1. Introduction
As one of the important aquaculture economic fish in China, large yellow croakers (
Larimichthys crocea), also known as yellow croakers, cucumber croakers, etc., are mainly distributed in Fujian, Zhejiang, Shandong, and Guangdong [
1]. The large yellow croaker has a reputation as a “National Fish” because of its delicious meat, rich in vitamins, trace elements such as selenium, phosphorus, potassium, and unsaturated fatty acids that are beneficial to the human body [
2]. In recent years, the aquaculture industry of large yellow croakers in China had been growing rapidly, with aquaculture production reaching 281,000 tons in 2023 [
3]. Nevertheless, large yellow croakers are susceptible to microorganisms, protein denaturation, and autolytic enzymes during storage, transportation, and marketing, which easily caused spoilage and deterioration and deteriorated their quality [
4]. Traditional sales methods hardly meet the current market demands, making it crucial to explore novel preservation technologies.
Nano-bubbles (NB)s are spherical bubbles < 200 nm in diameter with properties such as strong oxidation and mechanical sterilization [
5]. A highly reactive hydroxyl radical (–OH) is released from NBs upon rupture, which can disrupt microbial biofilms and other structures, thereby achieving a sterilizing effect [
6,
7]. Also, the pressure changes from NB rupture could have a direct mechanical damaging effect on microorganisms [
8]. In
Vibrio parahaemolyticus and
Listeria monocytogenes biofilm removal studies, NBs could reduce microorganisms by one to three orders of magnitude [
9]. When NBs were used to treat
Vibrio parahaemolyticus in aquaculture water, the bacterial reduction rate was 31% [
10]. Although NBs destroyed microbial biofilms and other structures, the bacterial reduction effect was not obvious when acting alone. It was shown that NB treatment alone was not effective at inhibiting
Vibrio parahaemolyticus and
Aeromonas hydrophila [
11]. Combining the application of NBs with green bacterial reduction agents becomes an important direction to enhance the effectiveness of bacterial reduction.
Slightly acidic electrolyzed water (SAEW) is produced as a non-thermal sterilization technique by electrolysis of an aqueous solution of sodium chloride or hydrochloric acid [
12]. Based on a low pondus hydrogenii, high oxidation reduction potential, and available chlorine, SAEW achieves sterilization by disrupting microbial cell membranes, inhibiting enzyme activities, and through oxidative reactions [
13]. The efficient and broad-spectrum, non-residue characteristics of SAEW have been used widely in the field of aquatic product preservation. In
Thunnus obesus [
14],
Teuthida [
15] and
Pampus [
16] studies, SAEW treatment reduced the number of microorganisms on the surface of aquatic products by 2~3 logarithmic levels, significantly inhibited the growth of total viable counts, TVB-N, and TBA values during the storage period, and prolonged the shelf-life by 2~3 days, while maintaining a better organoleptic quality. Meanwhile, the effect of SAEW, combined with NB treatment, on the sensory quality, microbial changes, taste, and volatile flavor compounds of vacuum-packed large yellow croaker during refrigerated storage has rarely been reported on.
