Hypoglycemic Effects of Sechium edule (Chayote) in Older Adults: A Systematic Review and Meta-Analysis of Clinical and Preclinical Trials
Abstract
1. Introduction
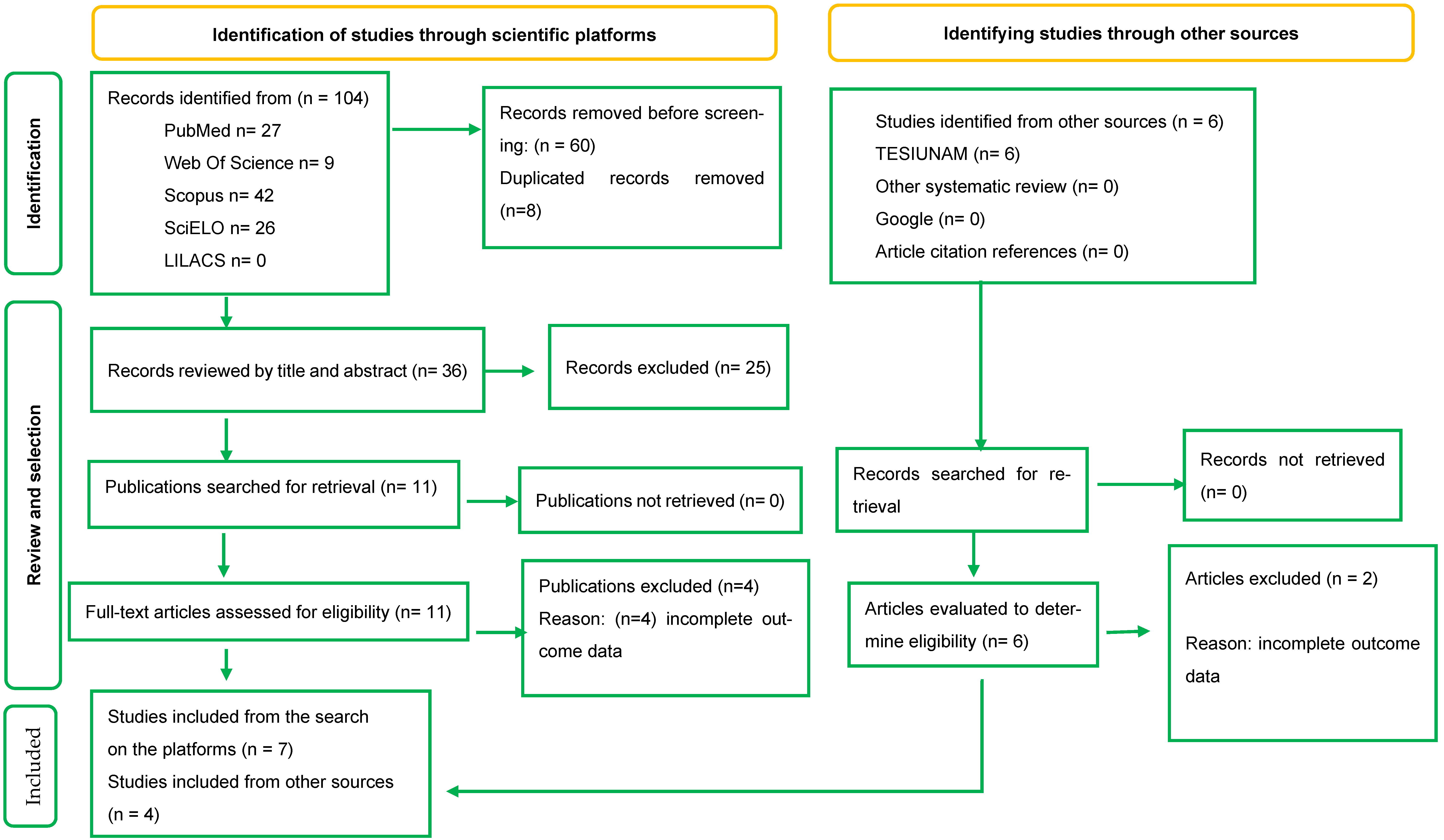
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Statistical Analysis
3. Results
3.1. Included Studies Characteristics
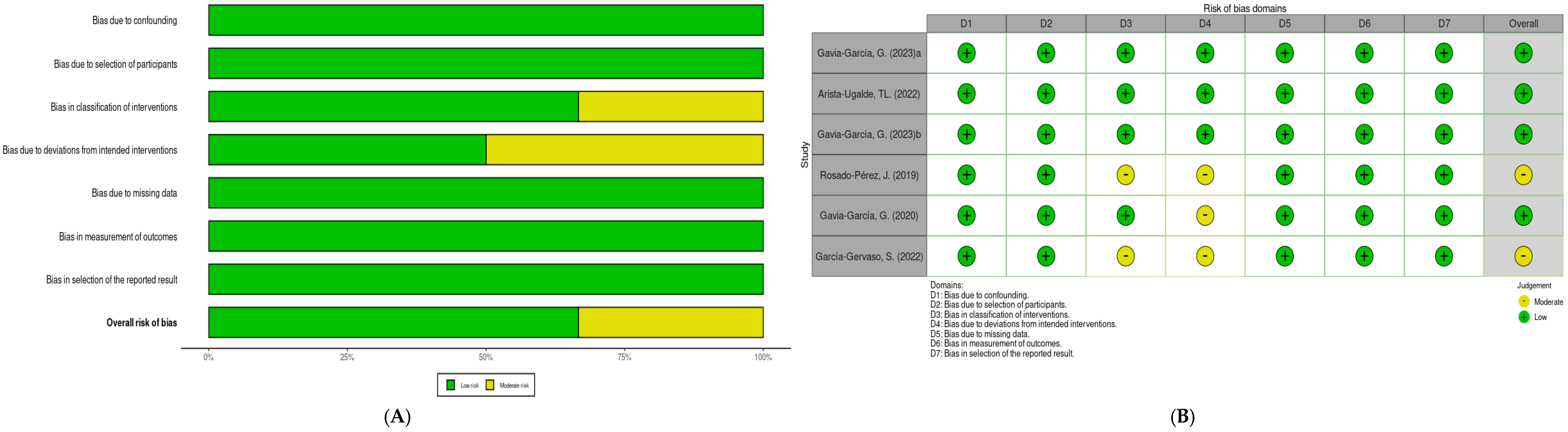
3.2. Risk of Bias Analysis
| Author (Year) | Design | Population (n) | Dosage and Treatment | Treatment | Outcome | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration | ||||||||||||||||||||||||||
| Gavia-García et al. (2023) [13] | Clinical trial | 48 elderly people with MS (PG n = 23, IG, n = 25), | IG: 1 capsule of 500 mg of dry powder of S. edule 3 times per day. PG: 500 mg placebo in 1 capsule 3 times per day. | 6 months | Mean and SD pre- and post-intervention by study group | |||||||||||||||||||||
| Serum glucose (mg/dL) | %HbA1c | |||||||||||||||||||||||||
| Placebo | S. edule | Placebo | S. edule | |||||||||||||||||||||||
| Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | |||||||||||||||||||
| 118 ± 67 | 123 ± 48 | 117 ± 73 | 108 ± 52 | 6.98 ± 1.6 | 6.94 ± 2 | 7.2 ± 1.2 | 6.28 ± 1.2 | |||||||||||||||||||
| MD: −14 [CI 95%: −38.00, 10.00] | MD: −0.96 [CI 95%: −1.53, −0.39] | |||||||||||||||||||||||||
| Arista-Ugalde et al. (2022) [14] | Clinical trial | 81 adults over 60 year with MS (PG n = 40, IG n = 41) | IG: 1 capsule of 500 mg of dry powder of S. edule 3 times per day. PG: 500 mg placebo in 1 capsule 3 times per day. | 3 and 6 months | Mean and SD pre- and post-intervention by study group | |||||||||||||||||||||
| Serum glucose (mg/dL) | %HbA1c | |||||||||||||||||||||||||
| Placebo | S. edule | Placebo | S. edule | |||||||||||||||||||||||
| Baseline | 3 month | 6 month | Baseline | 3 month | 6 month | Baseline | 3 month | 6 month | Baseline | 3 month | 6 month | |||||||||||||||
| 113 ± 43 | 123 ± 43 | 118 ± 40 | 113 ± 37 | 105 ± 29 | 103 ± 28 | 6.8 ± 1.9 | 6.5 ± 1.8 | 6.7 ± 1.9 | 6.3 ± 1.2 | 5.6 ± 1.2 | 5.9 ± 0.8 | |||||||||||||||
| MD (0–3 Months): −18 [CI 95% −28.82, −7.18] | MD (0–3 Months): −1.00 [CI 95% −1.43, −0.57] | |||||||||||||||||||||||||
| MD (0–6 Months): −15 [CI 95% −25.65, −4.35] | MD (0–6 Months): −0.50 [CI 95% −0.93, −0.07] | |||||||||||||||||||||||||
| Gavia-García et al. (2023) [15] | Clinical trial | 46 elderly people with MS (PG n = 20, IG, n = 26), | IG: 1 capsule of 500 mg of dry powder of S. edule 3 times per day. PG: 500 mg placebo in 1 capsule 3 times per day. | 6 months | Mean and SD pre- and post-intervention by study group | |||||||||||||||||||||
| Serum glucose (mg/dL) | %HbA1c | |||||||||||||||||||||||||
| Placebo | S. edule | Placebo | S. edule | |||||||||||||||||||||||
| Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | |||||||||||||||||||
| 137.3 ± 61.3 | 139.4 ± 69.1 | 140.7 ± 48.1 | 140 ± 57.3 | N/D | N/D | N/D | N/D | |||||||||||||||||||
| MD: −2.80 [CI 95% −25.44, 19.84] | ||||||||||||||||||||||||||
| Rosado-Pérez et al. (2019) [16] | Pre-experimental exploratory | 12 elderly people with MS. | 500 mg of Sechium edule of dry poder in a capsule, 3 times per day, | 1.5 months | Mean and SD values of baseline and post-treatment | |||||||||||||||||||||
| Serum glucose (mg/dL) | %HbA1c | |||||||||||||||||||||||||
| Sechium edule | Sechium edule | |||||||||||||||||||||||||
| Baseline | 1.5 months | Baseline | 1.5 months | |||||||||||||||||||||||
| 107 ± 38 | 95 ± 5 | 5.9 ± 2.7 | 6 ± 2.7 | |||||||||||||||||||||||
| MD: −12 [SD: 34.3] | MD: −0.1 [SD: 1.7] | |||||||||||||||||||||||||
| Gavia-García et al. (2020) [17] | Clinical trial | 75 elderly people (PG n = 30, IG:, n = 45), with 67 ± 6 years of age with MS. | IG: 1 capsule of 500 mg of dry powder of Sechium edule 3 times per day. PG: 500 mg placebo in 1capsule 3 times per day. | 3 months | Mean and SD values of baseline and post-treatment by study group | |||||||||||||||||||||
| Serum glucose (mg/dL) | %HbA1c | |||||||||||||||||||||||||
| Placebo | Sechium edule | Placebo | Sechium edule | |||||||||||||||||||||||
| Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | |||||||||||||||||||
| 111 ± 57 | 121 ± 48 | 137 ± 72 | 125 ± 54 | 6.7 ± 1.9 | 6.3 ± 1.7 | 7.1 ± 1.9 | 6.2 ± 1.8 | |||||||||||||||||||
| MD: −22 [C.I 95% −39.63, −4.37] | MD: −1.3 [C.I 95% −1.83, −0.77] | |||||||||||||||||||||||||
| García-Gervasio (2022) [18] | Clinical trial | 20 elderly people PG n = 10, IG:, n = 10) 67–69 years old with MS. | IG: 1 capsule of 500 mg of dry powder of Sechium edule 3 times per day. PG, 500 mg placebo in 1capsule 3 times per day. | 3 months | Mean and SD pre and post intervention by study group | |||||||||||||||||||||
