Enhancing Stability of Boesenbergia rotunda Bioactive Compounds: Microencapsulation via Spray-Drying and Its Physicochemical Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Core Materials Preparations
2.2. Preparation of the Wall Materials
2.3. Microencapsulation by Spray-Drying
2.4. Physical Property Analysis
2.4.1. Powder Flow-Related Properties
2.4.2. Hygroscopicity
2.4.3. Degree of Powder Caking
2.4.4. Microcapsule Solubility
2.4.5. Particle Size Distribution
2.4.6. Powder Morphology Analysis
2.5. Physicochemical Property Analysis
2.5.1. Determination of Water Activity
2.5.2. Moisture Content of Powder
2.5.3. Total Phenolic and Flavonoid Contents
2.5.4. Major Encapsulated Compounds Detection
2.5.5. Encapsulation Efficiency and Surface Phenolic Analysis
2.6. Microcapasule Thermal Stability Analysis
3. Results and Discussions
3.1. Effects of Wall Materials on Fingerroot Powders Flow Properties
3.2. Fingerroot Powder Characteristics of Various Wall Materials
3.3. Bioactive Content in Fingerroot Microcapsules
3.4. Thermal Stability of Fingerroot Extract Microcapsules
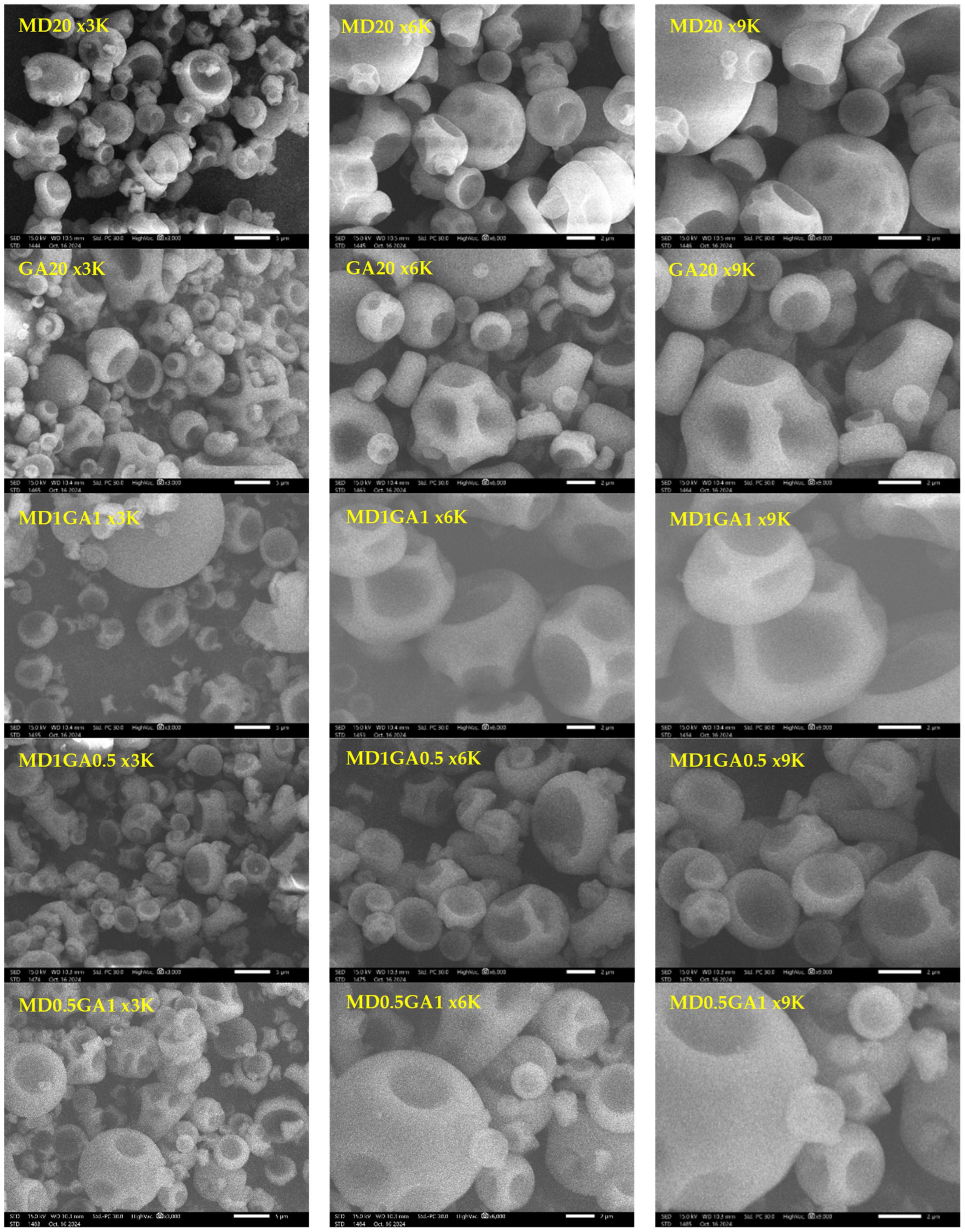
3.5. Morphology of Fingerroot Extract Microcapsules
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaewjai, C.; Tonsomboon, A.; Pawiwongchai, J.; Prommano, O. Antiprotozoal Activity of Boesenbergia rotunda (L.) Mansf and Ganoderma lucidum (Fr.) Kart Extracts Against Blastocystis Hominis. Vet. World 2023, 16, 187–193. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, J.; Liu, F.; Peng, X.; Xu, G.; Zhang, M.; Huang, Z. Traditional Usages, Chemical Metabolites, Pharmacological Activities, and Pharmacokinetics of Boesenbergia rotunda (L.) Mansf.: A Comprehensive Review. Front. Pharmacol. 