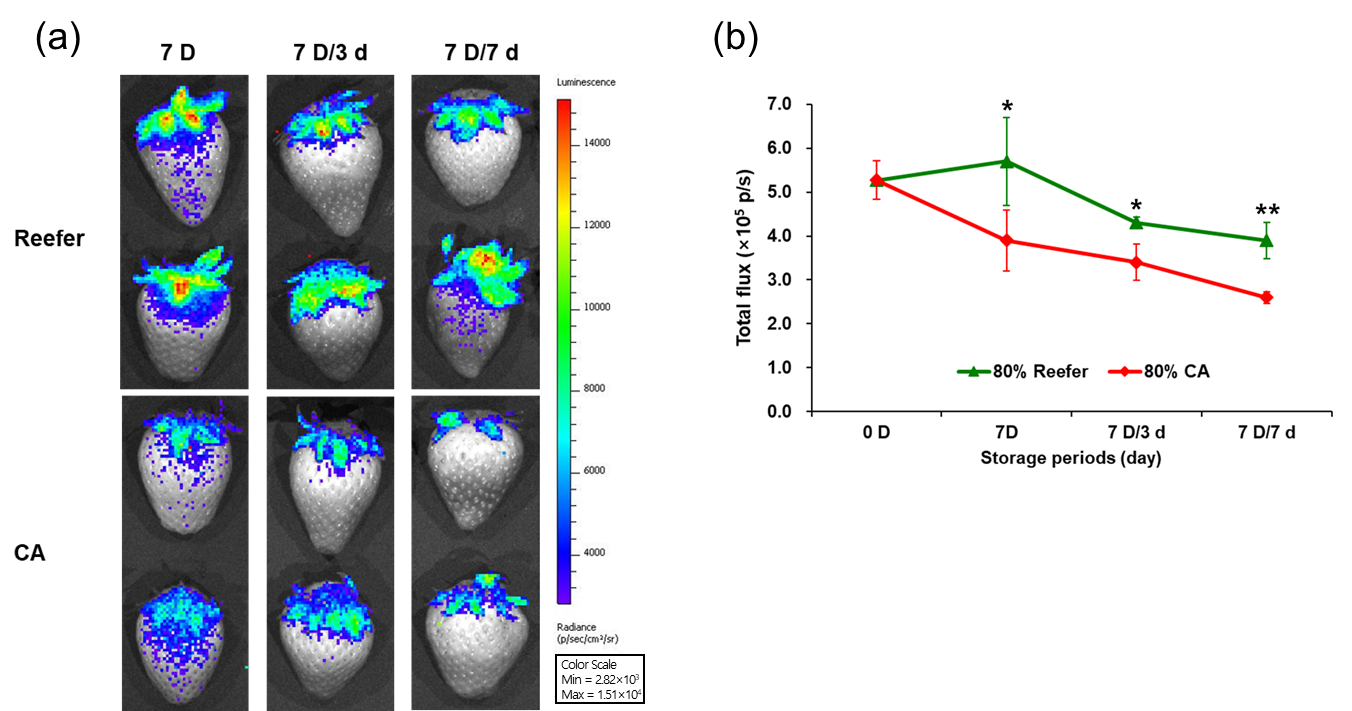
3.6. Bioluminescence-Based Verification of Lipid Peroxidation and Correlation Between Secondary Metabolites and Biophoton Emission Values According to Maturity Stage and Container Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | controlled atmosphere |
| VOCs | volatile organic compounds |
| MAP | modified atmosphere packaging |
| IVIS | In Vivo Imaging System |
| CCD | charge-coupled device |
| GC-MS | gas chromatography mass spectrometry |
| UPLC | ultra-performance liquid chromatography |
| QTOF | quadrupole time-of-flight |
| PG | polygalacturonase |
| PME | pectin methylesterase |
| PL | pectate lyase |
| PDH | pyruvate dehydrogenase |
| SDH | succinate dehydrogenase |
References
- Expert Market Research (EMR). Global Fresh Strawberry Market Report 2024. 2024. Available online: https://www.expertmarketresearch.com/reports/fresh-strawberry-market (accessed on 15 August 2025).
- Zacharaki, A.K.; Monaghan, J.M.; Bromley, J.R.; Vickers, L.H. Opportunities and challenges for strawberry cultivation inurban food production systems. Plants People Planet 2024, 6, 611–621. [Google Scholar] [CrossRef]
- KOSTAT Statistics Korea (KOSTAT). Crop Production Survey. 2025. Available online: http://www.kostat.go.kr (accessed on 6 July 2025).