| Serum glucose (mg/dL) | %HbA1c (mg/dL) | |||||||||||||||||||||||||
| Placebo | Sechium edule | Placebo | Sechium edule | |||||||||||||||||||||||
| Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | |||||||||||||||||||
| 108 ± 47 | 123 ± 47 | 143 ± 56 | 123 ± 58 | N/D | N/D | N/D | N/D | |||||||||||||||||||
| MD: −35 [C.I 95% −63.99, −6.01] | ||||||||||||||||||||||||||
| Author (Year) | Design | Population (n) | Dosage and Treatment | Treatment Duration | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mohammad et al. (2024) [19] | Experimental | 18 albino Wistar rats weighing 180–220g | Diabetic control (n = 6) with 60 mg/kg STZ. Two intervention groups with Sechium edule at 200 mg/kg (n = 6) and 400 mg/kg (n = 6). | 28 days | Mean and SD pre- and post-intervention by study group | |||||||||
| Serum glucose (mg/dL) | ||||||||||||||
| Control with T2DM | S. edule | |||||||||||||
| 60 mg/kg | 200 mg/kg | 400 mg/kg | ||||||||||||
| Day 1 | Day 28 | Day 1 | Day 28 | Day 1 | Day 28 | |||||||||
| 281.00 ± 2.56 | 369.35 ± 2.00 | 276.45 ± 3.01 | 165.88 ± 2.60 | 276.00 ± 1.95 | 152.00 ± 2.77 | |||||||||
| Aguiñiga-Sánchez et al. (2017) [20] | Experimental | 20 CD-1 mice, 10 to 12 weeks old | A dose of 800 mg/kg of S. edule var. nigrum spinosum monitored for 7 days. | 7 days | Mean and SD pre- and post-intervention by study group | |||||||||
| Serum glucose (mg/dL) | ||||||||||||||
| Control | S. edule | |||||||||||||
| Day 7 | Day 7 | |||||||||||||
| 115.72 ± 16.6 | 72.27 ± 3.82 | |||||||||||||
| Gómez-García (2013) [21] | Experimental | 12 CD mice—1 female, 2 to 3 months old | A dose of 785 mg/kg of S. edule var. nigrum spinosum administered intraperitoneally every 48 h. | 7 days | Mean and SD pre- and post-intervention by study group | |||||||||
| Serum glucose (mg/dL) | ||||||||||||||
| Control | S. edule | |||||||||||||
| Day 7 | Day 7 | |||||||||||||
| 105 ± 20 | 75 ± 16 | |||||||||||||
| Montiel-García (2023) [22] | Experimental | 10 Mus musculus L CD—1 mice, 8 to 10 weeks old. | Diabetic control with 175 mg/kg STZ. Four intervention groups with doses of 8 mg/kg, 50 mg/kg, 125 mg/kg, and 250 mg/kg of the hybrid S. edule H387-07. | 30 days | Mean and SD pre- and post-intervention by study group | |||||||||
| Serum glucose (mg/dL) | ||||||||||||||
| Control | S. edule | |||||||||||||