2025, 16, 1527210. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Q.; Wang, X.; Jaisi, A.; Olatunji, O.J. Boesenbergia rotunda Displayed Anti-Inflammatory, Antioxidant and Anti-Apoptotic Efficacy in Doxorubicin-induced Cardiotoxicity in Rats. Sci. Rep. 2023, 13, 11398. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Fuentes, J.C.; Chavez-Santoscoy, R.A. Nanotechnology as a Key to Enhance the Benefits and Improve the Bioavailability of Flavonoids in the Food Industry. Foods 2021, 10, 2701. [Google Scholar] [CrossRef] [PubMed]
- Sujana, D.; Sumiwi, S.A.; Saptarini, N.M.; Levita, J. The Nephroprotective Activity of Boesenbergia rotunda Rhizome by Reducing Creatinine, Urea Nitrogen, Glutamic Pyruvic Transaminase, and Malondialdehyde Levels in the Blood and Attenuating the Expression of Havcr1 (KIM-1), Lcn2 (NGAL), Casp3, and Casp7 Genes in the Kidney Cortex of Cisplatin-Induced Sprague-Dawley Rats. J. Exp. Pharmacol. 2024, 16, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals 2023, 16, 362. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Ferreira, S.; Nicoletti, V.R. Turmeric Oleoresin Encapsulated by Spray Drying in Maltodextrin/Gelatin and Starch/Gelatin Blends: Storage Stability and Water Sorption. Acta Sci. Technol. 2022, 44, e56950. [Google Scholar] [CrossRef]
- Fahrudin, F.I.; Phongthai, S.; Wirjantoro, T.I.; Intipunya, P. Synergistic Effects of Pressure, Temperature, CO2 Flow Rate and Co-Solvent on Bioactive Contents of Thai Fingerroot (Boesenbergia rotunda (L.) Mansf.) Extracts. Foods 2025, 14, 2189. [Google Scholar] [CrossRef]
- Fahrudin, F.I.; Sulaiman, R.; