Metabolomic Profile of Vaccinium corymbosum Leaves: Exploiting Diversity Among Ten Different Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. The Samples
2.2. Extraction Procedure
2.3. UHPLC-QTOF MS
2.4. Data Analysis
3. Results
3.1. Metabolic Profiling of V. corymbosum Cultivars by HPLC-QTOF MS
3.2. Metabolome Semi-Quantitative Evaluation
3.2.1. Global Metabolome Cultivars Comparison
3.2.2. Specific Metabolites’ Variation Between Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, S.M.; Soe, K.H.; Lee, T.H.; Kim, I.S.; Lee, Y.M.; Lim, B.O. Cognitive improving effects by highbush blueberry (Vaccinium corymbosum L.) vinegar on scopolamine-induced amnesia mice model. J. Agric. Food Chem. 2018, 66, 99–107. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, M.A.; Lim, J.H.; Lee, Y.M.; Kim, E.O.; Um, B.H.; Lim, B.O. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J. Nutr. Biochem. 2016, 28, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Heberlé, G.; Dos Santos, M.A.; Magri, S. Cosmetic formulations containing blueberry extracts (Vaccinium myrtillus L.). TOJSAT 2012, 2, 1–6. [Google Scholar]
- Tian, Y.; Puganen, A.; Alakomi, H.-L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves and branches of berry plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S.; Finn, C.E.; Hancock, J.F. Genotypic and environmental variation in antioxidant activity, total phenolic content, and anthocyanin content among blueberry cultivars. J. Am. Soc. Hortic. Sci. 2002, 127, 89–97. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Variation of phenolic profile and antioxidant activity of North American highbush blueberry leaves with variation of time and cultivar. Ind. Crops Prod. 2014, 62, 147–155. [Google Scholar] [CrossRef]
- Akšić, M.; Zagorac, D.; Sredojević, M.; Milivojević, J.; Gašić, U.; Meland, M.; Natić, M. Chemometric characterization of strawberries and blueberries according to their phenolic profile: Combined effect of cultivar and cultivation system. Molecules 2019, 24, 4310. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Barnackas, S.; Kazernavic, R.; Mazdzieriene, R.; Pukalskas, A.; Sipailiene, A.; Labokas, J.; Loziene, K.; Abrutiene, G. Variations in antioxidant capacity and phenolics in leaf extracts isolated by different polarity solvents from seven blueberry (Vaccinium L.) genotypes at three phenological stages. Acta Physiol. Plant. 2016, 38, 33. [Google Scholar] [CrossRef]
- Wu, H.; Chaia, Z.; Tathagat, R.P.; Zeng, Q.; Niu, L.; Li, D.; Yu, H.; Huang, W. Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 2019, 122, 548–560. [Google Scholar] [CrossRef]
- Mekky, R.H.; Contreras, M.M.; El-Gindi, M.R.; Abdel-Monem, A.R.; Abdel-Sattar, E.; Segura-Carretero, A. Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study. RSC Adv. 2015, 5, 17751–17767. [Google Scholar] [CrossRef]