| 175 mg/kg | 8 mg/kg | 50 mg/kg | 125 mg/kg | 250 mg/kg | ||||||||||
| Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 | |||||
| 433 ± 20 | 397 ± 18 | 451 ± 21 | 312 ± 19 | 372 ± 25 | 261 ± 20 | 440 ± 18 | 189 ± 12 | 420 ± 20 | 323 ± 18 | |||||
| Coria-Bárcenas (2017) [23] | Experimental | 35 young male Wistar rats. | Two intervention groups in which 500 mg/kg of different S. edule extracts were tested every 48 h. | 9 days | Mean and SD pre- and post-intervention by study group | |||||||||
| Serum glucose (mg/dL) | ||||||||||||||
| Control | 500 mg/kg of S. edule skin extract | 500 mg/kg of S. edule pulp extract | ||||||||||||
| Day 1 | Day 1 | Day 5 | Day 7 | Day 1 | Day 5 | Day 7 | ||||||||
| 103 ± 3.33 | 80 ± 5.25 | 82 ± 5.12 | 90 ± 13.3 | 75 ± 10 | 80 ± 12 | 81 ± 15 | ||||||||
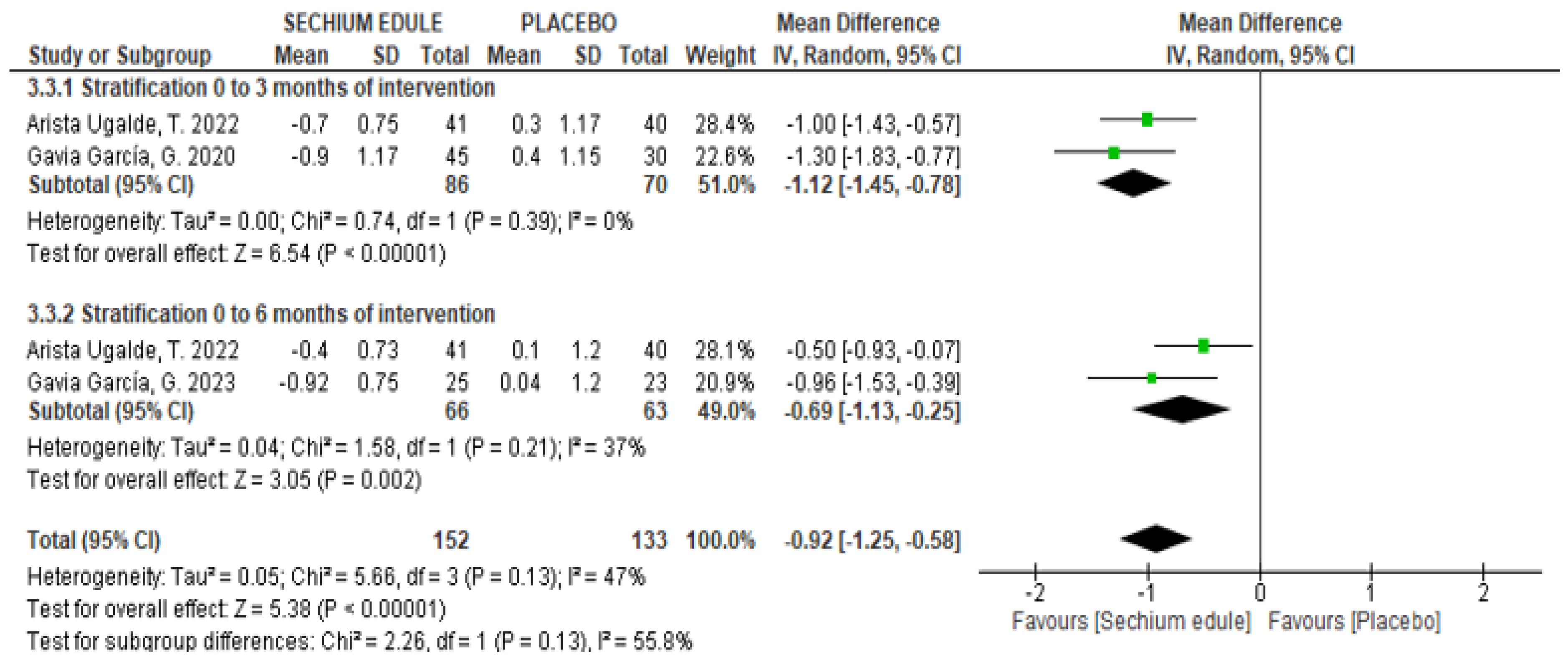
3.3. Serum Glucose
3.4. Glycosylated Hemoglobin (HbA1c)
4. Discussion
4.1. Practical Implications
4.2. Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| DPP-4 | Dipeptidyl peptudase-4 |
| EG | Experimental group |
| GA | Gallic acid |
| HDL | High-density lipoprotein |
| INS1-E | Insulin-secreting rat insulinoma |
| MA | Meta-analyses |
| MD | Mean difference |
| MS | Metabolic syndrome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| S. edule | Sechium edule |
| SD | Standard deviation |
| SR | Systematic review |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
References
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the global burden of disease study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Romero-Martínez, M.; Castro-Porras, L.; Gómez-Velasco, D.; Mehta, R. Trends in the prevalence of metabolic syndrome and its components in Mexican adults, 2006–2018. Salud Publica Mex. 2021, 63, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Gloyn, A.L.; Evans-Molina, C.; Joseph, J.J.; Misra, S.; Pajvani, U.V.; Simcox, J.; Susztak, K.; Drucker, D.J. Diabetes mellitus-progress and opportunities in the evolving epidemic. Cell 2024, 187, 3789–3820. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Galicia-García, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; Ali, I.I.; Adeghate, J.O.; Tekes, K.; Kalász, H.; Adeghate, E.A. An update on the molecular and cellular basis of pharmacotherapy in type 2 diabetes mellitus. Int. J. Mol. Sci. 2023, 24, 9328. [Google Scholar] [CrossRef]
