Curcumin Attenuates Zearalenone-Induced Reproductive Damage in Mice by Modulating the Gut Microbe–Testis Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Treatment
2.3. Testicular Index
2.4. Testosterone Levels in the Serum
2.5. Histopathological Assessment
2.6. Sperm Parameters Evaluation
2.7. Real-Time Quantitative PCR Analysis
2.8. Western Blot Analysis
2.9. Gut Microbiota Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of ZEN and CUR on Testicular Injury in Mice
3.2. ZEN and CUR on Sperm Survival in Mice
3.3. Effects of ZEN and CUR on Mouse Sperm Synthesis Pathway Genes
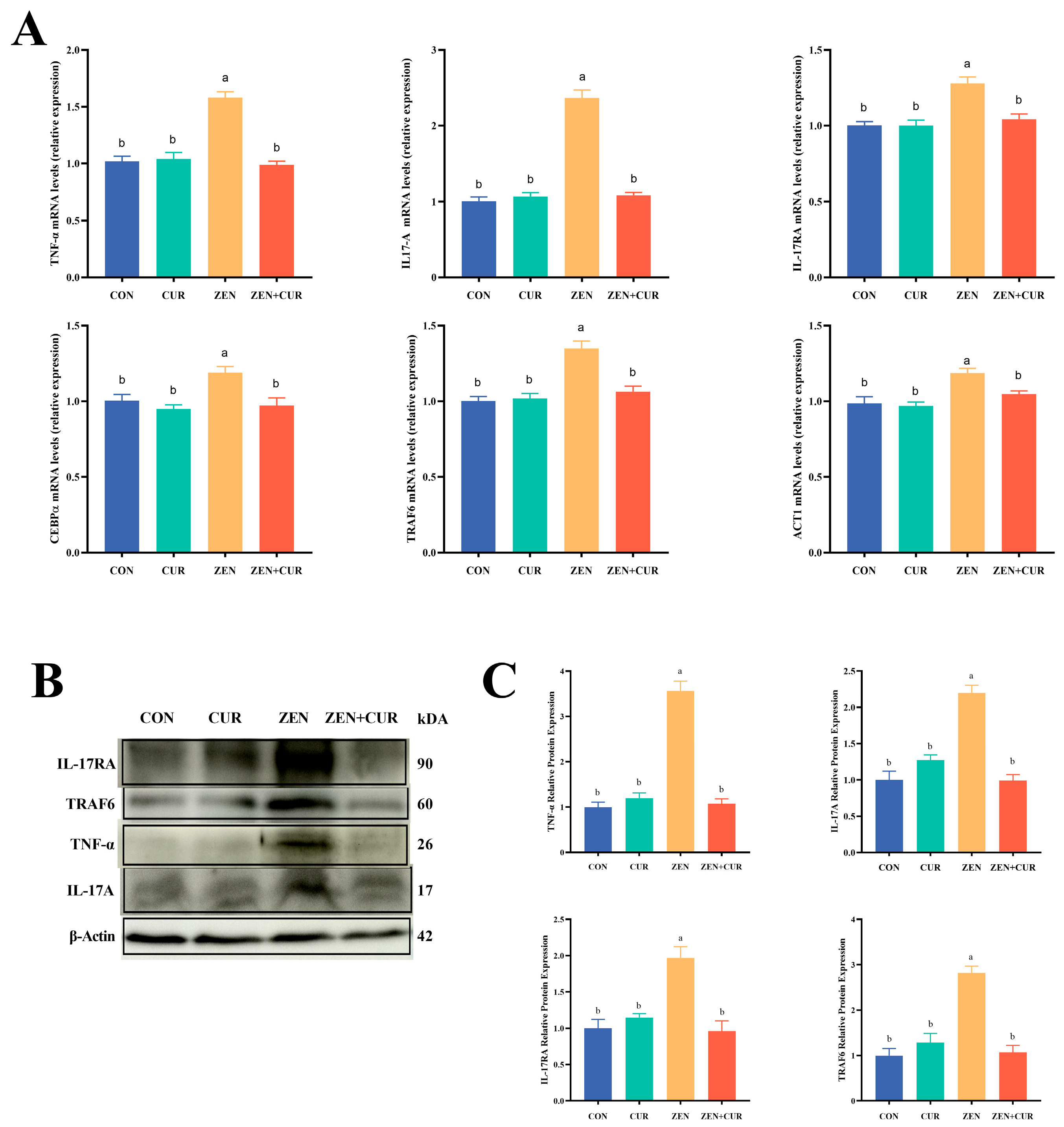
3.4. Expression of IL-17A Signaling Pathway Genes and Proteins
3.5. Effects of ZEN and CUR on the Gut Microbiota of Mice
3.6. Correlation Analysis Between Differential Bacteria and Sperm Synthesis Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nahle, S.; El Khoury, A.; Atoui, A. Current status on the molecular biology of zearalenone: Its biosynthesis and molecular detection of zearalenone producing Fusarium species. Eur. J. Plant Pathol. 2020, 159, 247–258. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, A.; Wang, J.; An, G.; Guan, S. Mycotoxin Contamination of Feeds and Raw Materials in China in Year 2021. Front. Vet. Sci. 2022, 9, 929904. [Google Scholar] [CrossRef]
- Binder, S.; Schwartz-Zimmermann, H.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of Zearalenone and Its Major Modified Forms in Pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajęcka, M.; Gajęcki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Deng, J.; Zuo, Z.; Wang, Y.; Shen, L. Individual and combined effects of deoxynivalenol and zearalenone on mouse kidney. Environ. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef]
- Reddy, K.; Song, J.; Lee, H.-J.; Kim, M.; Kim, D.-W.; Jung, H.; Kim, B.; Lee, Y.; Yu, D.; Kim, D.-W.; et al. Effects of High Levels of Deoxynivalenol and Zearalenone on Growth Performance, and Hematological and Immunological Parameters in Pigs. Toxins 2018, 10, 114. [Google Scholar] [CrossRef]
- Cool, J.; DeFalco, T.; Capel, B. Testis formation in the fetal mouse: Dynamic and complex de novo tubulogenesis. WIREs Dev. Biol. 2012, 1, 847–859. [Google Scholar] [CrossRef]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef]
- de Rooij, D.G.; Griswold, M.D. Questions About Spermatogonia Posed and Answered Since 2000. J. Androl. 2013, 33, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef]
- Goldman, A.L.; Bhasin, S.; Wu, F.C.W.; Krishna, M.; Matsumoto, A.M.; Jasuja, R. A Reappraisal of Testosterone’s Binding in Circulation: Physiological and Clinical Implications. Endocr. Rev. 2017, 38, 302–324. [Google Scholar] [CrossRef]
- Chen, W.; Zou, H.; Xu, H.; Cao, R.; Zhang, H.; Zhang, Y.; Zhao, J. The potential influence and intervention measures of gut microbiota on sperm: It is time to focus on testis-gut microbiota axis. Front. Microbiol. 2024, 15, 1478082. [Google Scholar] [CrossRef]
- Dubey, I.; Nandheeswari, K.; Vigneshwaran, G.; Rohilla, G.; Lalruatmawii; Naxine, P.; Jayapradha, P.; Rachamalla, M.; Kushwaha, S. Exploring the hypothetical links between environmental pollutants, diet, and the gut-testis axis: The potential role of microbes in male reproductive health. Reprod. Toxicol. 2024, 130, 108732. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Feng, Y.; Yan, X.; Chen, L.; Zhong, R.; Tang, X.; Shen, W.; Sun, Q.; Sun, Z.; Ren, Y.; et al. Gut microbiota-testis axis: FMT improves systemic and testicular micro-environment to increase semen quality in type 1 diabetes. Mol. Med. 2022, 28, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sayed, H.; Zhang, Q.; Tang, Y.; Wang, Y.; Guo, Y.; Zhang, J.; Ji, C.; Ma, Q.; Zhao, L. Alleviative Effect of Rutin on Zearalenone-Induced Reproductive Toxicity in Male Mice by Preventing Spermatogenic Cell Apoptosis and Modulating Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis. Toxins 2024, 16, 121. [Google Scholar] [CrossRef]
- Wang, L.; Deng, Z.; Huang, J.; Li, T.; Jiang, J.; Wang, W.; Sun, Y.; Deng, Y. Zearalenone-induced hepatointestinal toxicity in laying hens: Unveiling the role of gut microbiota and fecal metabolites. Poult. Sci. 2024, 103, 104221. [Google Scholar] [CrossRef]
- Yan, J.; Kong, L.; Zhang, X.; Yu, M.; Zhu, K.; Zhao, A.; Shi, D.; Sun, Y.; Wang, J.; Shen, W.; et al. Maternal Zearalenone Exposure Affects Gut Microbiota and Follicular Development in Suckled Offspring. J. Agric. Food Chem. 2022, 70, 15570–15582. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Shan, A.; Xu, L. Changes in intestinal barrier functions and gut microbiota in rats exposed to zearalenone. Ecotoxicol. Environ. Saf. 2020, 204, 111072. [Google Scholar] [CrossRef]
- Tan, S.; Ge, W.; Wang, J.; Liu, W.; Zhao, Y.; Shen, W.; Li, L. Zearalenone-induced aberration in the composition of the gut microbiome and function impacts the ovary reserve. Chemosphere 2020, 244, 125493. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Jagannathan, R.; Abraham, P.M.; Poddar, P. Temperature-Dependent Spectroscopic Evidences of Curcumin in Aqueous Medium: A Mechanistic Study of Its Solubility and Stability. J. Phys. Chem. B 2012, 116, 14533–14540. [Google Scholar] [CrossRef]
- Changlek, S.; Rana, M.N.; Phyu, M.P.; Karim, N.; Majima, H.J.; Tangpong, J. Curcumin Suppresses Lead-Induced Inflammation and Memory Loss in Mouse Model and In Silico Molecular Docking. Foods 2022, 11, 856. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Wan Mohd Tajuddin, W.N.B.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer. Nutrients 2019, 11, 2989. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Y.; Chen, H.; Jiang, H.; Zhou, F.; Lv, B.; Xu, M.; Dominguez Perles, R. Curcumin Alleviates DSS-Induced Anxiety-Like Behaviors via the Microbial-Brain-Gut Axis. Oxid. Med. Cell. Longev. 2022, 2022, 6244757. [Google Scholar] [CrossRef]
- Tsao, C.-W.; Ke, P.-S.; Yang, H.-Y.; Chang, T.-C.; Liu, C.-Y. Curcumin Remedies Testicular Function and Spermatogenesis in Male Mice with Low-Carbohydrate-Diet-Induced Metabolic Dysfunction. Int. J. Mol. Sci. 2022, 23, 10009. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, H.; Niu, J.; Chen, X.; Li, H.; Rao, Z.; Guo, Y.; Zhang, W.; Wang, Z. Curcumin alleviates zearalenone-induced liver injury in mice by scavenging reactive oxygen species and inhibiting mitochondrial apoptosis pathway. Ecotoxicol. Environ. Saf. 2024, 277, 116343. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Yang, M.; Li, J.; Wu, Y.; Fan, H.; Kong, X.; Ning, C.; Wang, S.; Xiao, W.; et al. Betulinic acid alleviates zearalenone-induced uterine injury in mice. Environ. Pollut. 2022, 316, 120435. [Google Scholar] [CrossRef]