- Mekky, R.H.; Abdel-Sattar, E.; Segura-Carretero, A.; Contreras, M.M. Phenolic compounds from Sesame cake and antioxidant activity: A new insight for Agri-food residues’ significance for sustainable development. Foods 2019, 8, 432. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, J.; Mattila, S.; Jakkola, L.; Pirttilä, A.M.; Tolonen, A. Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium Ruthe L.) leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.; Yang, B. Characterization of metabolite profiles of leaves of bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, H.; Huang, Z.; Zhang, C.; Lyu, L.; Li, W.; Li, W.; Wu, W. Metabolite profiling and classification of highbush blueberry leaves under different shade treatments. Metabolites 2022, 12, 79. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Antioxidant capacity of black currant varies with organ, season and cultivar. J. Agric. Food Chem. 2006, 54, 6271–6276. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Motiekaityte, V.; Vainoriene, R.; Raudone, L. Promising cultivars and intraspecific taxa of lingonberries (Vaccinium vitis-idaea L.): Profiling of phenolics and triterpenoids. J. Food Compos. Anal. 2022, 114, 104796. [Google Scholar] [CrossRef]
- Fan, M.; Lian, W.; Li, T.; Fan, Y.; Rao, Z.; Li, Y.; Qian, H.; Zhang, H.; Wu, G.; Qi, X.; et al. Metabolomics approach reveals discriminatory metabolites associating with the blue pigments from Vaccinium bracteatum thunb. leaves at different growth stages. Ind. Crops Prod. 2020, 147, 112252. [Google Scholar] [CrossRef]
- Shamilov, A.A.; Olennikov, D.N.; Pozdnyakov, D.I.; Bubenchikova, V.N.; Garsiya, E.R.; Larskii, M.V. Caucasian blueberry: Comparative study of phenolic compounds and neuroprotective and antioxidant potential of Vaccinium myrtilus and Vaccinium arctostaphylos leaves. Life 2022, 12, 2079. [Google Scholar] [CrossRef]
- Wang, H.; Xia, X.; An, L. Metabolomics analysis reveals the mechanism of hydrogen cyanamide in promoting flower bud break in blueberry. Agronomy 2021, 11, 102. [Google Scholar] [CrossRef]
- Umino, M.; Onozato, M.; Sakamoto, T.; Koishi, M.; Fukushima, T. Analyzing citramalic acid enantiomers in apples and commercial fruit juice by liquid chromatography-tandem mass spectrometry with pre-column derivatization. Molecules 2023, 28, 1556. [Google Scholar] [CrossRef]
- Luengwilai, K.; Saltveit, M.; Beckles, D.M. Metabolite content of harvested Micro-Tom tomato (Solanum lycopersicum L.) fruit is altered by chilling and protective heat-shock treatments as shown by GC–MS metabolic profiling. Postharvest Biol. Technol. 2012, 63, 116–122. [Google Scholar] [CrossRef]