- Cadena–Iñiguez, J.; Arévalo-Galarza, L.; Avendaño-Arrazate, C.H.; Soto-Hernández, M.; Ruiz-Posadas, L.M.; Santiago-Osorio, E.; Acosta-Ramos, M.; Cisneros-Solano, V.; Aguirre-Medina, J.; Ochoa-Martínez, D. Production, genetics, postharvest management and pharmacological characteristics of Sechium edule (Jacq.) Sw. Fresh Prod. 2007, 1, 41–53. [Google Scholar]
- Niewiadomska, J.; Gajek-Marecka, A.; Gajek, J.; Noszczyk-Nowak, A. Biological potential of polyphenols in the context of metabolic syndrome: An analysis of studies on animal models. Biology 2022, 11, 559. [Google Scholar] [CrossRef]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M.P.L.V.O.; Delerue-Matos, C. Chayote (Sechium edule): A review of nutritional composition, bioactivities and potential applications. Food Chem. 2019, 275, 557–568. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I. Food polyphenols and type II diabetes mellitus: Pharmacology and mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. The consumption of Sechium edule (chayote) has antioxidant effect and prevents telomere attrition in older adults with metabolic syndrome. Redox Rep. 2023, 28, 2207323. [Google Scholar] [CrossRef] [PubMed]
- Arista-Ugalde, T.L.; Santiago-Osorio, E.; Monroy-García, A.; Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Cadena-Iñiguez, J.; Gavia-García, G.; Mendoza-Núñez, V.M. Antioxidant and anti-inflammatory effect of the consumption of powdered concentrate of Sechium edule var. nigrum spinosum in Mexican older adults with metabolic syndrome. Antioxidants 2022, 11, 1076. [Google Scholar] [CrossRef]
- Gavia-García, G.; Hernández-Álvarez, D.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M.; Rosado-Pérez, J. The supplementation of Sechium edule var. nigrum spinosum (Chayote) promotes Nrf2-mediated antioxidant protection in older adults with metabolic syndrome. Nutrients 2023, 15, 4106. [Google Scholar] [CrossRef]
- Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Sechium edule var. nigrum spinosum (chayote) on oxidative stress and pro-inflammatory markers in older adults with metabolic syndrome: An exploratory study. Antioxidants 2019, 8, 146. [Google Scholar] [CrossRef]
- Gavia-García, G.; Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of Sechium edule var. nigrum spinosum (chayote) on telomerase levels and antioxidant capacity in older adults with metabolic syndrome. Antioxidants 2020, 9, 634. [Google Scholar] [CrossRef]
- García-Gervasio, S. Efecto del Tratamiento de Sechium edule var. nigrum spinosum Sobre la Fragmentación del ADN de Leucocitos de Adultos Mayores con Síndrome Metabólico. Bachelor’s Thesis, FES Zaragoza, UNAM, México City, Mexico, 2022. [Google Scholar]
- Mohammad, F.S.; Das, U.; Samanta, S.K.; Irfan, Z.; Gopinath, S.C.B.; Mostafa, M.A.H.; Al-Haidari, R.; Abdellatif, A.A.H.; Shehata, A.M.; Gouda, M.M. Evaluation of Sechium edule fruit attenuation impact on the cardiomyopathy of the STZ-induced diabetic rats. Heliyon 2024, 10, e30440. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Cadena-Íñiguez, J.; Santiago-Osorio, E.; Gómez-García, G.; Mendoza-Núñez, V.M.; Rosado-Pérez, J.; Ruíz-Ramos, M.; Cisneros-Solano, V.M.; Ledesma-Martínez, E.; Delgado-Bordonave, A.J.; et al. Chemical analyses and in vitro and in vivo toxicity of fruit methanol extract of Sechium edule var. nigrum spinosum. Pharm. Biol. 2017, 55, 1638–1645. [Google Scholar] [CrossRef]
- Gómez-García, G. Valoración de Parámetros Bioquímicos y Hematológicos en Ratones Sanos Tratados con Extractos de Sechium spp. Bachelor’s Thesis, FES Zaragoza, UNAM, México City, Mexico, 2013. [Google Scholar]
- Montiel-García, L. Participación del Extracto de Híbrido de Sechium H387-07 como Hipoglucemiante en un Modelo de Ratón Diabético. Bachelor’s Thesis, FES Zaragoza, UNAM, México City, Mexico, 2023. [Google Scholar]
- Coria-Bárcenas, A. Evaluación del Efecto Hipoglucémico de Sechium edule (Jacq.) y Su Interacción con la Metformina. Bachelor’s Thesis, FES Cuautitlán, UNAM, México City, Mexico, 2017. [Google Scholar]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Arghavani, P.; Mahdavi, M.; Khatibi, A.; García-Jiménez, C.; Moosavi-Movahedi, A.A. Hyperglycemia-driven signaling bridges between diabetes and cancer. Biochem. Pharmacol. 2024, 229, 116450. [Google Scholar] [CrossRef]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Agbabiaka, T.B.; Wider, B.; Watson, L.K.; Goodman, C. Concurrent use of prescription drugs and herbal medicinal products in older adults: A systematic review. Drugs Aging 2017, 34, 891–905. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Verardo, V.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. New insight into phenolic composition of chayote (Sechium edule (Jacq.) Sw.). Food Chem. 2019, 295, 514–519. [Google Scholar] [CrossRef]
- Vieira, E.F.; Fontoura, A.Q.; Delerue-Matos, C. Chayote (Sechium edule (Jacq.) Swartz) seed as an unexploited protein source: Bio-functional and nutritional quality of protein isolates. Foods 2023, 12, 2949. [Google Scholar] [CrossRef]
- Weykamp, C. HbA1c: A review of analytical and clinical aspects. Ann. Lab. Med. 2013, 33, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Little, R.R. Glycated hemoglobin standardization-National Glycohemoglobin Standardization Program (NGSP) perspective. Clin. Chem. Lab. Med. 2003, 41, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Hafliðadóttir, S.H.; Juhl, C.B.; Nielsen, S.M.; Henriksen, M.; Harris, I.A.; Bliddal, H.; Christensen, R. Placebo response and effect in randomized clinical trials: Meta-research with focus on contextual effects. Trials 2021, 22, 493. [Google Scholar] [CrossRef] [PubMed]
- Kaptchuk, T.J.; Miller, F.G. Placebo effects in medicine. N. Engl. J. Med. 2015, 373, 8–9. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Tang, W. Correlations between glycosylated hemoglobin and glucose levels in Chinese older adults with newly diagnosed type 2 diabetes mellitus. Turk. J. Med. Sci. 2022, 52, 1207–1215. [Google Scholar] [CrossRef]
- Munir, K.M.; Chandrasekaran, S.; Gao, F.; Quon, M.J. Mechanisms for food polyphenols to ameliorate insulin resistance and endothelial dysfunction: Therapeutic implications for diabetes and its cardiovascular complications. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E679–E686. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The therapeutic effects and mechanisms of quercetin on metabolic diseases: Pharmacological data and clinical evidence. Oxid. Med. Cell Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, S.J.; Gross, R.; Petit, P.; et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef]