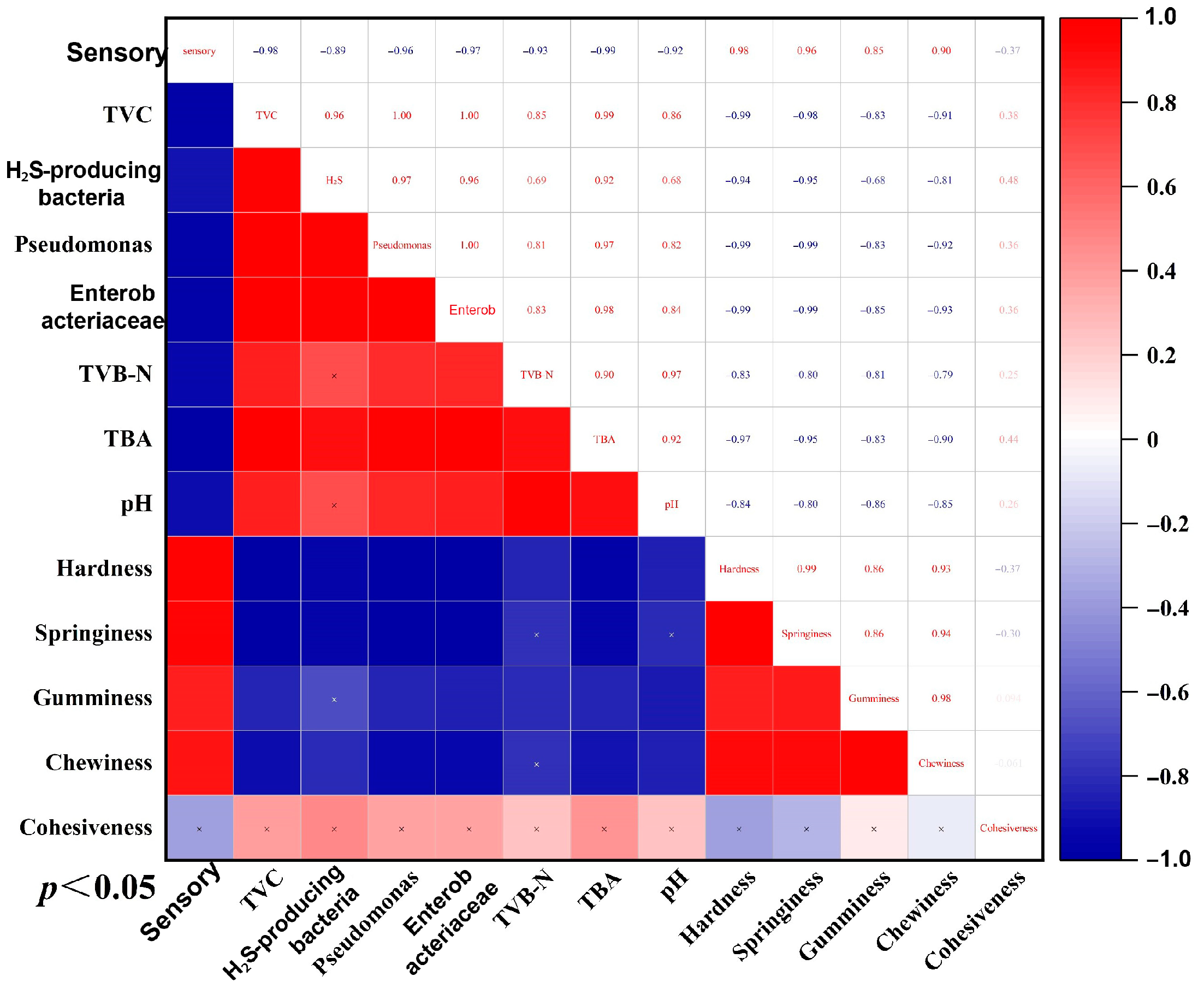
In this experiment, large yellow croakers were treated with SAEW combined with NBs and then vacuum-packed at 4 °C for refrigeration. By optimizing the parameters of the SAEW-NB treatment, this study systematically evaluated its effects on the physicochemical properties (TVB-N, TBA, and pH), microbial communities (H2S-producing bacteria, Pseudomonas, and Enterobacteriaceae), taste, and volatile flavor compounds of large yellow croakers. And combined with correlation analysis to reveal the trend of quality change in large yellow croaker. This study provided critical theoretical support and practical guidance for the research and development of preservation technologies for aquatic products.
2. Materials and Methods
2.1. Large Yellow Croaker
At night, farming large yellow croakers were caught in the sea of Sanduwan, Ningde, China. Then, large yellow croakers were placed on ice and immediately transported by refrigerated truck within 2 h to Cai Shi Aquatic Co. (Ningde, China). A total of 136 large yellow croakers were selected and transported to the laboratory within 1 h in stratified ice–fish layers. The scales, gills, and viscus of the large yellow croakers were removed and cleaned with fresh water within 2 h [
17]. The total elapsed time between capture and treatment was 5 h. Of the selected fish, 16 samples were for comparative experiments, 72 samples were for single-factor experiments, and 48 samples were randomly selected to be divided into the control group (CG) and the slightly acidic electrolyzed water combined with nano-bubbles treatment group (SAEW-NB). After vacuum packing, the large yellow croakers were stored in a low-temperature incubator (4 ± 1 °C) (HWS-70B, Taisite Instrument Co., Tianjin, China) and sampled at 0, 2, 4, 6, 8, and 10 days, respectively. The basic information and composition of the large yellow croaker (
Table 1) were determined with reference to the methods described by Deng et al. [
18].
2.2. Preparation of SAEW and NB
The SAEW-NB treatment system was designed with reference to the experimental approaches of Lan et al. [
19] and Jhunkeaw et al. [
20].
SAEW: The SAEW of different available chlorine concentrations (ACCs) was generated by adjusting the electric current magnitude of the electrolyzed water generator (Harmong-1000, Rui Andre Environmental Equipment Co., Ltd., Beijing, China) to electrolyze a solution containing 5% sodium chloride (NaCl). This was pre-cooled for storage away from the light. Before the experiment, the pondus hydrogenii (pH), oxidation reduction potential (ORP), and ACC were measured by using a pH meter (PHS-3E, Inase Scientific Instrument Co., Ltd., Shanghai, China), ORP meter (ORP-BL, Qiwei Instrument Co., Ltd., Hangzhou, China), and ACC meter (GDYS-104SM, Jida-Little Swan Instrument Co., Ltd., Changchun, China), respectively.
NB: The NBs were generated by using a nano-bubble generator (HM-KS-3G, Huankong Research Institute, Kaifeng, China). The average diameter of the NBs was <200 nm, and the density was 1 × 108 particles/mL. The pump flow rate of the nano-bubble generator was 5 L/min. The pump was connected to an acidic electrolyzed water tank with a diameter of 50 cm and a capacity of 30 L.
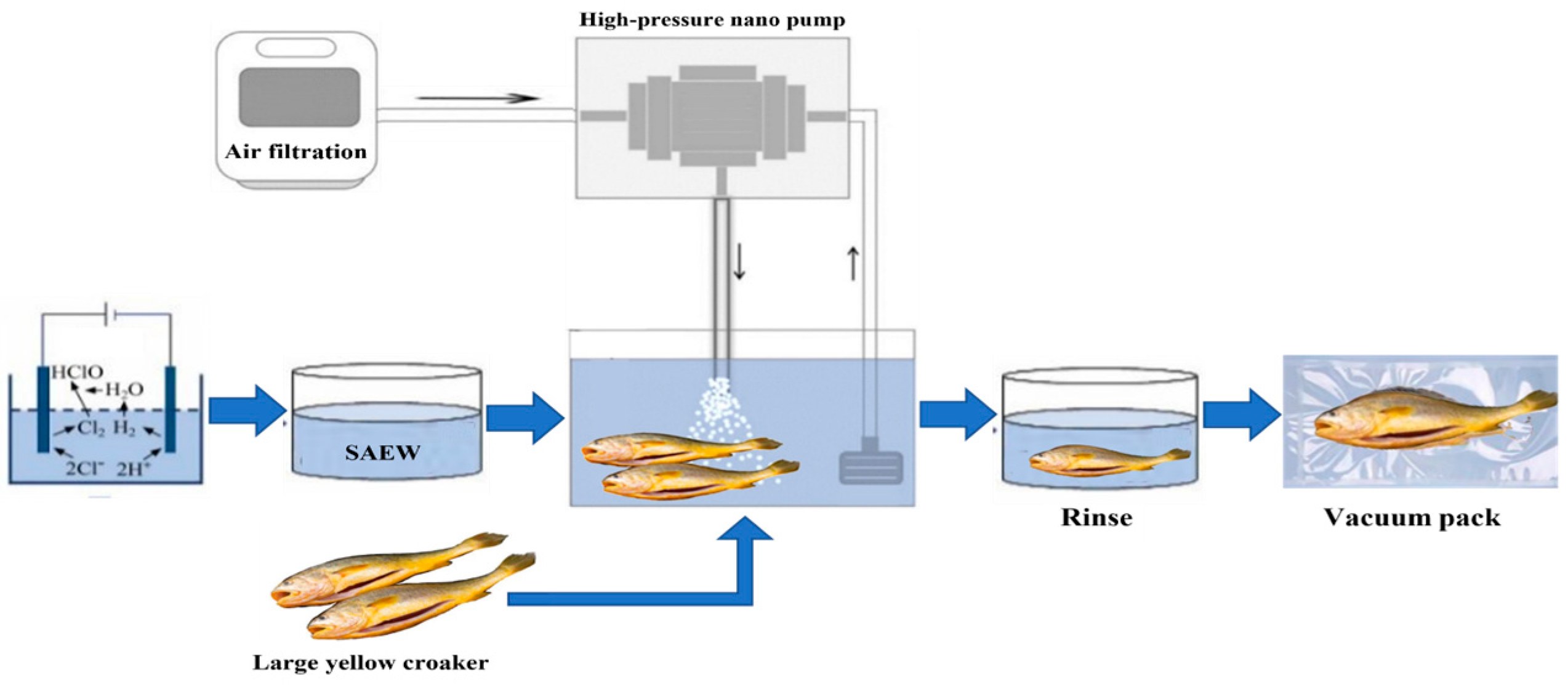
SAEW-NB operation is illustrated in
Figure 1. Firstly, SAEW with the target available chlorine concentration was generated using an electrolyzed water generator, transferred to a holding tank, and pre-cooled to the required water temperature. Secondly, the nano-bubble generator was connected. Its pump drew water from the electrolyzed water tank, then mixed purified air and SAEW in the nano-bubble generator to produce SAEW-NB water. The SAEW-NB water flowed back into the water tank. Finally, rinse and vacuum pack.
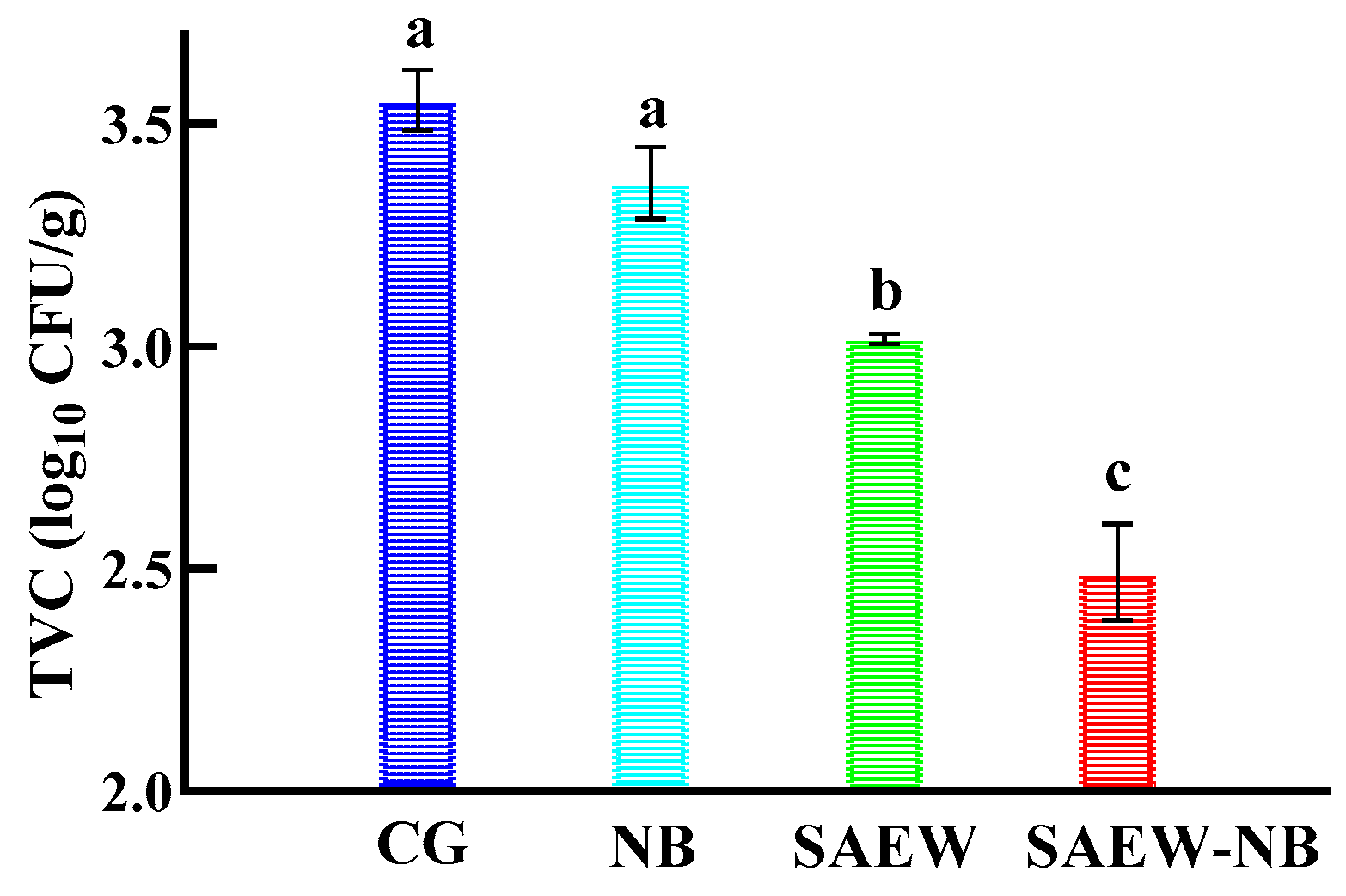
2.3. Comparative Experiments
Sixteen large yellow croakers were randomly divided into a CG, SAEW, NB, and SAEW-NB group, with four samples in each group. Referring to the results of Jin et al. and Liu et al. [
21,
22], the ACC was taken as 40 mg/L, solid–liquid ratio of 1:6 and the processing time was 15 min for the pre-experiment (
