Assessment of Fluoride Intake Risk via Infusions of Commercial Leaf Teas Available in Poland Using the Target Hazard Quotient Index Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Groups
2.2. Methodology for Preparing Tea Infusions
2.3. The Measurement of the pH Value of Tea Infusions
2.4. Procedure for Measurement of the Buffering Capacity of Tea Infusions
- pH1 is the pH of the brewed tea;
- pH2 is the resultant pH value subsequent to the addition of 0.1 M HCl.
2.5. Titratable Acidity of Tea Infusions Determination
2.6. The Method for the Determination of Calcium and Inorganic Phosphorus in Tea Infusions
2.7. The Method for the Quantitation of Fluoride in Tea Infusions
2.8. The Health Risk Assessment
2.9. Statistical Analysis
3. Results
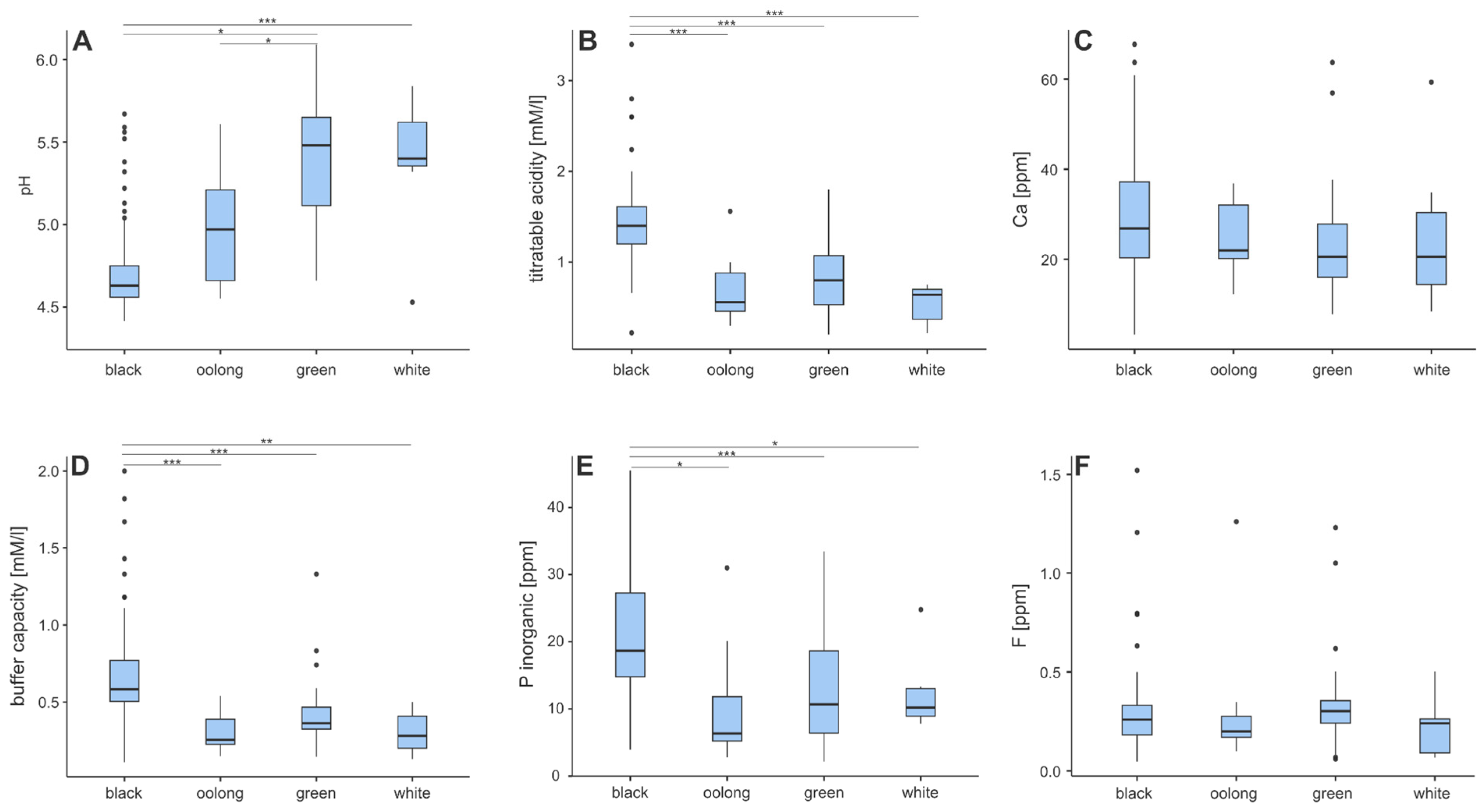
3.1. Physicochemical Properties of Teas
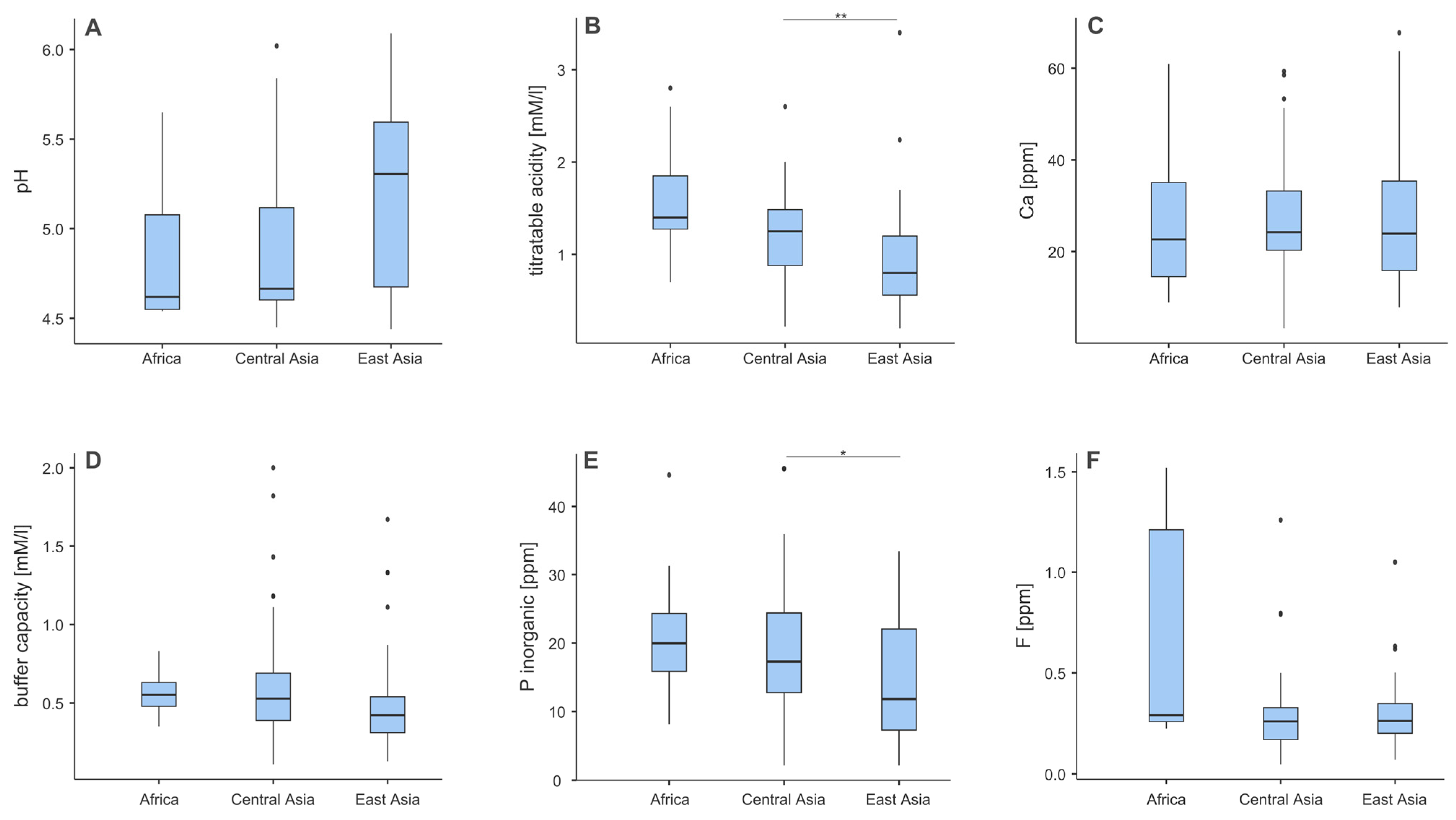
3.2. Chemical Composition of Teas Across Different Regions
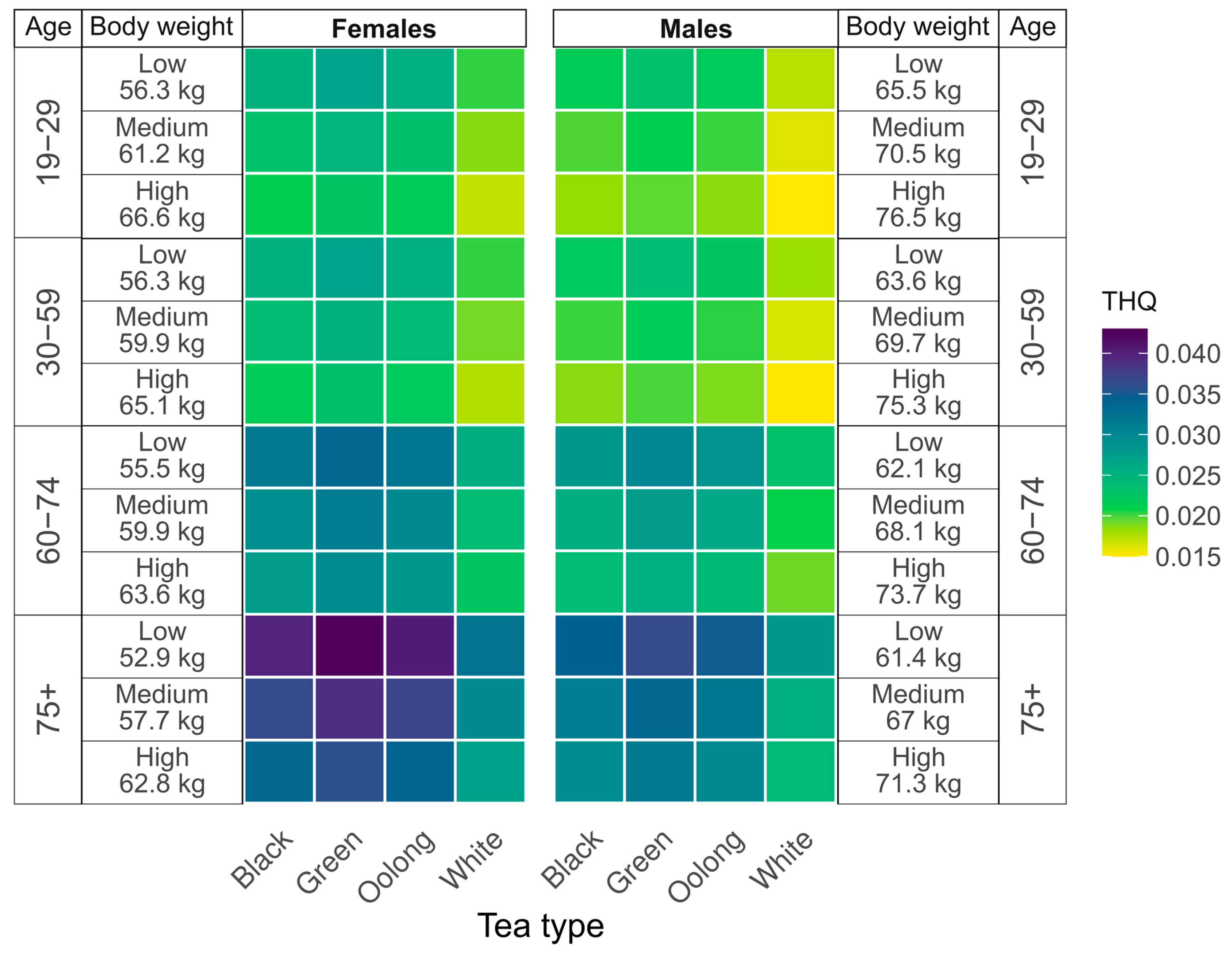
3.3. Target Hazard Quotient Analysis
3.4. THQ Calculator
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Małyszek, A.; Kiryk, S.; Kensy, J.; Kotela, A.; Michalak, M.; Kiryk, J.; Janeczek, M.; Matys, J.; Dobrzyński, M. Identification of Factors Influencing Fluoride Content in Tea Infusions: A Systematic Review. Appl. Sci. 2025, 15, 5974. [Google Scholar] [CrossRef]
- Lakshmanan, L.; Gurunathan, D.; Shanmugam, R. Effectiveness of White Tea-Mediated Silver Nanoparticles as an Intracanal Irrigant against Enterococcus Faecalis: An in vitro Study. Dent. Med. Probl. 2024, 61, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Luo, L.; Zhao, J.; Wang, Y.; Luo, H. Biological Potential and Mechanisms of Tea’s Bioactive Compounds: An Updated Review. J. Adv. Res. 2024, 65, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xia, G.; Luo, Z.; Liu, S. UHPLC Analysis of Major Functional Components in Six Types of Chinese Teas: Constituent Profile and Origin Consideration. LWT 2019, 102, 52–57. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Haufe, T.C.; Ferruzzi, M.G.; Neilson, A.P. Production and Polyphenolic Composition of Tea. Nutr. Today 2018, 53, 268–278. [Google Scholar] [CrossRef]
- Dąbrowska, J.; Dmowski, P.; Śmiechowska, M. Behavior of Polish and English Consumers on the Tea Market. Zesz. Nauk. Uniw. Szczecińskiego Probl. Zarządzania Finans. Mark. 2015, 38, 219–228. [Google Scholar] [CrossRef]
- Szmagara, A.; Krzyszczak, A.; Stefaniak, E.A. Determination of Fluoride Content in Teas and Herbal Products Popular in Poland. J. Environ. Health Sci. Eng. 2022, 20, 717–727. [Google Scholar] [CrossRef]
- Rybowska, A.; Newerli-Guz, J. Behaviours of Consumers Aged 60+ in the Tricity Market for Luxurious Food Products. Handel Wewnętrzny 2015, 2, 357–369. [Google Scholar]
- Guelinckx, I.; Ferreira-Pêgo, C.; Moreno, L.A.; Kavouras, S.A.; Gandy, J.; Martinez, H.; Bardosono, S.; Abdollahi, M.; Nasseri, E.; Jarosz, A.; et al. Intake of Water and Different Beverages in Adults across 13 Countries. Eur. J. Nutr. 2015, 54 (Suppl. S2), 45–55. [Google Scholar] [CrossRef]
- Rocznik Statystyczny Rzeczypospolitej Polskiej 2024 = Statistical Yearbook of the Republic of Poland 2024; Główny Urza̦d Statystyczny: Warszawa, Poland, 2024.
- Drywień, M.; Podkowska, J.; Frąckiewicz, J.; Górnicka, M. Consumption of Black and Green Teas as a Dietary Source of Polyphenols in Polish Inhabitants of the Mazovian Region. Ann. Natl. Inst. Hyg. 2015, 66, 35–38. [Google Scholar]
- Waugh, D.; Potter, W.; Limeback, H.; Godfrey, M. Risk Assessment of Fluoride Intake from Tea in the Republic of Ireland and Its Implications for Public Health and Water Fluoridation. Int. J. Environ. Res. Public Health 2016, 13, 259. [Google Scholar] [CrossRef]
- Fung, K.F.; Zhang, Z.Q.; Wong, J.W.C.; Wong, M.H. Fluoride Contents in Tea and Soil from Tea Plantations and the Release of Fluoride into Tea Liquor during Infusion. Environ. Pollut. 1999, 104, 197–205. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, W.-F.; Yang, X.-Q. Fluoride Content in Tea and Its Relationship with Tea Quality. J. Agric. Food Chem. 2004, 52, 4472–4476. [Google Scholar] [CrossRef] [PubMed]
- Lubojanski, A.; Piesiak-Panczyszyn, D.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Mielan, B.; Wiglusz, R.J.; Watras, A.; Dobrzynski, M. The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review. Materials 2023, 16, 1242. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride Contamination, Consequences and Removal Techniques in Water: A Review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Jha, S.K.; Mishra, V.K.; Sharma, D.K.; Damodaran, T. Fluoride in the Environment and Its Metabolism in Humans. Rev. Environ. Contam. Toxicol. 2011, 211, 121–142. [Google Scholar]
- Choubisa, S.L. Is Industrial Fluoride Pollution Harmful to Agricultural Crops? Farmers Need to Know. Environ. Anal. Ecol. Stud. 2023, 11, 1261–1266. [Google Scholar] [CrossRef]
- Fita, K.; Dobrzyński, M.; Ziętek, M.; Diakowska, D.; Watras, A.; Wiglusz, R.J. Assessment of Microstructure and Release of Fluoride Ions from Selected Fissure Sealants: An In Vitro Study. Materials 2021, 14, 4936. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The Influence of Fluoride Gels on the Physicochemical Properties of Tooth Tissues and Dental Materials—A Systematic Review. Gels 2024, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Kosior, P.; Dobrzyński, M.; Korczyński, M.; Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Piesiak-Pańczyszyn, D.; Fita, K.; Janeczek, M. Long-Term Release of Fluoride from Fissure Sealants—In Vitro Study. J. Trace Elem. Med. Biol. 2017, 41, 107–110. [Google Scholar] [CrossRef]
- Olczak-Kowalczyk, D.; Szczepańska, J.; Kaczmarek, U. Modern Dentistry of Developmental Age; Med Press International: Otwock, Poland, 2017. [Google Scholar]
- Kosior, P.; Dobrzynski, M.; Zakrzewska, A.; Diakowska, D.; Nienartowicz, J.; Blicharski, T.; Nagel, S.; Sikora, M.; Wiglusz, K.; Watras, A.; et al. Comparison of the Fluoride Ion Release from Composite and Compomer Materials under Varying PH Conditions—Preliminary In Vitro Study. Appl. Sci. 2022, 12, 12540. [Google Scholar] [CrossRef]
- ten Cate, J.M. Current Concepts on the Theories of the Mechanism of Action of Fluoride. Acta Odontol. Scand. 1999, 57, 325–329. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. The Science and Practice of Caries Prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Whitford, G.M. The Metabolism and Toxicity of Fluoride. Monogr. Oral Sci. 1996, 16, 1–153. [Google Scholar]
- Buzalaf, M.A.R.; Whitford, G.M. Fluoride Metabolism. Monogr. Oral Sci. 2011, 22, 20–36. [Google Scholar]
- Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorine; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2003.
- Shanmugam, T.; Selvaraj, M. Sources of Human Overexposure to Fluoride, Its Toxicities, and Their Amelioration Using Natural Antioxidants. In Fluoride; IntechOpen: London, UK, 2022. [Google Scholar]
- Jiménez-Farfán, M.D.; Hernández-Guerrero, J.C.; Juárez-López, L.A.; Jacinto-Alemán, L.F.; De la Fuente-Hernández, J. Fluoride Consumption and Its Impact on Oral Health. Int. J. Environ. Res. Public Health 2011, 8, 148–160. [Google Scholar] [CrossRef]
- Kanduti, D.; Sterbenk, P.; Artnik, B. Fluoride: A Review of Use and Effects on Health. Mater. Sociomed. 2016, 28, 133–137. [Google Scholar] [CrossRef]
- Tu, J.; Liu, K.-J.; Ran, L.-J.; Pan, X.-P. Fluoride Burden of Aluminum Plant Workers. Iran. J. Public Health 2015, 44, 583–585. [Google Scholar]
- Lavanya, S.; Hema Shree, K.; Ramani, P. Fluoride Effect on Renal and Hepatic Functions: A Comprehensive Decade Review of In Vitro and In Vivo Studies. J. Oral Biol. Craniofac. Res. 2024, 14, 735–745. [Google Scholar] [CrossRef]
- Gumińska, M.; Sterkowicz, J. Effect of Sodium Fluoride on Glycolysis in Human Erythrocytes and Ehrlich Ascites Tumour Cells In Vitro. Acta Biochim. Pol. 1976, 23, 285–291. [Google Scholar] [PubMed]
- Skórka-Majewicz, M.; Goschorska, M.; Żwierełło, W.; Baranowska-Bosiacka, I.; Styburski, D.; Kapczuk, P.; Gutowska, I. Effect of Fluoride on Endocrine Tissues and Their Secretory Functions—Review. Chemosphere 2020, 260, 127565. [Google Scholar] [CrossRef] [PubMed]
- Veneri, F.; Iamandii, I.; Vinceti, M.; Birnbaum, L.S.; Generali, L.; Consolo, U.; Filippini, T. Fluoride Exposure and Skeletal Fluorosis: A Systematic Review and Dose-Response Meta-Analysis. Curr. Environ. Health Rep. 2023, 10, 417–441. [Google Scholar] [CrossRef]
- Du, C.; Xiao, P.; Gao, S.; Chen, S.; Chen, B.; Huang, W.; Zhao, C. High Fluoride Ingestion Impairs Bone Fracture Healing by Attenuating M2 Macrophage Differentiation. Front. Bioeng. Biotechnol. 2022, 10, 791433. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachari, K.A. Skeletal Fluorosis in Humans: A Review of Recent Progress in the Understanding of the Disease. Prog. Food Nutr. Sci. 1986, 10, 279–314. [Google Scholar] [PubMed]
- Kurdi, M. Chronic Fluorosis: The Disease and Its Anaesthetic Implications. Indian J. Anaesth. 2016, 60, 157–162. [Google Scholar] [CrossRef]
- Mazurek, A.; Kowalska, G.; Włodarczyk-Stasiak, M.; Wyrostek, J.; Kowalski, R. The Influence of the Preparation of Tea Infusion on the Content of Fluoride and the Assessment of Health Risk for the Consumer in Poland. Appl. Sci. 2023, 13, 5075. [Google Scholar] [CrossRef]
- Donohue, J.M.; Duke, T.; Opresko, D.; Watson, A.; Tomkins, B. Fluoride: Exposure and Relative Source Contribution Analysis; U.S. Environmental Protection Agency: Washington, DC, USA, 2010.
- Manjunathappa, T.H.; Devegowda, D.; Mysore, N.K.; Vishwanath, P.; Narayana, P.S. Association between Drinking Water Fluoride and the Serum Alkaline Phosphatase and Phosphate Levels in Pregnant Women and Newborn Infants. Dent. Med. Probl. 2023, 60, 569–575. [Google Scholar] [CrossRef]
- Rocznik Statystyczny Rzeczypospolitej Polskiej 2023 = Statistical Yearbook of the Republic of Poland 2023; Główny Urząd Statystyczny: Warszawa, Poland, 2023.
- Average Values of Basic Water Quality Parameters. Available online: https://www.mpwik.wroc.pl/strefa-klienta/uslugi/uslugi-laboratoryjne/parametry-wody/parametry-wody-archiwum/#tabs-26048-4-iv-kwartal (accessed on 31 July 2025).
- Małyszek, A.; Zawiślak, I.; Kulus, M.; Watras, A.; Kensy, J.; Kotela, A.; Styczyńska, M.; Janeczek, M.; Matys, J.; Dobrzyński, M. Fluoride Content in Infusions of Selected Teas Available on the Polish Market—An In Vitro Study. Foods 2025, 14, 2452. [Google Scholar] [CrossRef]
- Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Dobrzyński, M. Assessment of the Influence of Vegetarian Diet on the Occurrence of Erosive and Abrasive Cavities in Hard Tooth Tissues. Postep. Hig. Med. Dosw. 2011, 65, 764–769. [Google Scholar] [CrossRef]
- Pattaravisitsate, N.; Phetrak, A.; Denpetkul, T.; Kittipongvises, S.; Kuroda, K. Effects of Brewing Conditions on Infusible Fluoride Levels in Tea and Herbal Products and Probabilistic Health Risk Assessment. Sci. Rep. 2021, 11, 14115. [Google Scholar] [CrossRef]
- Risk Assessment: Guidance for Superfund. Volume I Human Health Evaluation Manual (Part A); Office of Emergency and Remedial Response, U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- Rychlik, E.; Stoś, K.; Woźniak, A.; Mojska, H. (Eds.) Normy Żywienia Dla Populacji Polski; Narodowy Instytut Zdrowia Publicznego PZH—Państwowy Instytut Badawczy: Warszawa, Poland, 2024.
- Kowalska, J.; Marzec, A.; Domian, E.; Galus, S.; Ciurzyńska, A.; Brzezińska, R.; Kowalska, H. Influence of Tea Brewing Parameters on the Antioxidant Potential of Infusions and Extracts Depending on the Degree of Processing of the Leaves of Camellia Sinensis. Molecules 2021, 26, 4773. [Google Scholar] [CrossRef]
- de Bocanera, M.E.L.; de Stisman, M.A.K.; de Labanda, E.B.; de Gepner, A.C. Statistical Analysis of Salivary PH Changes after the Intake of Black Tea and Yerba Mate Supplemented with Sweeteners. J. Oral Sci. 1999, 41, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, R.; Liu, K.; Chen, Q.; Bo, N.; Wang, Q.; Xiao, Y.; Sha, G.; Chen, S.; Lei, X.; et al. Changes in Sensory Characteristics, Chemical Composition and Microbial Succession during Fermentation of Ancient Plants Pu-Erh Tea. Food Chem. X 2023, 20, 101003. [Google Scholar] [CrossRef] [PubMed]
- Ocieczek, A.; Pukszta, T.; Żyłka, K.; Kirieieva, N. The Influence of Storage Conditions on the Stability of Selected Health-Promoting Properties of Tea. LWT 2023, 184, 115029. [Google Scholar] [CrossRef]
- Ing, M.E.; Magnuson, B.E.; Frantz, D.L. Fluoride Content in Asian Produced Green Teas. J. Can. Dent. Assoc. 2021, 87, l3. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Abrams, S.A.; Belizán, J.M.; Boy, E.; Cormick, G.; Quijano, C.D.; Gibson, S.; Gomes, F.; Hofmeyr, G.J.; Humphrey, J.; et al. Interventions to Improve Calcium Intake through Foods in Populations with Low Intake. Ann. N. Y. Acad. Sci. 2022, 1511, 40–58. [Google Scholar] [CrossRef]
- Jing, T.; Li, J.; He, Y.; Shankar, A.; Saxena, A.; Tiwari, A.; Maturi, K.C.; Solanki, M.K.; Singh, V.; Eissa, M.A.; et al. Role of Calcium Nutrition in Plant Physiology: Advances in Research and Insights into Acidic Soil Conditions—A Comprehensive Review. Plant Physiol. Biochem. 2024, 210, 108602. [Google Scholar] [CrossRef]
- Ruxton, C.H.S. The Suitability of Caffeinated Drinks for Children: A Systematic Review of Randomised Controlled Trials, Observational Studies and Expert Panel Guidelines. J. Hum. Nutr. Diet. 2014, 27, 342–357. [Google Scholar] [CrossRef]
- Disler, P.B.; Lynch, S.R.; Torrance, J.D.; Sayers, M.H.; Bothwell, T.H.; Charlton, R.W. The Mechanism of the Inhibition of Iron Absorption by Tea. S. Afr. J. Med. Sci. 1975, 40, 109–116. [Google Scholar]
- Delimont, N.M.; Haub, M.D.; Lindshield, B.L. The Impact of Tannin Consumption on Iron Bioavailability and Status: A Narrative Review. Curr. Dev. Nutr. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Rocha-Amador, D.O.; González-Martell, A.D.; Pérez-Vázquez, F.J.; Cilia López, V.G. Health Risk Assessment in Mexican Children Exposed to Fluoride from Sweetened Beverages. Biol. Trace Elem. Res. 2023, 201, 2250–2257. [Google Scholar] [CrossRef]
- Pérez-Vázquez, F.J.; González-Martell, A.D.; Fernández-Macias, J.C.; Rocha-Amador, D.O.; González-Palomo, A.K.; Ilizaliturri-Hernández, C.A.; González-Mille, D.J.; Cilia-Lopez, V.G. Health Risk Assessment in Children Living in an Urban Area with Hydrofluorosis: San Luis Potosí Mexico Case Study. J. Trace Elem. Med. Biol. 2021, 68, 126863. [Google Scholar] [CrossRef] [PubMed]
- Girolametti, F.; Annibaldi, A.; Illuminati, S.; Damiani, E.; Carloni, P.; Ajdini, B.; Fanelli, M.; Truzzi, C. Unlocking the Elemental Signature of European Tea Gardens: Implications for Tea Traceability. Food Chem. 2024, 453, 139641. [Google Scholar] [CrossRef] [PubMed]
- Girolametti, F.; Annibaldi, A.; Illuminati, S.; Damiani, E.; Carloni, P.; Truzzi, C. Essential and Potentially Toxic Elements (PTEs) Content in European Tea (Camellia Sinensis) Leaves: Risk Assessment for Consumers. Molecules 2023, 28, 3802. [Google Scholar] [CrossRef]
- Taşcioğlu, S.; Kök, E. Temperature Dependence of Copper, Iron, Nickel and Chromium Transfers into Various Black and Green Tea Infusions. J. Sci. Food Agric. 1998, 76, 200–208. [Google Scholar] [CrossRef]
- Maleki, A.; Abulmohammadi, P.; Teymouri, P.; Zandi, S.; Daraei, H.; Mahvi, A.H.; Shahsawari, S. Effect of Brewing Time and Water Hardness on Fluoride Release from Different Iranian Teas. Fluoride 2016, 49, 263–273. [Google Scholar]
- Zohoori, F.V.; Maguire, A. Development of a Database of the Fluoride Content of Selected Drinks and Foods in the UK. Caries Res. 2016, 50, 331–336. [Google Scholar] [CrossRef]
- Townsend, J.A.; Thompson, T.; Vaughn, S.; Wang, Y.; Yu, Q.; Xu, X.; Wen, Z.T. Analysis of Fluoride Content in Alternative Milk Beverages. J. Clin. Pediatr. Dent. 2019, 43, 388–392. [Google Scholar] [CrossRef]
- Casaglia, A.; Cassini, M.A.; Condò, R.; Iaculli, F.; Cerroni, L. Dietary Fluoride Intake by Children: When to Use a Fluoride Toothpaste? Int. J. Environ. Res. Public Health 2021, 18, 5791. [Google Scholar] [CrossRef]
- Basak, S.S.; Dokumacioglu, E. Evaluation of the Effects of Different Mouthrinses on Dental Remineralization. Dent. Med. Probl. 2023, 60, 219–225. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, S.; Kiryk, J.; Kensy, J.; Michalak, M.; Matys, J.; Dobrzyński, M. Fluoride Release from Two Commercially Available Dental Fluoride Gels—In Vitro Study. Gels 2025, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Herman, K.; Wujczyk, M.; Dobrzynski, M.; Diakowska, D.; Wiglusz, K.; Wiglusz, R.J. In Vitro Assessment of Long-Term Fluoride Ion Release from Nanofluorapatite. Materials 2021, 14, 3747. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, W.; Nikodem, A.; Diakowska, D.; Wiglusz, R.J.; Watras, A.; Dobrzyński, M.; Mikulewicz, M. Comparison of the Fluoride Ion Release from Nanofluoroapatite-Modified Orthodontic Cement under Different PH Conditions—An In Vitro Study. Acta Bioeng. Biomech. 2023, 25, 159–176. [Google Scholar] [CrossRef]
- Suba, D.S.S.; Arvina, A.R. Antibacterial and Anti-Inflammatory Effects of a Novel Herb-Mediated Nanocomposite Mouthwash in Plaque-Induced Gingivitis: A Randomized Controlled Trial. Dent. Med. Probl. 2023, 60, 445–451. [Google Scholar] [CrossRef]
- Piszko, P.J.; Kulus, M.; Piszko, A.; Kiryk, J.; Kiryk, S.; Kensy, J.; Małyszek, A.; Michalak, M.; Dobrzyński, W.; Matys, J.; et al. The Influence of Calcium Ions and PH on Fluoride Release from Commercial Fluoride Gels in an In Vitro Study. Gels 2025, 11, 486. [Google Scholar] [CrossRef]
| No | Brand | Tea Name | Type | Country | Region |
|---|---|---|---|---|---|
| 1 | Ahmad Tea | Assam Tea | black | India | Central Asia |
| 2 | Ahmad Tea | Ceylon Tea | black | UAE | Africa |
| 3 | Ahmad Tea | English Breakfast | black | UAE | Africa |
| 4 | Ahmad Tea | Green Tea | green | UAE | Africa |
| 5 | Ahmad Tea | Jasmine Green Tea | green | UAE | Africa |
| 6 | Akbar | Earl Grey | black | Sri Lanka | Central Asia |
| 7 | Akbar | Pure Ceylon Tea | black | India | Central Asia |
| 8 | Astra | Safari | black | Kenya | Africa |
| 9 | Big-Active | Ceylon | black | UAE | Africa |
| 10 | Big-Active | Pure Green | green | China | East Asia |
| 11 | Chelton | English Green Tea | green | Sri Lanka | Central Asia |
| 12 | Chelton | Gunpowder Tea | green | Sri Lanka | Central Asia |
| 13 | Dilmah | Ceylon Gold | black | Sri Lanka | Central Asia |
| 14 | Dilmah | Ceylon Premium Tea | black | Sri Lanka | Central Asia |
| 15 | Dilmah | Ceylon Supreme | black | Sri Lanka | Central Asia |
| 16 | Dilmah | English Breakfast | black | Sri Lanka | Central Asia |
| 17 | Dilmah | Gourmet Earl Grey tea | black | Sri Lanka | Central Asia |
| 18 | Eternal | Finest Top | black | Sri Lanka | Central Asia |
| 19 | Five o’clock | Assam Halmari GTGFBOP | black | India | Central Asia |
| 20 | Five o’clock | Assam Halmari GTGFOP1CL | black | India | Central Asia |
| 21 | Five o’clock | Assam Satrupa | black | India | Central Asia |
| 22 | Five o’clock | Assam Singlijan | black | India | Central Asia |
| 23 | Five o’clock | Assam Tonganagaon | black | India | Central Asia |
| 24 | Five o’clock | Ceylon Ahinsa | black | Sri Lanka | Central Asia |
| 25 | Five o’clock | Ceylon Lumbini | black | India | Central Asia |
| 26 | Five o’clock | Ceylon Vithanakande | black | Taiwan | East Asia |
| 27 | Five o’clock | China Wild Buds | white | China | East Asia |
| 28 | Five o’clock | China Moonlight White | white | China | East Asia |
| 29 | Five o’clock | China Oolong Ti Kuan Yin | oolong | China | East Asia |
| 30 | Five o’clock | China Panyong Golden Needle | black | China | East Asia |
| 31 | Five o’clock | China White Monkey | white | China | East Asia |
| 32 | Five o’clock | China Wuyi Rou Gui | oolong | China | East Asia |
| 33 | Five o’clock | Darjeeling Flower Balasun | black | India | Central Asia |
| 34 | Five o’clock | Darjeeling Gielle | black | India | Central Asia |
| 35 | Five o’clock | Darjeeling Liza Hill DJ5/21 | black | India | Central Asia |
| 36 | Five o’clock | Darjeeling Musk Puttabang | black | India | Central Asia |
| 37 | Five o’clock | Darjeeling Nagr DJ2 | black | India | Central Asia |
| 38 | Five o’clock | Darjeeling Shree Dwarika | black | India | Central Asia |
| 39 | Five o’clock | Darjeeling Teesta Valley DJ11 | black | India | Central Asia |
| 40 | Five o’clock | Formosa Finest Oolong | oolong | Taiwan | East Asia |
| 41 | Five o’clock | Formosa Lapsang Souchong | black | Taiwan | East Asia |
| 42 | Five o’clock | Formosa Oolong Dung Ting | oolong | Taiwan | East Asia |
| 43 | Five o’clock | Formosa Oolong High Mountain | oolong | Sri Lanka | Central Asia |
| 44 | Five o’clock | Formosa Pi Lo Chun | green | China | East Asia |
| 45 | Five o’clock | Formosa Supreme Gancy Oolong | oolong | Taiwan | East Asia |
| 46 | Five o’clock | Golden Yunan | black | China | East Asia |
| 47 | Five o’clock | Golden Yunan Superior | black | China | East Asia |
| 48 | Five o’clock | Gunpowder Temple of Heaven | green | China | East Asia |
| 49 | Five o’clock | Japan Bancha Tenryu | green | Japan | East Asia |
| 50 | Five o’clock | Japan Black Tea | black | Japan | East Asia |
| 51 | Five o’clock | Japan Gabalong | green | Japan | East Asia |
| 52 | Five o’clock | Japan Gyokuro Tohei | green | Japan | East Asia |
| 53 | Five o’clock | Japan Gyokuro Uji | green | Japan | East Asia |
| 54 | Five o’clock | Japan Kabuse Kagashima | green | Japan | East Asia |
| 55 | Five o’clock | Japan Sencha Fukujyu | green | Japan | East Asia |
| 56 | Five o’clock | Japan Sencha Shizuoka | green | Japan | East Asia |
| 57 | Five o’clock | Keemun | black | China | East Asia |
| 58 | Five o’clock | Nilgiri Platinum Needles | white | India | Central Asia |
| 59 | Five o’clock | Oolong Sun Moon Lake Ruby | oolong | Taiwan | East Asia |
| 60 | Five o’clock | Pai Mu Tan Superior | white | China | East Asia |
| 61 | Five o’clock | South India Nilgiri Bamboo | green | India | Central Asia |
| 62 | Five o’clock | South India Nilgiri Kukicha Roasted | black | India | Central Asia |
| 63 | Five o’clock | South India Nilgiri Long Jing | green | India | Central Asia |
| 64 | Five o’clock | South India Nilgiri Slender | green | India | Central Asia |
| 65 | Five o’clock | South India Nilgiri White Tea Peony | white | India | Central Asia |
| 66 | Five o’clock | South India Nilgiri Wulong | oolong | India | Central Asia |
| 67 | Five o’clock | Yunan Special Green | green | China | East Asia |
| 68 | Five o’clock | Yunnan Green Superior | green | China | East Asia |
| 69 | HAYB | Cui Min Spring | white | China | East Asia |
| 70 | HAYB | Oolong Formosa | oolong | Taiwan | East Asia |
| 71 | HAYB | Sencha | green | China | East Asia |
| 72 | Impra Tea | Royal Elixir Tea (green packaging) | green | Sri Lanka | Central Asia |
| 73 | Lipton | Yellow Label | black | Kenya | Africa |
| 74 | Lord Nelson | Green | green | China | East Asia |
| 75 | Lord Nelson | Assam | black | India | Central Asia |
| 76 | Lord Nelson | Earl Grey with Lemon Peel | black | China | East Asia |
| 77 | Loyd | Earl Grey | black | Sri Lanka | Central Asia |
| 78 | Loyd | Green | green | China | East Asia |
| 79 | Loyd | Madras | black | India | Central Asia |
| 80 | Loyd | Yunnan | black | China | East Asia |
| 81 | Remsey | Green Pure Leaf | green | Kenya | Africa |
| 82 | Sir Adalbert’s Tea | Black Tropical Tea | black | Sri Lanka | Central Asia |
| 83 | Sir Adalbert’s Tea | Earl Grey | black | Sri Lanka | Central Asia |
| 84 | Vintage Teas | Dimbula | black | Sri Lanka | Central Asia |
| 85 | Vintage Teas | Earl Grey | black | Sri Lanka | Central Asia |
| 86 | Vintage Teas | Kandy | black | Sri Lanka | Central Asia |
| 87 | Vintage Teas | Natural Green Tea | green | Sri Lanka | Central Asia |
| 88 | Vintage Teas | Nuwara Eliya | black | Sri Lanka | Central Asia |
| 89 | Vintage Teas | Organic Black Tea | black | Sri Lanka | Central Asia |
| 90 | Vintage Teas | Organic Green Tea | green | Sri Lanka | Central Asia |
| 91 | Vintage Teas | Ruhuna | black | Sri Lanka | Central Asia |
| 92 | Vintage Teas | Sabaragamuwa | black | Sri Lanka | Central Asia |
| 93 | Yunnan | Black Tea | black | China | East Asia |
| 94 | Yunnan | Green Tea | green | China | East Asia |
| 95 | ZAS | Assam | black | India | Central Asia |
| 96 | ZAS | Madras | black | India | Central Asia |
| 97 | ZAS | Yunnan | black | China | East Asia |
| 98 | ZAS | Yunan Black Tea | black | China | East Asia |
| Gender | Age (Years) | Reference Body Weight (kg) * | ||
|---|---|---|---|---|
| 10th Percentile | Median | 90th Percentile | ||
| Men | 19–29 | 65.5 | 70.5 | 76.5 |
| 30–59 | 63.6 | 69.7 | 75.3 | |
| 60–74 | 62.1 | 68.1 | 73.7 | |
| ≥75 | 61.4 | 67 | 71.3 | |
| Women | 19–29 | 56.3 | 61.2 | 66.6 |
| 30–59 | 56.3 | 59.9 | 65.1 | |
| 60–74 | 55.5 | 59.9 | 63.6 | |
| ≥75 | 52.9 | 57.7 | 62.8 | |
| Age (Years) | Annual Tea Consumption (g) | Annual Tea Infusion Intake (L) * | Daily Tea Infusion Intake (L) * |
|---|---|---|---|
| 19–29 | 480 | 48 | 0.132 |
| 30–59 | 480 | 48 | 0.132 |
| 60–74 | 600 | 60 | 0.164 |
| ≥75 | 720 | 72 | 0.197 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małyszek, A.; Zawiślak, I.; Kulus, M.; Watras, A.; Kensy, J.; Kotela, A.; Styczyńska, M.; Janeczek, M.; Matys, J.; Dobrzyński, M. Assessment of Fluoride Intake Risk via Infusions of Commercial Leaf Teas Available in Poland Using the Target Hazard Quotient Index Approach. Foods 2025, 14, 2944. https://doi.org/10.3390/foods14172944
Małyszek A, Zawiślak I, Kulus M, Watras A, Kensy J, Kotela A, Styczyńska M, Janeczek M, Matys J, Dobrzyński M. Assessment of Fluoride Intake Risk via Infusions of Commercial Leaf Teas Available in Poland Using the Target Hazard Quotient Index Approach. Foods. 2025; 14(17):2944. https://doi.org/10.3390/foods14172944
Chicago/Turabian StyleMałyszek, Agata, Ireneusz Zawiślak, Michał Kulus, Adam Watras, Julia Kensy, Agnieszka Kotela, Marzena Styczyńska, Maciej Janeczek, Jacek Matys, and Maciej Dobrzyński. 2025. "Assessment of Fluoride Intake Risk via Infusions of Commercial Leaf Teas Available in Poland Using the Target Hazard Quotient Index Approach" Foods 14, no. 17: 2944. https://doi.org/10.3390/foods14172944
APA StyleMałyszek, A., Zawiślak, I., Kulus, M., Watras, A., Kensy, J., Kotela, A., Styczyńska, M., Janeczek, M., Matys, J., & Dobrzyński, M. (2025). Assessment of Fluoride Intake Risk via Infusions of Commercial Leaf Teas Available in Poland Using the Target Hazard Quotient Index Approach. Foods, 14(17), 2944. https://doi.org/10.3390/foods14172944