- Bardy, G.; Virsolvy, A.; Quignard, J.F.; Ravier, M.A.; Bertrand, G.; Dalle, S.; Cros, G.; Magous, R.; Richard, S.; Oiry, C. Quercetin induces insulin secretion by direct activation of L-type calcium channels in pancreatic beta cells. Br. J. Pharmacol. 2013, 169, 1102–1113. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Z.; Dong, R.; Liu, P.; Zhang, X.; Li, Y.; Lai, X.; Cheong, H.F.; Wu, F.; Wang, Y.; et al. Rutin ameliorated lipid metabolism dysfunction of diabetic NAFLD via AMPK/SREBP1 pathway. Phytomedicine 2024, 126, 155437. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, J.R.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, I.S.; Park, J.; Kim, Y.; An, E.J.; Jang, H.J. Cucurbitacin B induces hypoglycemic effect in diabetic mice by regulation of AMP-activated protein kinase alpha and glucagon-like peptide-1 via bitter taste receptor signaling. Front. Pharmacol. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic potential of gallic acid from emblica officinalis: Improved glucose trans-porters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arista-Ugalde, T.L.; Delgado-Arroyo, S.; Gavia-García, G.; Hernández-Álvarez, D.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Rosado-Pérez, J.; Mendoza-Núñez, V.M. Hypoglycemic Effects of Sechium edule (Chayote) in Older Adults: A Systematic Review and Meta-Analysis of Clinical and Preclinical Trials. Foods 2025, 14, 2937. https://doi.org/10.3390/foods14172937
Arista-Ugalde TL, Delgado-Arroyo S, Gavia-García G, Hernández-Álvarez D, Aguiñiga-Sánchez I, Santiago-Osorio E, Rosado-Pérez J, Mendoza-Núñez VM. Hypoglycemic Effects of Sechium edule (Chayote) in Older Adults: A Systematic Review and Meta-Analysis of Clinical and Preclinical Trials. Foods. 2025; 14(17):2937. https://doi.org/10.3390/foods14172937
Chicago/Turabian StyleArista-Ugalde, Taide Laurita, Sebastián Delgado-Arroyo, Graciela Gavia-García, David Hernández-Álvarez, Itzen Aguiñiga-Sánchez, Edelmiro Santiago-Osorio, Juana Rosado-Pérez, and Víctor Manuel Mendoza-Núñez. 2025. "Hypoglycemic Effects of Sechium edule (Chayote) in Older Adults: A Systematic Review and Meta-Analysis of Clinical and Preclinical Trials" Foods 14, no. 17: 2937. https://doi.org/10.3390/foods14172937
APA StyleArista-Ugalde, T. L., Delgado-Arroyo, S., Gavia-García, G., Hernández-Álvarez, D., Aguiñiga-Sánchez, I., Santiago-Osorio, E., Rosado-Pérez, J., & Mendoza-Núñez, V. M. (2025). Hypoglycemic Effects of Sechium edule (Chayote) in Older Adults: A Systematic Review and Meta-Analysis of Clinical and Preclinical Trials. Foods, 14(17), 2937. https://doi.org/10.3390/foods14172937