Table 2).
Table 2.
Conditions of different treatment methods.
Table 2.
Conditions of different treatment methods.
| Conditions | Groups |
|---|
| CG | SAEW | NB | SAEW-NB |
|---|
| ACC (mg/L) | - | 40 | 0 | 40 |
| Solid—liquid (g: mL) | - | 1:6 | 1:6 | 1:6 |
| Processing time (min) | - | 15 | 15 | 15 |
| Water temperature (°C) | - | RT | RT | RT |
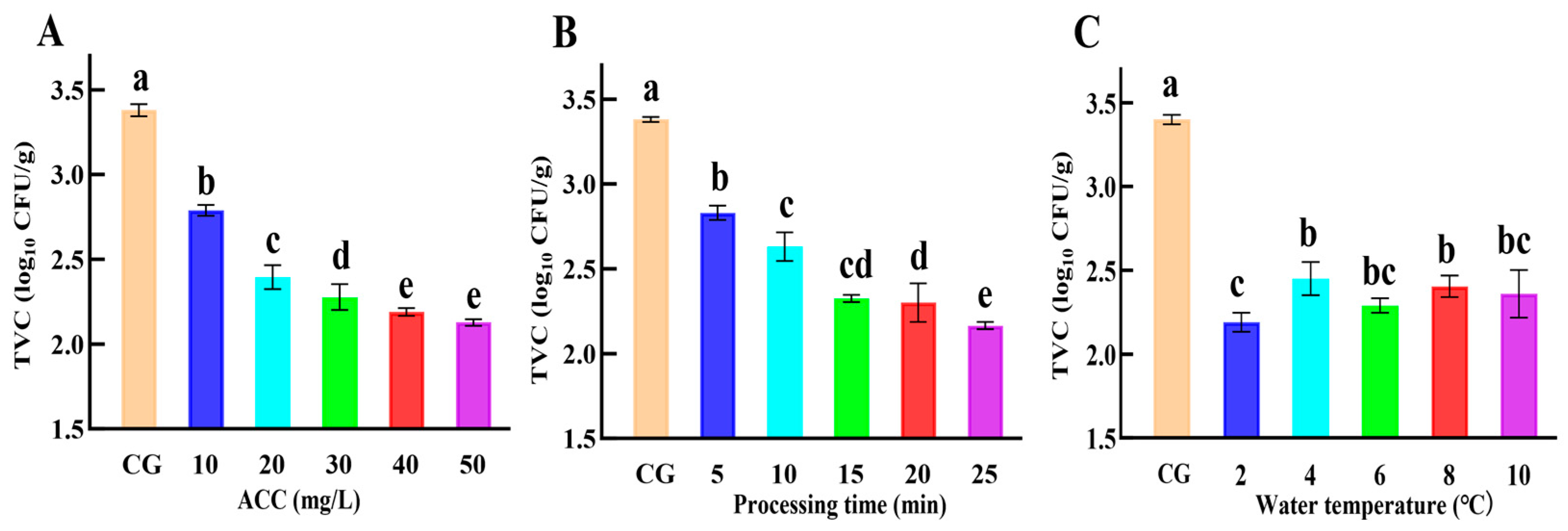
2.4. Single-Factor Experimental Design for SAEW-NB
The condition of a single factor is shown in
Table 3. The sterilization effect of single factor treatment was analyzed by setting ACC, processing time, and water temperature as single factor according to the solid–liquid ratio of 1:6. When a single factor was analyzed, the remaining factors were taken as fixed values, and total viable counts (TVCs) were determined for the SAEW-NB and CG.