- Berry, T.M.; Defraeye, T.; Ambaw, A. Exploring novel carton footprints for improved refrigerated containers usage and a more efficient supply chain. Biosyst. Eng. 2022, 220, 181–202. [Google Scholar] [CrossRef]
- Lukasse, L.J.S.; Schouten, R.E.; Castelein, R.B.; Lawton, R.; Paillart, M.J.M.; Guo, X.; Woltering, E.J.; Tromp, S.; Snels, J.C.M.A.; Defraeye, T. Perspectives on the evolution of reefer containers for transporting fresh produce. Trends Food Sci. Technol. 2023, 140, 104147. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, S.J.; Jeong, C.S.; Eum, H.L. Quality change during MAP storage of strawberry (Fragaria × ananassa Duch.) after forced-air cooling with silicone rubber pads. Hortic. Sci. Technol. 2021, 39, 86–95. [Google Scholar] [CrossRef]
- Ahn, D.; Kim, I.; Lim, J.H.; Choi, J.H.; Park, K.J.; Lee, J. The effect of high CO2 treatment on targeted metabolites of ‘Seolhyang’ strawberry (Fragaria × ananassa) fruits during cold storage. LWT Food Sci. Technol. 2021, 143, 111156. [Google Scholar] [CrossRef]
- Pott, D.M.; Lima, F.A.; Soria, C.; Willmitzer, L.; Fernie, A.R.; Nikoloski, Z.; Osorio, S.; Vallarino, J.G. Metabolic reconfiguration of strawberry physiology in response to postharvest practices. Food Chem. 2020, 321, 126747. [Google Scholar] [CrossRef]
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworki, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, J.H.; et al. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef]
- Hong, S.J.; Eum, H.L. Determination of the harvest date and ripening phase of ‘Seolhyang’ strawberry. Korean Soc. Bio-Environ. Control 2020, 29, 62–72. [Google Scholar] [CrossRef]
- Eum, H.L.; Han, S.H.; Lee, E.J. High-CO2 treatment prolongs the postharvest shelf life of strawberry fruits by reducing decay and cell wall degradation. Foods 2021, 10, 1649. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, M.; Luo, G.; Peng, J.; Vilas, M.S.; Guo, J. Effect of modified atmosphere packaging on the preservation of strawberry and the extension of its shelf-life. Int. Agrophysics 2004, 18, 195–201. [Google Scholar]
- Yang, M.; Ban, Z.; Luo, Z.; Li, J.; Lu, H.; Li, D.; Chen, C.; Li, L. Impact of elevated O2 and CO2 atmospheres on chemical attributes and quality of strawberry (Fragaria × ananassa Duch.) during storage. Food Chem. 2020, 307, 125550. [Google Scholar] [CrossRef]
- Wszelaki, A.L.; Mitcham, E.J. Effect of combinations of hot water dips, biological control and controlled atmospheres for control of gray mold on harvested strawberries. Postharvest Biol. Technol. 2003, 27, 255–264. [Google Scholar] [CrossRef]
- Tang, N.; Deng, W.; Hu, N.; Chen, N.; Li, Z. Metabolite and transcriptomic analysis reveals metabolic and regulatory features associated with Powell orange pulp deterioration during room temperature and cold storage. Postharvest Biol. Technol. 2016, 112, 75–86. [Google Scholar] [CrossRef]
- Bekele, E.A.; Ampofo-Asiama, J.; Alis, R.R.; Hertog, M.; Nicolai, B.M.; Geeraerd, A.H. Dynamics of metabolic adaptation during initiation of controlled atmosphere storage of ‘Jonagold’ apple: Effects of storage gas concentrations and conditioning. Postharvest Biol. Technol. 2016, 117, 9–20. [Google Scholar] [CrossRef]
- Blanch, M.; Rosales, R.; Palma, F.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. CO2-driven changes in energy and fermentative metabolism in harvested strawberries. Postharvest Biol. Technol. 2015, 110, 33–39. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Huang, X.; Zhang, L.; Zhao, P.; Ma, H.; Li, X.; Ban, Z.; Liu, X. Label-free quantitative proteomics to investigate strawberry fruit proteome changes under controlled atmosphere and low temperature storage. J. Proteom. 2015, 120, 44–57. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Li, L.; Aghdam, M.S.; Wei, X.; Liu, J.; Xu, Y.; Luo, Z. Elevated CO2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chem. 2019, 285, 163–170. [Google Scholar] [CrossRef]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant Activity of Phenolic Acids and Their Metabolites: Synthesis and Antioxidant Properties of the Sulfate Derivatives of Ferulic and Caffeic acids and of the acyl Glucuronide of Ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef]
- Zabetakis, I.; Holden, M.A. Strawberry Flavour: Analysis and Biosynthesis. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Hirivi, T. Mass fragmentographic and sensory analyses in the evaluation of the aroma of some strawberry varieties. LWT Food Sci. Technol. 1983, 16, 157–164. [Google Scholar]
- Pelayo, C.; Ebeler, S.E.; Kader, A.A. Postharvest life and flavor quality of three strawberry cultivars kept at 5 °C in air or air +20 kPa CO2. Postharvest Biol. Technol. 2003, 27, 171–183. [Google Scholar] [CrossRef]
- Jócsák, I.; Végvári, G.; Klász, K.; Andrássy-Baka, G.; Somfalvi-Tóth, K.; Varga-Visi, É. Analytical and bioluminescence-based non-invasive quality assessment of differentially grown strawberry (Fragaria × ananassa Duch. ‘Asia’) during household refrigeration storage. Heliyon 2023, 9, e18358. [Google Scholar] [CrossRef]
- Nematollahi, M.A.; Alinasab, Z.; Nassiri, S.M.; Khaneghah, A.M. Ultra-weak photon emission: A nondestructive detection tool for food quality and safety assessment. Qual. Assur. Saf. 2020, 12, 18–31. [Google Scholar] [CrossRef]
- Salin, M.L. Toxic oxygen species and protective systems of the chloroplast. Physiol. Plant. 1988, 72, 681–689. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, J.G.; Yang, K.Y.; Lim, S.; Lee, E.J. Postharvest fumigation of (E)-2-hexanal on kiwifruit (Actinidia chinensis cv. ‘Haegeum’) enhances resistance to Botrytis cinerea. Postharvest Biol. Technol. 2022, 187, 111854. [Google Scholar] [CrossRef]
- Jee, E.; Do, E.; Gil, C.S.; Kim, S.; Lee, S.Y.; Lee, S.; Ku, K.M. Analysis of volatile organic compounds in Korean-bred strawberries: Insights for improving fruit flavor. Front. Plant Sci. 2024, 15, 1360050. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Posé, S.; Morris, V.J.; Kirby, A.R.; Quesada, M.A.; Mercado, J.A. Fruit softening and pectin disassembly: An overview of nanostructural pectin modifications assessed by atomic force microscopy. Ann. Bot. 2014, 114, 1375–1383. [Google Scholar] [CrossRef]
- Moya-León, M.A.; Mattus-Araya, E.; Herrera, R. Molecular Events Occurring During Softening of Strawberry Fruit. Front. Plant Sci. 2019, 10, 615. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Harker, F.R.; Elgar, H.J.; Watkins, C.B.; Jackson, P.J.; Hallett, I.C. Physical and mechanical changes in strawberry fruit after high carbon dioxide treatments. Postharvest Biol. Technol. 2000, 19, 139–146. [Google Scholar] [CrossRef]
- Harker, F.R.; Redgwell, R.J.; Hallett, I.C.; Murray, S.H.; Carter, G. Texture of fresh fruit. Hort. Rev. 1997, 20, 121–224. [Google Scholar]
- Zhang, J.J.; Watkins, C.B. Fruit Quality, Fermentation Products, and Activities of Associated Enzymes During Elevated CO2 Treatment of Strawberry Fruit at High and Low Temperatures. J. Am. Soc. Hortic. Sci. 2005, 130, 124–130. [Google Scholar] [CrossRef]
- Kerbel, E.L.; Kader, A.A.; Romani, R.J. Respiratory and glycolytic response of suspension-cultured ‘Passe Crassane’ pear fruit cells to elevated CO2 concentrations. J. Am. Soc. Hortic. Sci. 1990, 115, 111–114. [Google Scholar] [CrossRef]
- Nakata, Y.; Izumi, H. Microbiological and Quality Responses of Strawberry Fruit to High CO2 CA and MA Storage. Hortscience 2020, 55, 386–391. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, K.J.; Choi, J.H.; Lim, J.H.; Kim, H.J. Metabolomic analysis of strawberries at different maturities according to postharvest storage period. Sci. Hortic. 2023, 321, 112283. [Google Scholar] [CrossRef]