- Cruz-Estrada, A.; Ruiz-Sánchez, E.; Cristobal-Alejo, J.; González-Coloma, A.; Andrés, M.F.; Gamboa-Angulo, M. Medium-chain fatty acids from Eugenia winzerlingii leaves causing insect settling deterrent, nematicidal, and phytotoxic effects. Molecules 2019, 24, 1724. [Google Scholar] [CrossRef]
- Weber, B.; Hoesch, L.; Rast, D.M. Protocatechualdehyde and other phenols as cell wall components of grapevine leaves. Phytochemistry 1995, 40, 433–437. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, T.H. Pancreatic lipase inhibitors in the roots of Taraxacum ohwianum, a herb used in Korean traditional medicine. Korean J. Food Preserv. 2011, 18, 53–58. [Google Scholar] [CrossRef]
- Pavlović, A.V.; Papetti, A.; Zagorac, D.; Gašić, U.; Mišić, D.; Tešić, Ž.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Stanoeva, J.; Stefova, M.; Andonovska, K.; Vankova, A.; Stafilov, T. Phenolics and mineral content in bilberry and bog bilberry from Macedonia. Int. J. Food Prop. 2017, 20, S863–S883. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlic-Maganja, D.; Bandelj, D.; Arbeiter, A.B.; Peeters, K.; Višnjevec, A.M.; Pražnikar, Z.J. HPLC-DAD-ESI-QTOF-MS determination of bioactive compounds and antioxidant activity comparison of the hydroalcoholic and water extracts from two Helichrysum italicum species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.-L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Petrikaite, V.; Pukalskas, A.; Sipailiene, A.; Raudone, L. Exploring Vaccinium vitis-idaea L. as a potential source of therapeutic agents: Antimicrobial, and anti-inflammatory activities of extracts and fractions. J. Ethnopharmacol. 2022, 292, 115207. [Google Scholar] [CrossRef]
- Chagas, M.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F.; Angeloni, C. Flavonols and flavones as potential ani-inflammatory, antioxidant, and antibacterial compounds. Oxidative Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Rawat, M.S.M.; Geeta Pant, G.; Devi Prasad, D.; Joshi, R.K.; Pande, C.B. Plant growth inhibitors (Proanthocyanidins) from Prunus armeniaca. Biochem. Syst. Ecol. 1998, 26, 13–23. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Biological potential of aromadendrin against human disorders: Recent development in pharmacological activities and analytical aspects. Pharmacol. Res. Mod. Chin. Med. 2024, 11, 100424. [Google Scholar] [CrossRef]
- Medic, A.; Smrke, T.; Hudina, M.; Veberic, R.; Zamljen, T. HPLC-Mass spectrometry analysis of phenolics comparing traditional bilberry and blueberry liqueurs. Food Res. Int. 2023, 173, 113373. [Google Scholar] [CrossRef]
- Brasseur, T.; Angenot, L. Six flavonol glycosides from leaves of Strychnos variabilis. Phytochemistry 1988, 27, 1487–1490. [Google Scholar] [CrossRef]