In the present experiment, the actual ACC, pH, and ORP results of SAEW-NB are shown in
Table 4.
2.5. Microbiological Enumeration
Referring to GB 4789.2-2022 [
23] for microbiological analysis, total viable counts,
Pseudomonas, H
2S-producing bacteria, and
Enterobacteriaceae were counted (colony counter, SN-JLQ, Shangpu Instrument Equipment Co., Ltd., Shanghai, China) using plate count agar,
Pseudomonas CFC selective agar, iron agar, and violet red bile glucose agar, respectively. Microorganisms of
Enterobacteriaceae were placed in a constant-temperature incubator at 37 °C for 48 h, and the rest of the microorganisms were placed in a constant-temperature incubator at 30 °C for 72 h for colony counting. The results were expressed as log
10 CFU/g.
2.6. Measurement of pH
After mashing skinless back muscle (5.0 g), distilled water was added and filtered after standing for 30 min. The supernatant was taken and measured three times in parallel using a pH meter (PHS-3E, Inase Scientific Instrument Co., Ltd., Shanghai, China).
2.7. Measurement of Total Volatile Base Nitrogen (TVB-N)
The content of TVB-N was determined by the semi-micro method with reference to the Determination of Total Volatile Basic Nitrogen in Foods (GB 5009.228-2016 [
24]).
2.8. Measurement of 2-Thiobarbituric Acid (TBA)
The skinless back muscle (5.0 g) was weighed and placed in a 150 mL conical flask. Then, the 25 mL trichloroacetic acid mixed solution (75 g/L, 0.1% EDTA) was added to the conical flask and shaken for 30 min. The sample mixture was centrifuged at 4 °C and 4500 r/min for 15 min. The supernatant (5 mL) was mixed with 2-thiobarbituric acid solution (5 mL) and heated in a boiling water bath for 30 min. After temperature reduction, the absorbance values were determined by spectrophotometer at 532 nm and 600 nm, respectively, three times in parallel for each sample. Based on the formula
, the TBA value was calculated. Here, A532 and A600 were the absorbance values at wavelength 532 nm and 600 nm, respectively.
2.9. Measurement of Texture
The skinless back muscles (length × width × height = 2 cm × 2 cm × 1 cm) were measured by using texture profile analysis (TPA mode, TMS-PRO, Food Technology Corporation Co., Sterling, TX, USA). It was equipped with a P/5 probe with a test speed of 50 mm/min, a deformation of 50%, and a return distance of 30 mm. The muscles in each group were measured in parallel four times. The indexes included hardness, cohesiveness, springiness, gumminess, and chewiness.
2.10. Measurement of Taste Profile
After shaking and mixing 50 g of stirred back muscle with 250 mL of ultrapure water, the mixture was centrifuged at 4 °C and 4500 r/min for 15 min. The supernatant was filtered and then transferred to a special container for the electronic tongue (SA-402B, INSENT Co., Tokyo, Japan). The electronic tongue was configured with 5 lipid membrane sensors (AAE, CT0, C00, AE1, and GL1, INSENT Co., Tokyo, Japan) and 2 Ag/AgCl reference electrodes (INSENT Co., Tokyo, Japan). The sensors are designed to detect 5 taste signal values of sample solutions, including umami, saltiness, bitterness, astringency, and sweetness, and 3 aftertaste signal values, namely umami aftertaste, bitter aftertaste, and astringent aftertaste. Each sample was set up for 4 cycles and the last 3 were selected for analysis. For the determination of sweetness, each sample was set to measure 5 cycles, and the last 3 were taken for analysis.