- Bang, J.; Lim, S.; Yi, G.; Lee, J.G.; Lee, E.J. Integrated transcriptomic-metabolomic analysis reveals cellular responses of harvested strawberry fruit subjected to short-term exposure to high levels of carbon dioxide. Postharvest Biol. Technol. 2019, 148, 120–131. [Google Scholar] [CrossRef]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolite Changes during Postharvest Storage: Effects on Fruit Quality Traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, Y.; Avigne, W.T.; Koch, K.E. Rapid repression of maize invertases by low oxygen. Invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiol. 1999, 121, 599–608. [Google Scholar] [CrossRef]
- Blanch, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. The relationship between bound water and carbohydrate reserves in association with cellular integrity in Fragaria vesca stored under different conditions. Food Bioprocess Technol. 2015, 8, 875–884. [Google Scholar] [CrossRef]
- Farcuh, M.; Rivero, R.M.; Sadka, A.; Blumwald, E. Ethylene regulation of sugar metabolism in climacteric and non-climacteric plums. Postharvest Biol. Technol. 2018, 139, 20–30. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, Y.; Zhang, Z.; Li, T.; Jiang, Y.; Gao, H.; Yun, Z. Effect of blue light on primary metabolite and volatile compound profiling in the peel of red pitaya. Postharvest Biol. Technol. 2020, 160, 111059. [Google Scholar] [CrossRef]
- Mulas, M.; Fadda, A.; Angioni, A. Effect of maturation and cold storage on the organic acid composition of myrtle fruits. J. Sci. Food Agric. 2013, 93, 37–44. [Google Scholar] [CrossRef]
- Wankier, B.N.; Salunkhe, D.K.; Campbell, W.F. Effects of Controlled Atmosphere Storage on Biochemical Changes in Apricot and Peach Fruit. J. Amer. Soc. Hort. Sci. 1970, 95, 604–609. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; Caverzan, A.; Rauber, R.; Ferreira, E.S.; Margis-Pinheiro, M.; Galina, A. Succinate dehydrogenase (mitochondrial complex II) is a source of reactive oxygen species in plants and regulates development and stress responses. New Phytol. 2015, 208, 776–789. [Google Scholar] [CrossRef]
- Cukrov, D.; Zermiani, M.; Brizzolara, S.; Cestaro, A.; Licausi, F.; Luchinat, C.; Santucci, C.; Tenori, L.; Veen, H.V.; Zuccolo, A.; et al. Extreme hypoxic conditions induce selective molecular responses and metabolic reset in detached apple fruit. Front. Plant Sci. 2016, 7, 146. [Google Scholar] [CrossRef]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary Metabolism in Fresh Fruits During Storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef]
- Luo, Z.; Li, D.; Du, R.; Mou, W. Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Sci. Hortic. 2015, 183, 144–151. [Google Scholar] [CrossRef]
- Bodelón, O.; Blanck, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. The effects of high CO2 levels on anthocyanin composition, antioxidant activity and soluble sugar content of strawberries stored at low non-freezing temperature. Food Chem. 2010, 122, 378–673. [Google Scholar] [CrossRef]
- Blanch, M.; Alvarez, I.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. Increasing catechin and procyanidin accumulation in high CO2-treated Fragaria vesca strawberries. J. Agric. Food Chem. 2012, 60, 7489–7496. [Google Scholar] [CrossRef]
- Carmona, L.; Alquézar, B.; Marques, V.V.; Peña, L. Anthocyanin biosynthesis and accumulation in blood oranges during postharvest storage at different low temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.; Qu, H.; Li, L.; Mao, B.; Xu, Y.; Lin, X.; Luo, Z. Delaying the biosynthesis of aromatic secondary metabolites in postharvest strawberry fruit exposed to elevated CO2 atmosphere. Food Chem. 2020, 306, 125611. [Google Scholar] [CrossRef]