- Qi, Z.; Feng, H.J.; Yan, C.; Yun, D.M.; Feng, Z.W.; Wei, Y.X.; Gonzalez, F.J.; Fei, L. Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome 2021, 9, 224. [Google Scholar] [CrossRef]
- Jin, H.; Yao, M.; Pan, C.; .Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef]
- Liu, J.-B.; Chen, K.; Li, Z.-F.; Wang, Z.-Y.; Wang, L. Glyphosate-induced gut microbiota dysbiosis facilitates male reproductive toxicity in rats. Sci. Total Environ. 2022, 805, 150368. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.; Dong, S.; Chen, X.; Zhang, Y.; He, J. Characterization of semen quality, testicular marker enzyme activities and gene expression changes in the blood testis barrier of Kunming mice following acute exposure to zearalenone. Environ. Sci. Pollut. Res. Int. 2017, 24, 27235–27243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, B.; Liu, T.; Wu, Y.; Wang, Z. Probiotics improve polystyrene microplastics-induced male reproductive toxicity in mice by alleviating inflammatory response. Ecotoxicol. Environ. Saf. 2023, 263, 115248. [Google Scholar] [CrossRef]
- Teng, M.; Sun, J.; Zhao, L.; Li, Y.; Zhang, Z.; Zhu, W.; Zhang, Y.; Xu, F.; Xing, S.; Zhao, X.; et al. Effects of BBIBP-CorV vaccine on gut microbiota and short-chain fatty acids in mice exposed to bis (2-ethylhexyl) phthalate and dioctyl terephthalate. Environ. Int. 2024, 190, 108851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Z.; Dong, W.; Wei, S.; Liu, Y.; Zuo, M. A Model for Predicting and Grading the Quality of Grain Storage Processes Affected by Microorganisms under Different Environments. Int. J. Env. Res. Public. Health 2023, 20, 4120. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Zhang, W.; Guang, C.; Xu, W.; Mu, W. An overview of chemical, physical and biological methods for zearalenone elimination: Recent advances and future prospective. Food Control 2023, 154, 110011. [Google Scholar] [CrossRef]
- Corona, G.; Maggi, M. The role of testosterone in male sexual function. Rev. Endocr. Metab. Disord. 2022, 23, 1159–1172. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Ren, X.; Li, B.; Wang, S. Male reproductive toxicity of zearalenone—Meta-analysis with mechanism review. Ecotoxicol. Environ. Saf. 2021, 221, 112457. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; Gao, X.; Kong, L.; Huang, Y.; Zhao, H.; Chen, Y.; Wen, L.; Li, R.; Wu, J.; et al. Ameliorative effect of betulinic acid against zearalenone exposure triggers testicular dysfunction and oxidative stress in mice via p38/ERK MAPK inhibition and Nrf2-mediated antioxidant defense activation. Ecotoxicol. Environ. Saf. 2022, 238, 113561. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. Bmj 2018, 361, k2179. [Google Scholar] [CrossRef]
- Lv, S.; Huang, J.; Luo, Y.; Wen, Y.; Chen, B.; Qiu, H.; Chen, H.; Yue, T.; He, L.; Feng, B.; et al. Gut microbiota is involved in male reproductive function: A review. Front. Microbiol. 2024, 15, 1371667. [Google Scholar] [CrossRef]