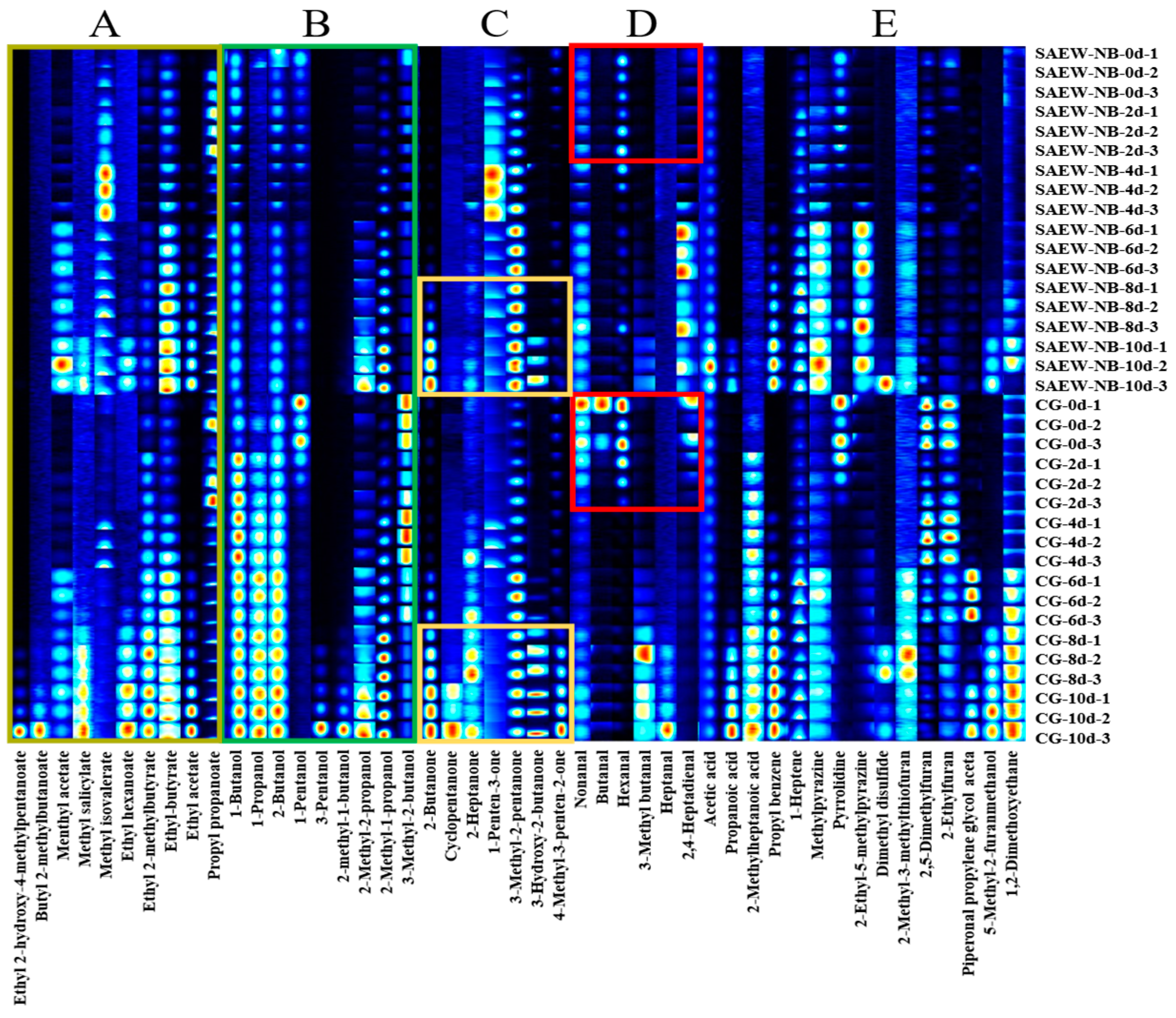
2.11. Measurement of Volatile Flavor Compounds
Sample processing: After the back muscle was stirred, 3 g of back muscle was weighed into a 20 mL headspace bottle.
Automatic headspace injection: The injection volume was 200 μL, the injection needle temperature was 85 °C, the incubation temperature of the headspace bottle was 40 °C, the incubation time was 20 min, and the incubation speed was set at 500 r/min.
Gas chromatography (GC) conditions: A MXT-WAX column (30 m × 0.53 mm, 1 μm) was used, the column temperature was 60 °C, high-purity nitrogen (N2, purity ≥ 99.999%) was used as the carrier gas, and the column flow rate was as follows: the initial column flow rate was 2 mL/min, held for 2 min, increased linearly to 100 mL/min from 2 min to 10 min, and to 150 mL from 10 min to 20 min.
Ion mobility spectrometry (IMS) conditions: The length of the drift tube was 98 mm, the temperature of the drift tube was 45 °C, the linear voltage inside the drift tube was 500 V/cm, the flow rate of the drift gas was 150 mL/min, and the drift gas was N2.
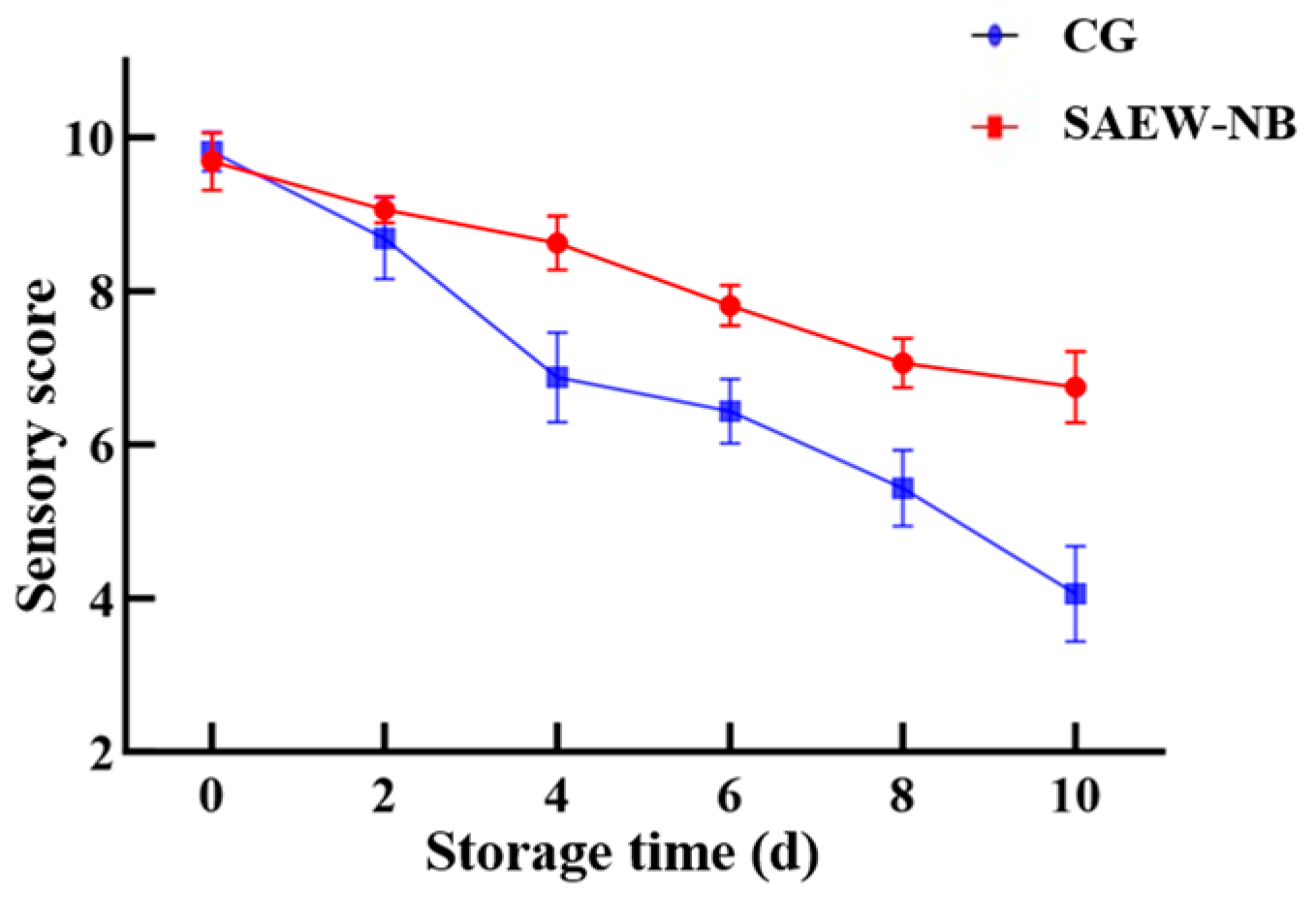
2.12. Sensory Evaluation
The sensory evaluation table is formulated according to GB/T 18108-2024, SC/T 3101-2010 and SC/T 3216-2016 (