- Chen, P.Y.; Kuo, Y.C.; Chen, C.H.; Kuo, Y.H.; Lee, C.K. Isolation and immunomodulatory effect of homoisoflavones and flavones from Agave sisalana Perrine ex Engelm. Molecules 2009, 14, 1789–1795. [Google Scholar] [CrossRef]
- Gu, R.-H.; Morcol, T.; Liu, B.; Shi, M.-J.; Kennelly, E.J.; Long, C.-L. GC-MS, UPLC-QTOF-MS, and bioactivity characterization of Acer truncatum seeds. Ind. Crops Prod. 2019, 138, 111480. [Google Scholar] [CrossRef]
- Mencherini, T.; Cau, A.; Bianco, G.; Della, L.R.; Aquino, R.P.; Autore, G. An extract of Apium graveolens var. dulce leaves: Structure of the major constituent, apiin, and its anti-inflammatory properties. J. Pharm. Pharmacol. 2007, 59, 891–897. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.K.; Liao, X.; Zhu, X.M.; Peng, S.L.; Ding, L.S. Rapid identification of compounds in Glycyrrhiza uralensis by liquid chromatography/tandem mass spectrometry. Chin. J. Anal. Chem. 2004, 32, 174–178. [Google Scholar]
- Fatoki, T.H.; Ajiboye, B.O.; Aremu, A.O. In silico evaluation of the antioxidant, antii-inflammatory, and dermatocosmetic activities of phytoconstituents in licorice (Glycyrrhiza glabra L.). Cosmetics 2023, 10, 69. [Google Scholar] [CrossRef]
- Aziz, I.M.; Alshalan, R.M.; Rizwana, H.; Alkhelaiwi, F.; Almuqrin, A.M.; Aljowaie, R.M.; Alkubaisi, N.A. Chemical composition, antioxidant, anticancer, and anibacterial activities of roots and seeds of Ammi visnaga L. methanol extracts. Pharmaceuticals 2024, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Duan, Y.; Wei, Z.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Integrated physiological and metabolomic analyses reveal the differences in the fruit quality of the blueberry cultivated in three soilless substrates. Foods 2023, 11, 3965. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jung, H.S.; Giang, P.M.; Jin, X.; Lee, S.; Son, P.T.; Lee, D.; Hong, Y.S.; Lee, K.; Lee, J.J. Blockade of nuclear factor-B signaling pathway and anti-Inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J. Pharmacol. Exp. Ther. 2005, 316, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, J.; Rasul, A.; Shah, M.A.; Hussain, G.; Riaz, A.; Sarfraz, I.; Zafar, S.; Adnan, M.; Khan, A.H.; Selamoglu, Z. Cardamini: A new player to fight cancer via multiple signaling pathways. Life Sci. 2020, 250, 117591. [Google Scholar] [CrossRef]
- Erst, A.S.; Chernonosov, A.A.; Petrova, N.V.; Kulikovskiy, M.S.; Maltseva, S.Y.; Wang, W.; Kostikova, V.A. Investigation of chemical constituents of Eranthis longistipitata (Ranunculaceae): Coumarins and furochromones. Int. J. Mol. Sci. 2022, 23, 406. [Google Scholar] [CrossRef]
- da Silva, A.P.G.; Sganzerla, W.G.; John, O.D.; Marchiosi, R. A comprehensive review of the classification, sources, biosynthesis, and biological properties of hydroxybenzoic and hydroxycinnamic acids. Phytochem. Rev. 2025, 24, 1061–1090. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I. Hydroxycinnamic Acids. In Handbook of Food Bioactive Ingredients; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Li, Y.C.; Li, B.X.; Geng, L.J. Hypolipidemic and antioxidant effects of total flavonoids from blueberry leaves. Eur. Food Res. Technol. 2011, 233, 897–903. [Google Scholar] [CrossRef]
- Mathivha, P.L.; Msagati, T.A.M.; Thibane, V.S.; Mudau, F.N. Phytochemical Analysis of Herbal Teas and Their Potential Health, and Food Safety Benefits: A Review. In Herbal Medicine in India; Sen, S., Chakraborty, R., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Song, G.Q. Blueberry (Vaccinium corymbosum L.). In Agrobacterium Protocols. Methods in Molecular Biology; Wang, K., Ed.; Springer: New York, NY, USA, 2015; Volume 1224. [Google Scholar]
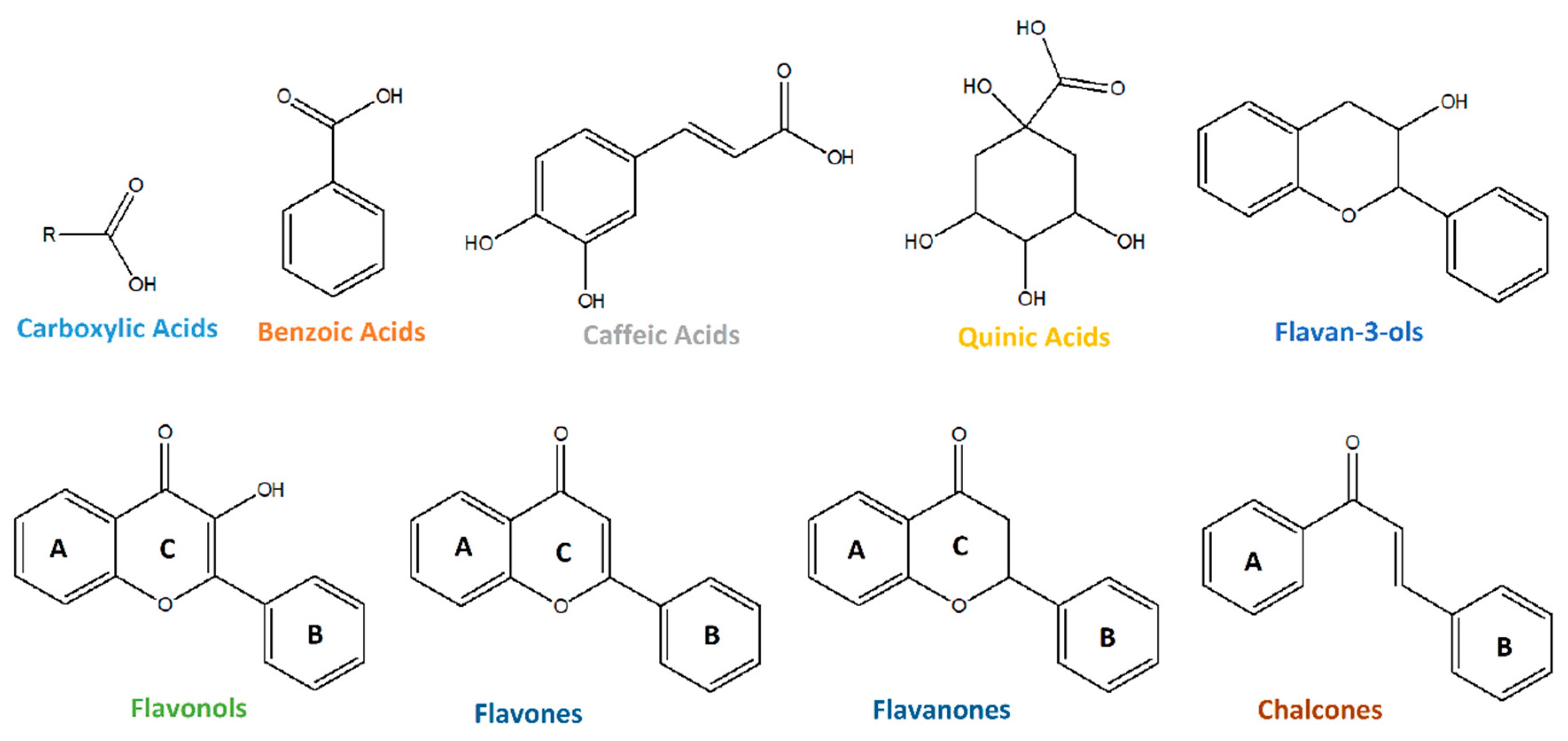
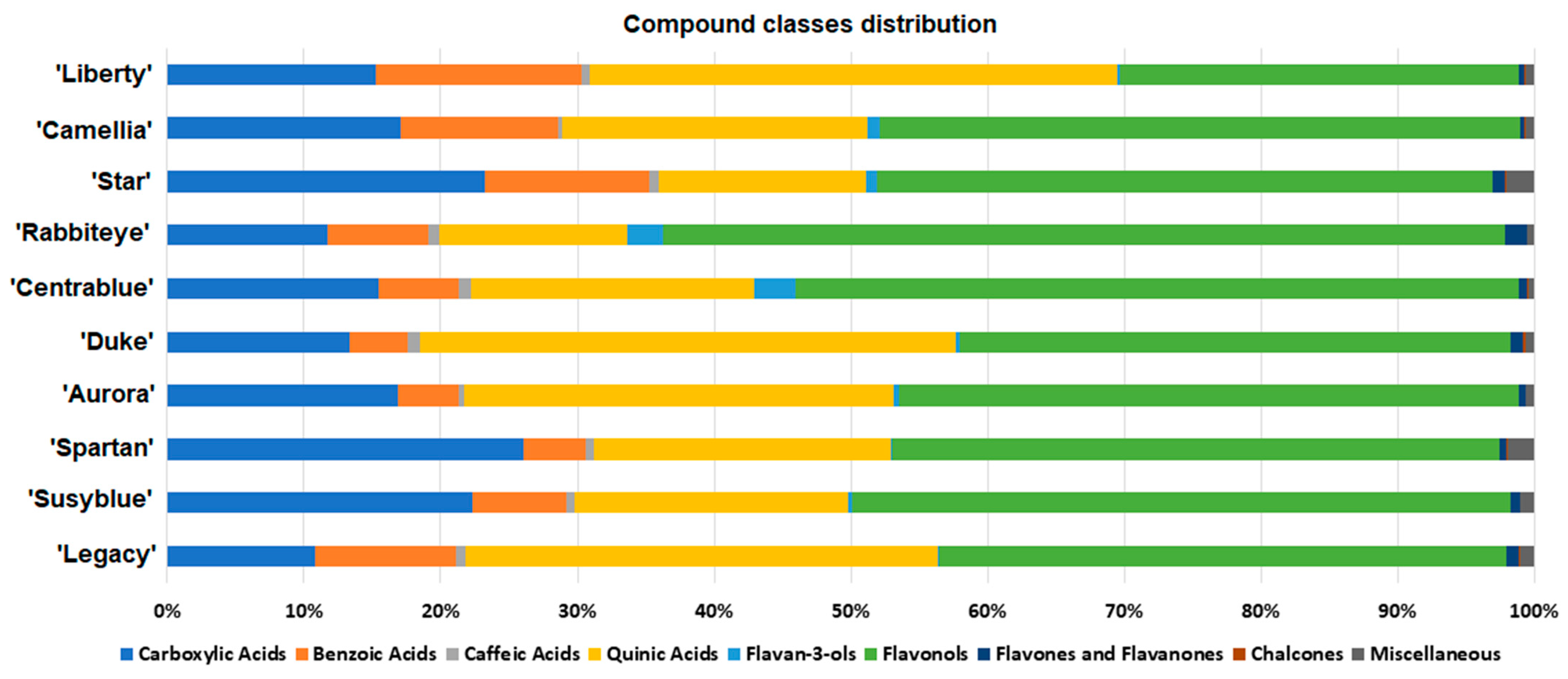
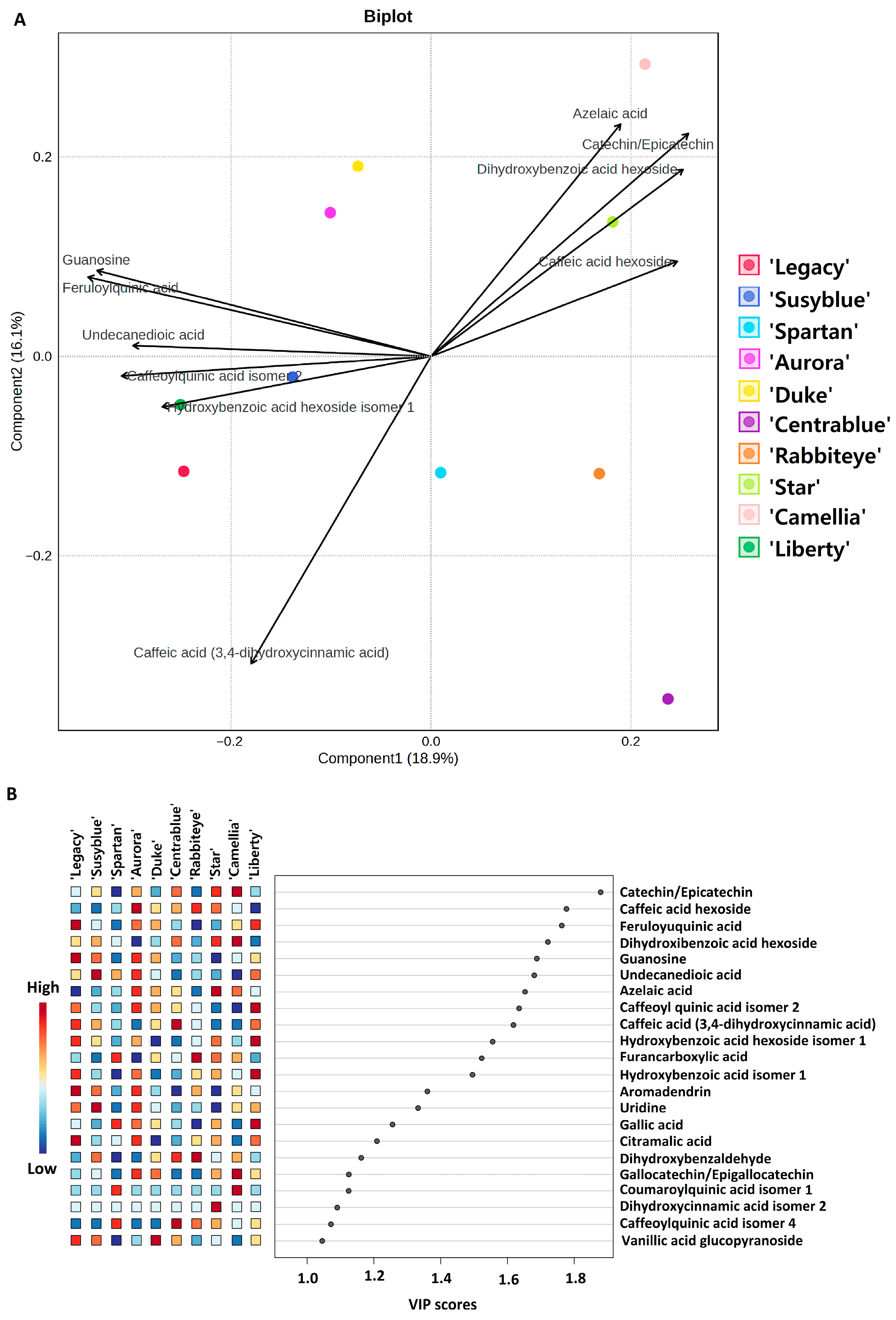
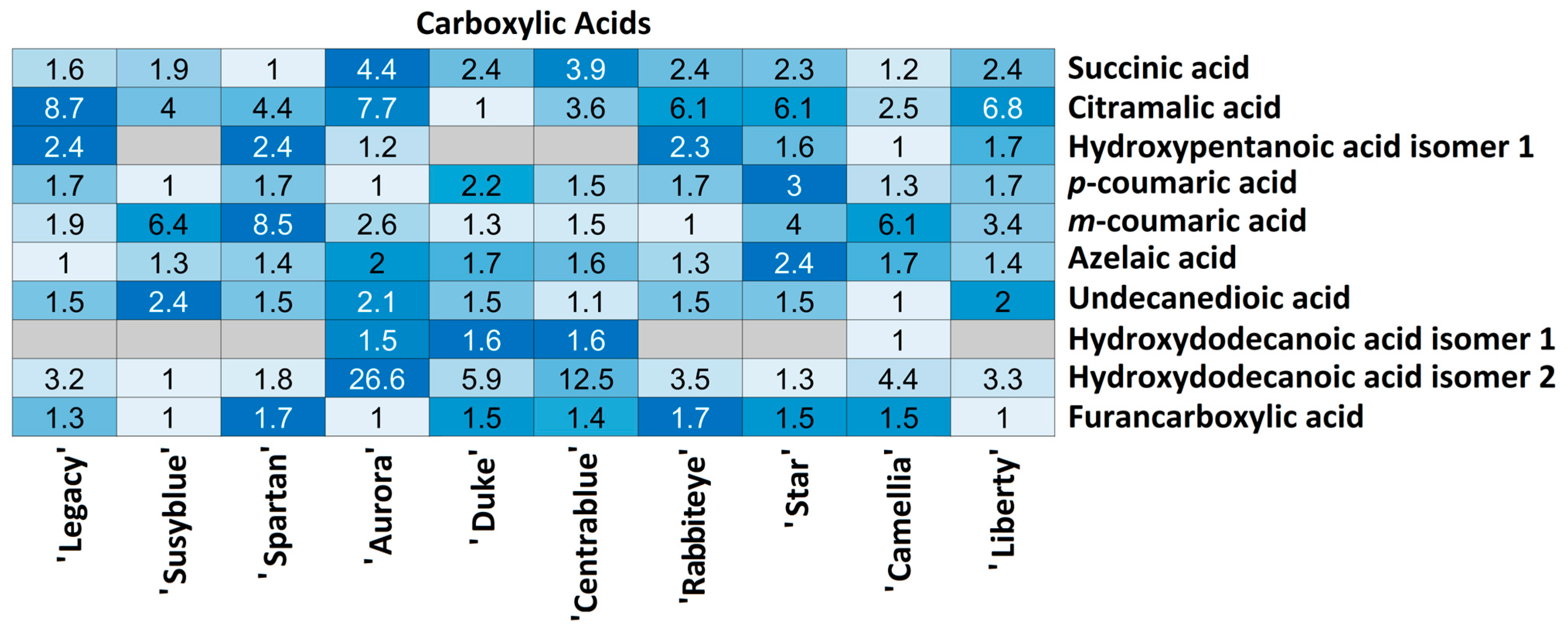


| # | Formula | [M−H]− | Tr | Fragments | mSigma | |Accuracy|(ppm) | Annotation | |
|---|---|---|---|---|---|---|---|---|
| Carboxylic Acids | C1 | C4H6O4 | 117.01904 | 1.98 | 73.029 | 4.7 | 2.48 | Succinic acid |
| C2 | C5H8O5 | 147.03024 | 1.97 | 87.009; 85.029; 129.019 | 7.1 | 2.38 | Citramalic acid | |
| C3 | C5H10O3 | 117.05536 | 2.95 | 73.029; 59.014 | 21.0 | 3.08 | Hydroxypentanoic acid isomer 1 | |
| C4 | C9H8O3 | 163.04052 | 4.95 | 119.050; 71.014 | 6.9 | 3.01 | p-coumaric acid | |
| C5 | 163.04048 | 10.28 | 119.050 | 11.8 | 2.45 | m-coumaric acid | ||
| C6 | C9H16O4 | 187.09791 | 13.31 | 125.097; 169.089; 123.082; 143.108 | 9.4 | 1.92 | Azelaic acid | |
| C7 | C11H20O4 | 215.12888 | 18.03 | 197.119; 153.129 | 17.2 | 0.01 | Undecanedioic acid | |
| C8 | C12H24O3 | 215.16519 | 17.40 | 169.157; 87.008; 171.103 | 8.3 | −0.93 | Hydroxydodecanoic acid isomer 1 | |
| C9 | 215.16526 | 19.21 | 169.160; 197.055; 199.133 | 8.4 | 0.88 | Hydroxydodecanoic acid isomer 2 | ||
| C10 | C14H22O4 | 253.14435 | 19.69 | 209.155; 210.159; 59.015; 209.120; 89.027 | 13.2 | 1.34 | Furancarboxylic acid | |
| Benzoic Acids | B11 | C7H6O3 | 137.02484 | 1.74 | 93.038; 111.008; 81.034 | 4.98 | 3.07 | Dihydoxybenzaldehyde |
| B12 | 137.02475 | 2.86 | 93.035; 94.038 | 7.86 | 2.26 | Hydroxybenzoic acid isomer 1 | ||
| B13 | 137.02479 | 11.99 | 93.035; 108.020 | >30 | 2.70 | Hydroxybenzoic acid isomer 2 | ||
| B14 | 137.02479 | 13.04 | 93.035; 108.022; 94.038 | >30 | 2.70 | Hydroxybenzoic acid isomer 3 | ||
| B15 | C7H6O4 | 153.01959 | 7.39 | 109.030; 108.022; 123.046 | 10.99 | 2.03 | Dihydroxybenzoic acid isomer 1 | |
| B16 | 153.01952 | 9.82 | 109.030 | >30 | 1.24 | Dihydroxybenzoic acid isomer 2 | ||
| B17 | C7H6O5 | 169.01453 | 2.51 | 125.025 | >30 | 1.66 | Gallic acid | |
| B18 | 169.01441 | 3.44 | 125.025; 124.017 | >30 | 0.95 | Trihydroxybenzoic acid isomer 1 | ||
| B19 | 169.01450 | 4.20 | 151.004; 125.024; 83.012 | >30 | 1.48 | Tri-hydroxybenzoic acid isomer 2 | ||
| B20 | C13H16O8 | 299.07716 | 2.86 | 137.025; 93.034; 139.040 | 15.17 | 0.50 | Hydroxybenzoic acid hexoside isomer 1 | |
| B21 | 299.07681 | 4.05 | 137.025; 179.036; 239.057; 151.041 | 16.62 | 1.17 | Hydroxybenzoic acid hexoside isomer 2 | ||
| B22 | 299.07666 | 5.64 | 137.024; 93.033 | >30 | 1.94 | Hydroxybenzoic acid hexoside isomer 3 | ||
| B23 | C13H16O9 | 315.07196 | 3.58 | 152.012; 108.022 | 16.92 | 0.63 | Dihydroxybenzoic acid hexoside | |
| B24 | C14H18O9 | 329.08707 | 8.45 | 167.036; 191.035; 209.047; 123.045 | 3.37 | 0.36 | Vanillic acid glucopyranoside | |
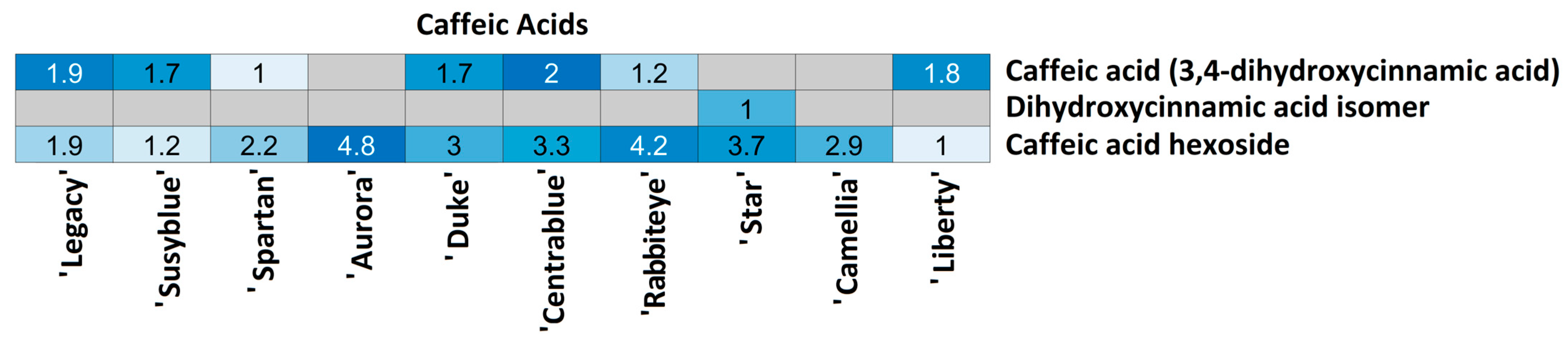
| Caffeic Acids | Caf25 | C9H8O4 | 179.03527 | 8.63 | 135.046; 134.038 | >30 | 1.62 | Caffeic acid (3,4-dihydroxycinnamic acid) |
| Caf26 | 179.03529 | 13.63 | 135.047; 137.060 | >30 | 1.56 | Dihydroxycinnamic acid isomer 2 | ||
| Caf27 | C15H18O9 | 341.08777 | 6.20 | 179.035; 135.035; 181.050 | 18.7 | 1.88 | Caffeic acid hexoside | |
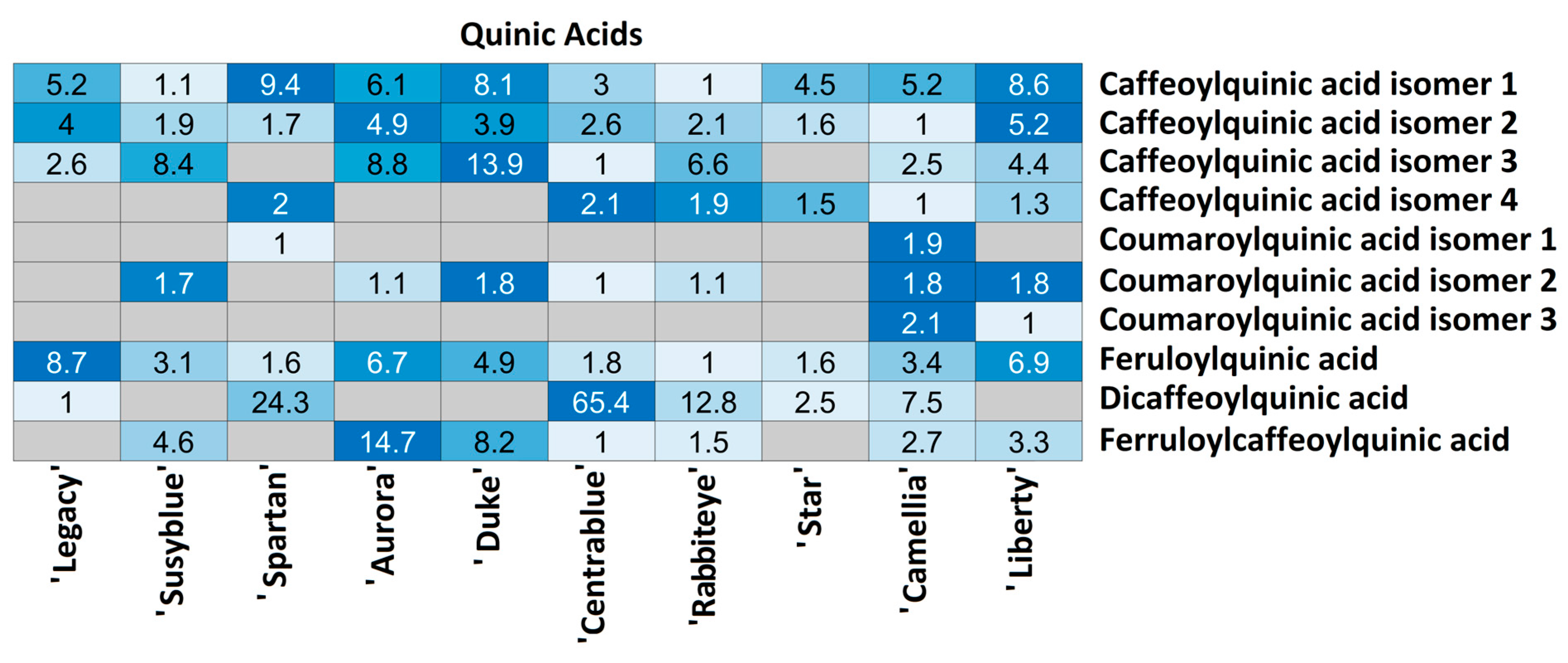
| Quinic Acids | Q28 | C16H18O9 | 353.08767 | 1.39 | 191.057; 179.036; 135.045; 173.046 | 15.7 | 0.20 | Caffeoylquinic acid isomer 1 |
| Q29 | 353.08695 | 9.09 | 191.057 | 27.6 | 2.44 | Caffeoylquinic acid isomer 2 | ||
| Q30 | 353.08689 | 12.65 | 191.057; 179.035; 135.046; 192.059; 165.018; 173.046 | 6.0 | 2.66 | Caffeoylquinic acid isomer 3 | ||
| Q31 | 353.08662 | 13.61 | 191.057; 173.046; 179.036; 135.045 | 26.2 | 3.37 | Caffeoylquinic acid isomer 4 | ||
| Q32 | C16H18O8 | 337.09237 | 10.59 | 191.057; 173.046 | 18.1 | 1.57 | Coumaroylquinic acid isomer 1 | |
| Q33 | 337.09225 | 14.27 | 163.041; 191.057; 119.051 | 4.0 | 1.51 | Coumaroylquinic acid isomer 2 | ||
| Q34 | 337.09249 | 16.12 | 163.041; 191.057; 173.047; 164.044; 119.050 | 21.5 | 1.54 | Coumaroylquinic acid isomer 3 | ||
| Q35 | C17H20O9 | 367.10304 | 9.14 | 191.057; 173.046; 193.053; 93.035 | 20.3 | 0.57 | Feruloylquinic acid | |
| Q36 | C25H24O12 | 515.11837 | 15.48 | 353.089; 173.046; 179.036; 191.057 | 1.2 | 2.19 | Dicaffeoylquinic acid | |
| Q37 | C26H26O12 | 529.13358 | 14.90 | 367.104; 193.051; 191.056; 353.088; 173.046; 179.037 | 10.7 | 3.27 | Feruloylcaffeoylquinic acid | |
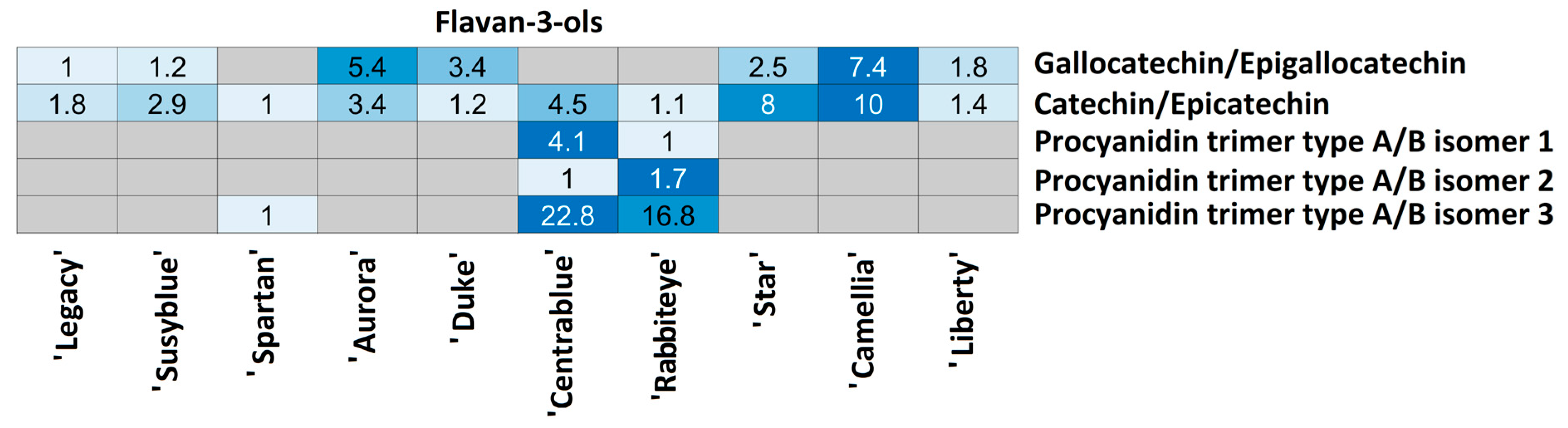
| Flavan-3-ols | Fla38 | C15H14O7 | 305.06639 | 3.88 | 125.025; 165.020; 167.036; 137.025 | 16.7 | 0.43 | Gallocatechin/Epigallocatechin |
| Fla39 | C15H14O6 | 289.07128 | 5.81 | 245.082; 203.071; 205.051; 137.025; 179.036 | 21.8 | 1.45 | Catechin/Epicatechin | |
| Fla40 | C45H36O18 | 863.18313 | 5.04 | 411.073; 289.072; 285.041;712.137; 451.104 | >30 | 0.28 | Procyanidin trimer type A/B isomer 1 | |
| Fla41 | 863.18424 | 7.95 | 289.072; 411.072; 451.104; 711.136; 573.104 | 25.8 | 1.15 | Procyanidin trimer type A/B isomer 2 | ||
| Fla42 | 863.18285 | 8.58 | 411.073; 289.073; 451.104; 711.134; 573.103 | >30 | 0.30 | Procyanidin trimer type A/B isomer 3 | ||
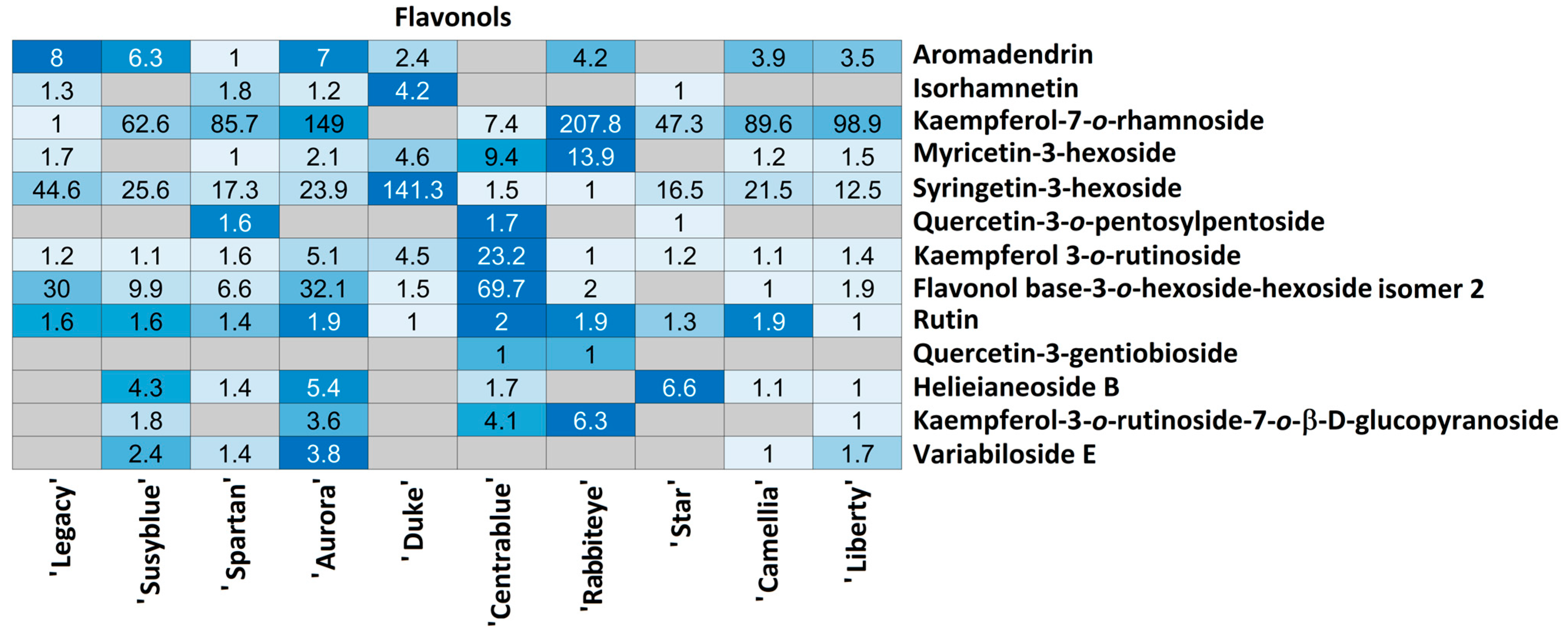
| Flavonols | Flo43 | C15H12O6 | 287.05553 | 13.52 | 259.060; 243.069; 125.024; 152.010 | 24.2 | 1.43 | Aromadendrin |
| Flo44 | C16H12O7 | 315.05058 | 18.35 | 300.028; 301.032; 151.004 | 16.9 | 1.46 | Isorhamnetin | |
| Flo45 | C21H20O10 | 431.09760 | 14.60 | 285.040; 284.033; 191.056 | 22.1 | 1.79 | Kaempferol-7-O-rhamnoside | |
| Flo46 | C21H20O13 | 479.08169 | 11.82 | 317.029; 191.056; 301.033 | >30 | 1.42 | Myricetin-3-hexoside | |
| Flo47 | C23H24O13 | 507.11269 | 13.11 | 344.054 | 18.5 | 1.77 | Syringetin-3-hexoside | |
| Flo48 | C25H26O15 | 565.11834 | 11.34 | 300.027; 463.087; 191.056 | >30 | 3.11 | Quercetin-3-O-pentosylpentoside | |
| Flo49 | C27H30O15 | 593.14989 | 11.81 | 284.033; 463.088; 327.050 | 13.9 | 2.24 | kaempferol 3-O-rutinoside | |
| Flo50 | 593.15105 | 12.38 | 285.042; 284.034 | 24.8 | 0.42 | Flavonol base-3-O-hexoside-hexoside isomer 2 | ||
| Flo51 | C27H30O16 | 609.14667 | 11.00 | 300.029 | >30 | 0.72 | Rutin | |
| Flo52 | C27H30O17 | 625.13923 | 13.20 | 445.077; 463.088; 301.035; 464.090; 300.029 | >30 | 2.86 | Quercetin-3-gentiobioside | |
| Flo53 | C32H38O20 | 741.18837 | 9.60 | 300.028; 191.057; 353.087; 417.123; 178.998 | >30 | 0.05 | Helieianeoside B | |
| Flo54 | C33H40O20 | 755.20502 | 12.40 | 593.152; 285.042 | >30 | 1.40 | Kaempferol-3-O-rutinoside-7-O-β-D-glucopyranoside | |
| Flo55 | C36H36O18 | 755.18240 | 16.96 | 593.130; 285.040 | 77.9 | 0.04 | Variabiloside E | |
| Flavones and Flavanones | Flav56 | C17H16O5 | 299.09344 | 18.24 | 179.035; 151.004; 135.046; 165.020; 121.030; 229.053; 149.063 | 15.7 | 3.81 | 3,9-dihydroeucomin |
| Flav57 | C21H22O11 | 449.10701 | 13.53 | 287.056; 191.057; 151.004; 257.082; 301.033; 135.045 | 29.9 | 4.14 | Miscanthoside | |
| Flav58 | C26H28O14 | 563.13989 | 7.74 | 191.057; 353.088 | >30 | 1.31 | Apiin | |
| Flav59 | C27H32O14 | 579.17045 | 8.66 | 417.118; 418.123; 307.083; 335.077; 191.057 | >30 | 2.56 | Glucoliquiritin | |
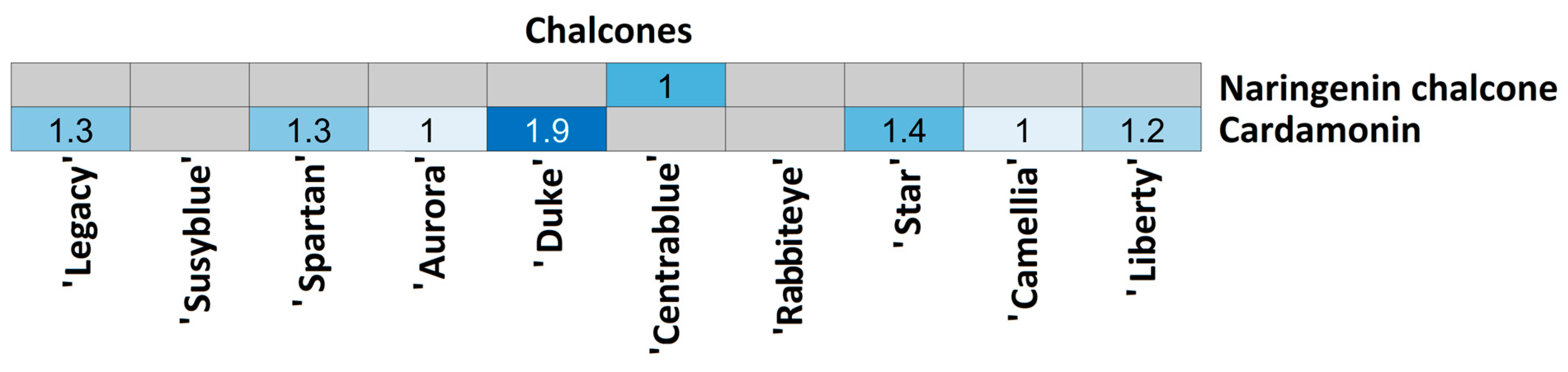
| Chalcones | Cha60 | C15H12O5 | 271.06069 | 13.03 | 151.003; 165.055; 119.050; 177.019; 228.076 | >30 | 1.88 | Naringenin chalcone |
| Cha61 | C16H14O4 | 269.08231 | 19.83 | 134.037; 178.028; 137.025; 133.029; 139.042 | 13.9 | 1.26 | Cardamonin | |
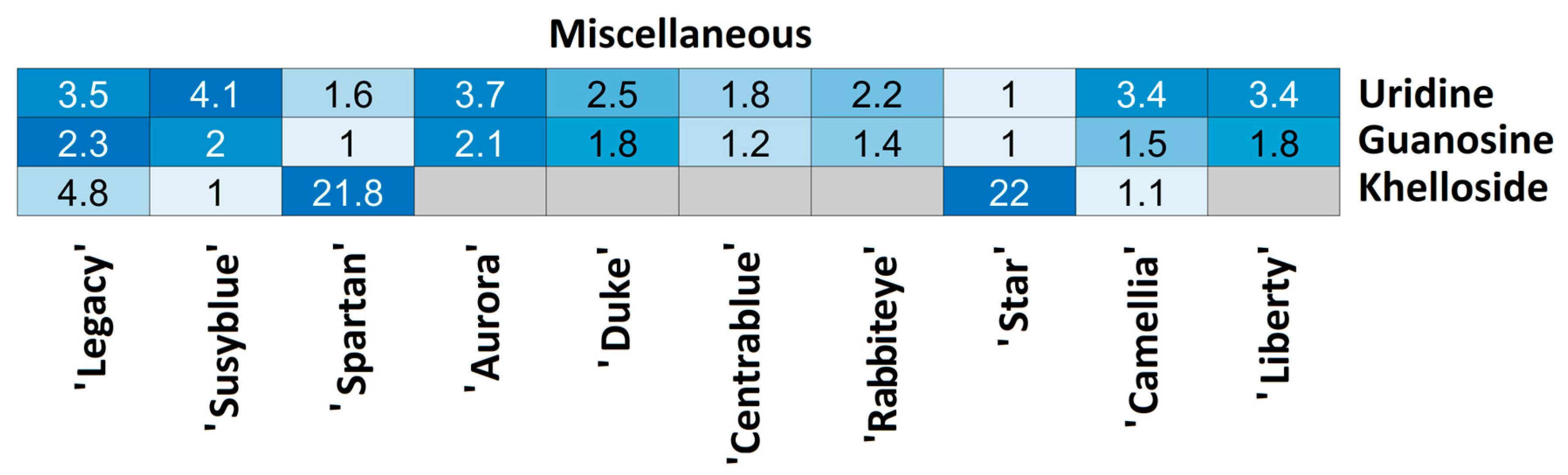
| Miscellaneous | M62 | C9H12N2O6 | 243.06211 | 1.80 | 200.055; 42.000; 153.029; 140.035; 71.013 | 10.1 | 0.45 | Uridine |
| M63 | C10H13N5O5 | 282.08430 | 2.00 | 150.043; 133.018; 117.019; 113.025; 191.055 | 10.8 | 0.21 | Guanosine | |
| M64 | C19H20O10 | 407.09745 | 5.77 | 245.045 | 12.0 | −2.65 | Khelloside |
| Compound | Cultivar 1 | Cultivar 2 | Corrected p-Value (Holm) |
|---|---|---|---|
| Azelaic acid | ‘Aurora’ | ‘Susyblue’ | 0.0042 |
| Azelaic acid | ‘Aurora’ | ‘Liberty’ | 0.0042 |
| Azelaic acid | ‘Camellia’ | ‘Susyblue’ | 0.0042 |
| Azelaic acid | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Azelaic acid | ‘Star’ | ‘Susyblue’ | 0.0042 |
| Azelaic acid | ‘Star’ | ‘Liberty’ | 0.0042 |
| Undecanedioic acid | ‘Camellia’ | ‘Susyblue’ | 0.0042 |
| Undecanedioic acid | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Hydroxybenzoic acid hexoside isomer 1 | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Dihydroxybenzoic acid hexoside | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Caffeic acid (3,4-dihydroxycinnamic acid) | ‘Aurora’ | ‘Susyblue’ | 0.0042 |
| Caffeic acid (3,4-dihydroxycinnamic acid) | ‘Aurora’ | ‘Liberty’ | 0.0042 |
| Caffeic acid (3,4-dihydroxycinnamic acid) | ‘Camellia’ | ‘Susyblue’ | 0.0042 |
| Caffeic acid (3,4-dihydroxycinnamic acid) | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Caffeic acid (3,4-dihydroxycinnamic acid) | ‘Star’ | ‘Susyblue’ | 0.0042 |
| Caffeic acid (3,4-dihydroxycinnamic acid) | ‘Star’ | ‘Liberty’ | 0.0042 |
| Caffeic acid hexoside | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Caffeoylquinic acid isomer 2 | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Feruloylquinic acid | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Catechin/Epicatechin | ‘Camellia’ | ‘Liberty’ | 0.0042 |
| Guanosine | ‘Camellia’ | ‘Liberty’ | 0.0042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.; Pintado, M.E.; Sousa, C. Metabolomic Profile of Vaccinium corymbosum Leaves: Exploiting Diversity Among Ten Different Cultivars. Foods 2025, 14, 2846. https://doi.org/10.3390/foods14162846
Ribeiro T, Pintado ME, Sousa C. Metabolomic Profile of Vaccinium corymbosum Leaves: Exploiting Diversity Among Ten Different Cultivars. Foods. 2025; 14(16):2846. https://doi.org/10.3390/foods14162846
Chicago/Turabian StyleRibeiro, Tânia, Manuela E. Pintado, and Clara Sousa. 2025. "Metabolomic Profile of Vaccinium corymbosum Leaves: Exploiting Diversity Among Ten Different Cultivars" Foods 14, no. 16: 2846. https://doi.org/10.3390/foods14162846
APA StyleRibeiro, T., Pintado, M. E., & Sousa, C. (2025). Metabolomic Profile of Vaccinium corymbosum Leaves: Exploiting Diversity Among Ten Different Cultivars. Foods, 14(16), 2846. https://doi.org/10.3390/foods14162846