- Cai, H.; Cao, X.; Qin, D.; Liu, Y.; Liu, Y.; Hua, J.; Peng, S. Gut microbiota supports male reproduction via nutrition, immunity, and signaling. Front. Microbiol. 2022, 13, 977574. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.; Xu, T.; Huang, K.; He, X. Zearalenone exacerbates lipid metabolism disorders by promoting liver lipid droplet formation and disrupting gut microbiota. Ecotoxicol. Environ. Saf. 2025, 289, 117664. [Google Scholar] [CrossRef]
- Zhang, R.; Huangfu, B.; Xu, T.; Opatola, V.O.; Ban, Q.; Huang, K.; He, X. Zearalenone enhances TSST-1 production by intestinal Staphylococcus and increases uterine immune stress in rats. Food Chem. Toxicol. 2025, 196, 115140. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The Modulatory Effects of Curcumin on the Gut Microbiota: A Potential Strategy for Disease Treatment and Health Promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Xu, H.; Ban, W.; Tian, J.; Xu, J.; Tan, Z.; Li, S.; Chen, K.; Ou, M.; Li, K. The New Roles of traf6 Gene Involved in the Development of Zebrafish Liver and Gonads. Mar. Biotechnol. 2024, 26, 917–930. [Google Scholar] [CrossRef]
- Wu, P.; Yang, K.; Sun, Z.; Zhao, Y.; Manthari, R.K.; Wang, J.; Cao, J. Interleukin-17A knockout or self-recovery alleviated autoimmune reaction induced by fluoride in mouse testis. Sci. Total Environ. 2023, 884, 163616. [Google Scholar] [CrossRef]
- Pérez, C.V.; Pellizzari, E.H.; Cigorraga, S.B.; Galardo, M.N.; Naito, M.; Lustig, L.; Jacobo, P.V. IL17A impairs blood–testis barrier integrity and induces testicular inflammation. Cell Tissue Res. 2014, 358, 885–898. [Google Scholar] [CrossRef]
- Douzandeh-Mobarrez, B.; Kariminik, A. Gut Microbiota and IL-17A: Physiological and Pathological Responses. Probiotics Antimicrob. Proteins 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhao, Y.; Liu, S.; Yuan, H.; You, T.; Xu, H. Microplastics-perturbed gut microbiota triggered the testicular disorder in male mice: Via fecal microbiota transplantation. Environ. Pollut. 2022, 309, 119789. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhou, Q.; Sun, X.; Li, L.; Zhou, B.; Zeng, F.; Zhao, Y.; Shen, W.; Sun, Z. Effect of low-dose zearalenone exposure on reproductive capacity of male mice. Toxicol. Appl. Pharmacol. 2017, 333, 60–67. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.; Guo, S.; Niu, J.; Guo, Y.; Wang, Z.; Zhang, W. Curcumin Attenuates Zearalenone-Induced Reproductive Damage in Mice by Modulating the Gut Microbe–Testis Axis. Foods 2025, 14, 2703. https://doi.org/10.3390/foods14152703
Peng B, Guo S, Niu J, Guo Y, Wang Z, Zhang W. Curcumin Attenuates Zearalenone-Induced Reproductive Damage in Mice by Modulating the Gut Microbe–Testis Axis. Foods. 2025; 14(15):2703. https://doi.org/10.3390/foods14152703
Chicago/Turabian StylePeng, Bangwang, Shuaiju Guo, Junlong Niu, Yongpeng Guo, Zhixiang Wang, and Wei Zhang. 2025. "Curcumin Attenuates Zearalenone-Induced Reproductive Damage in Mice by Modulating the Gut Microbe–Testis Axis" Foods 14, no. 15: 2703. https://doi.org/10.3390/foods14152703
APA StylePeng, B., Guo, S., Niu, J., Guo, Y., Wang, Z., & Zhang, W. (2025). Curcumin Attenuates Zearalenone-Induced Reproductive Damage in Mice by Modulating the Gut Microbe–Testis Axis. Foods, 14(15), 2703. https://doi.org/10.3390/foods14152703