Table 5) [
25,
26,
27]. Large yellow croakers were removed from a 4 °C low-temperature incubator, rinsed with fresh water, and their mid-sections were steamed in boiling water for 8 min. A group of eight professionally trained evaluators (20–30 years old, 4 males and 4 females) were selected to assess the appearance, flavor, morphology, and texture of large yellow croakers based on the sensory evaluation table criteria (water as a neutralizer). For these, the score of 8 to 10 was high quality, the score of 4 to 8 was medium quality, and the score of 2 to 4 was low quality.
2.13. Statistical Analysis
The results are expressed as mean ± standard deviation (SD). Microsoft Excel 2021 and IBM SPSS Statistics 21.0 were used to analyze the data.
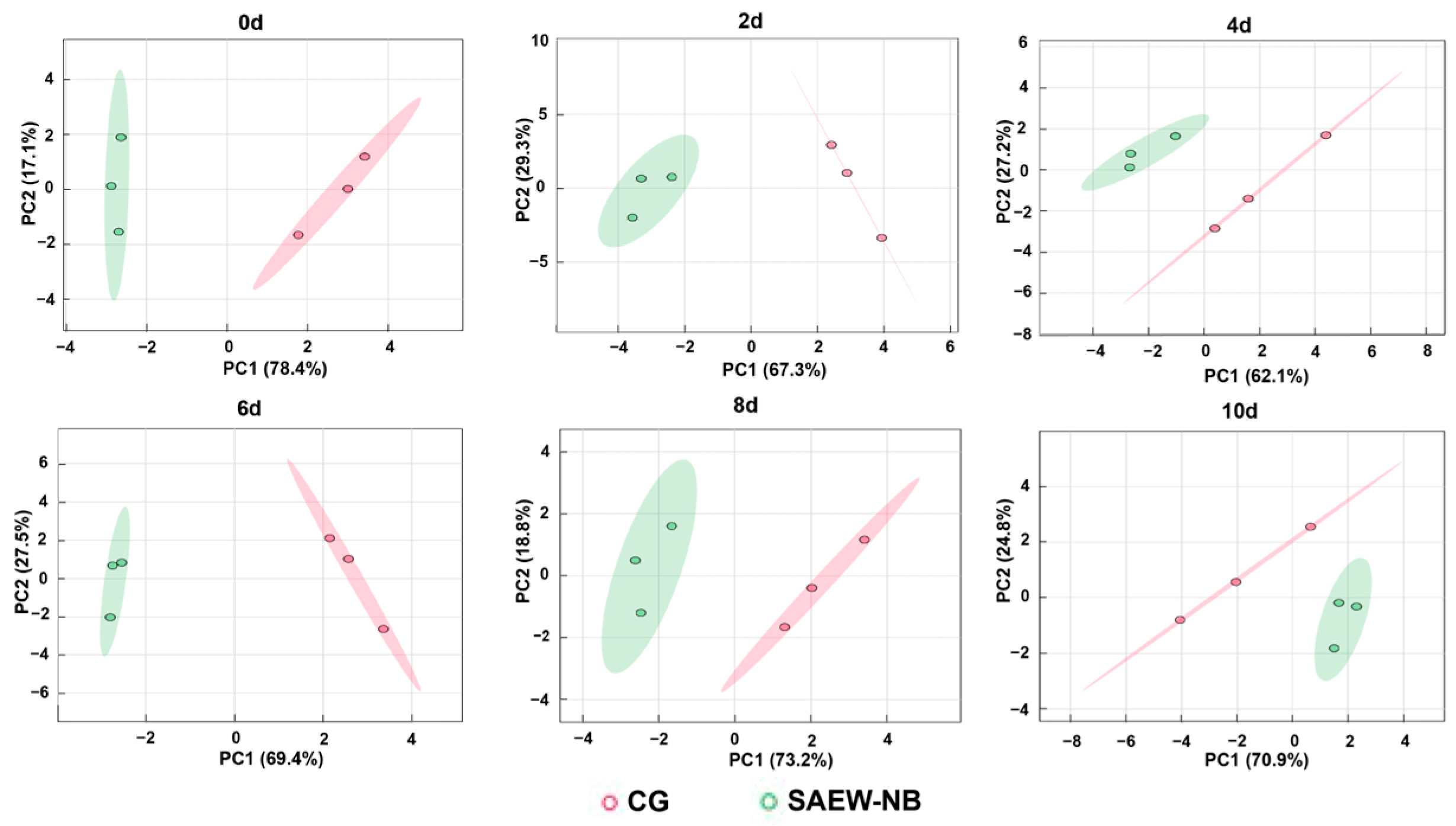
p ≤ 0.05 indicates significant differences. GraphPad Prism 10.0 was used to perform drawing editing. The GC-IMS was used to characterize the characteristic flavor compounds using the built-in NIST database and IMS database, and the fingerprints of volatile flavor compounds were constructed using the Gallery plug-in. Principal component analysis (PCA) was performed using MetaboAnalyst 6.0 (