- Yu, D.; Huang, T.; Tian, B.; Zhan, J. Advances in biosynthesis and biological functions of proanthocyanidins in horticultural plants. Foods 2020, 9, 1774. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.G.; Olías, R.; Sanz, C.; Olías, J.M. Furanones in strawberries: Evolution during ripening and postharvest shelf life. J. Agric. Food Chem. 1996, 44, 3620–3624. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Olias, R.; Olias, J.M. Lipoxygenase and hydroperoxide lyase activities in ripening strawberry fruits. J. Agric. Food Chem. 1999, 47, 249–253. [Google Scholar] [CrossRef]
- Cozzolino, R.; Pace, B.; Palumbo, M.; Laurino, C.; Picariello, G.; Siano, F.; Giulio, B.; Pelosi, S.; Cefola, M. Profiles of Volatile and Phenolic Compounds as Markers of Ripening Stage in Candonga Strawberries. Foods 2021, 10, 3102. [Google Scholar] [CrossRef]
- Wszelaki, A.L.; Mitcham, E.J. Effects of superatmospheric oxygen on strawberry fruit quality and decay. Postharvest Biol. Technol. 2000, 20, 125–133. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.H.; Szulejko, J.E.; Parker, D. Quantitative Analysis of Fragrance and Odorants Released from Fresh and Decaying Strawberries. Sensors 2013, 13, 7939–7978. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.R.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 66. [Google Scholar] [CrossRef]
- Lu, H.; Luo, Z.; Li, D.; Jiang, Y.; Li, L. FaMYB11 promotes the accumulation of volatile esters by regulating FaLOX5 during strawberry (Fragaria × ananassa) ripening. Postharvest Biol. Technol. 2021, 178, 111560. [Google Scholar] [CrossRef]
- Ménager, I.; Jost, M.; Aubert, C. Changes in physicochemical characteristics and volatile constituents of strawberry (Cv. Cigaline) during maturation. J. Agric. Food Chem. 2004, 52, 1248–1254. [Google Scholar] [CrossRef]
- Yan, J.; Ban, Z.; Lu, H.; Li, D.; Poverenov, E.; Luo, Z.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef] [PubMed]
- Sudheeran, P.K.; Feygenberg, O.; Maurer, D.; Alkan, N. Improved Cold Tolerance of Mange Fruit with Enhanced Anthocyanin and Flavonoid Contents. Molecules 2018, 23, 1832. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Liu, C.; Wang, C.; Qiao, Y.; Zhang, B. Changes of Sensory Quality, Flavor-Related Metabolites and Gene Expression in Peach Fruit Treated by Controlled Atmosphere (CA) under Cold Storage. Int. J. Mol. Sci. 2022, 23, 7141. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, Z.; Zhou, Q.; Shi, F.; Yao, M.; Wei, B.; Cheng, S.; Ji, S. Effect of intermittent warming on aroma-related esters of ‘Nanguo’ pears through regulation of unsaturated fatty acid synthesis after cold storage. Food Bioprocess Technol. 2020, 13, 1119–1130. [Google Scholar] [CrossRef]
- Besada, C.; Llorca, E.; Novillo, P.; Hernando, I.; Salvador, A. Short-term high CO2 treatment alleviates chilling injury of persimmon cv. Fuyu by preserving the parenchyma structure. Food Control 2015, 51, 163–170. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eum, H.L.; Lee, J.-H.; Lee, J.G.; Chang, M.-S.; Do, K.-R.; Yang, H.; Ku, K.-M.; Kim, D.-S. Impact of Harvest Maturity and Controlled Atmosphere on Strawberry Quality Under Simulated Export Conditions. Foods 2025, 14, 2959. https://doi.org/10.3390/foods14172959
Eum HL, Lee J-H, Lee JG, Chang M-S, Do K-R, Yang H, Ku K-M, Kim D-S. Impact of Harvest Maturity and Controlled Atmosphere on Strawberry Quality Under Simulated Export Conditions. Foods. 2025; 14(17):2959. https://doi.org/10.3390/foods14172959
Chicago/Turabian StyleEum, Hyang Lan, Ji-Hyun Lee, Jeong Gu Lee, Min-Sun Chang, Kyung-Ran Do, Haejo Yang, Kang-Mo Ku, and Dong-Shin Kim. 2025. "Impact of Harvest Maturity and Controlled Atmosphere on Strawberry Quality Under Simulated Export Conditions" Foods 14, no. 17: 2959. https://doi.org/10.3390/foods14172959
APA StyleEum, H. L., Lee, J.-H., Lee, J. G., Chang, M.-S., Do, K.-R., Yang, H., Ku, K.-M., & Kim, D.-S. (2025). Impact of Harvest Maturity and Controlled Atmosphere on Strawberry Quality Under Simulated Export Conditions. Foods, 14(17), 2959. https://doi.org/10.3390/foods14172959