https://www.metaboanalyst.ca/) (accessed on 17 May 2025).
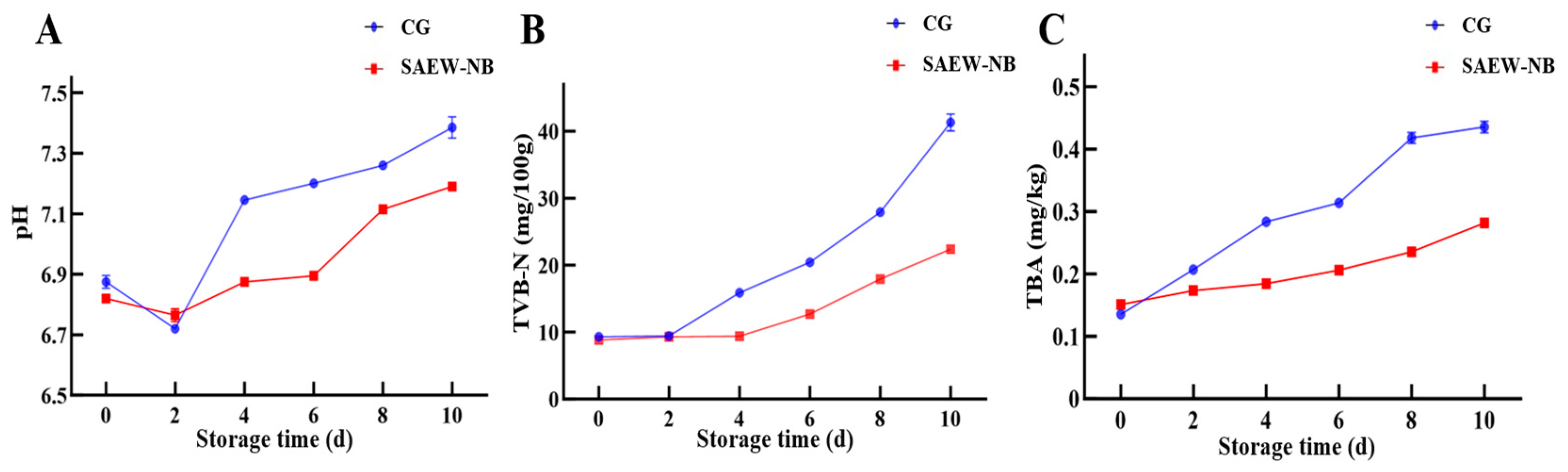
4. Conclusions
This research optimized the parameters for combining SAEW with NB, and their effect on the preservation of large yellow croaker during storage. The results demonstrate that this novel treatment slowed down the pH value increase; reduced the generation of TVB-N and TBA; maintained better texture, taste, and sensory qualities; and effectively mitigated spoilage compared with the CG. In addition, the combined treatment demonstrated potent bacterial inhibition, keeping the total viable count within acceptable limits by day 10 of storage and effectively suppressing spoilage-related bacterial counts, such as Pseudomonas and H2S-producing bacteria. These findings confirm that SAEW-NB’s physico-chemical sterilization mechanism enhances product quality and extends shelf life by approximately 2 days under refrigerated conditions. The optimized parameters provide critical, theoretical, and operational foundations for developing efficient green preservation technologies in aquatic products, with demonstrated potential to drive technological innovation in industrial seafood processing.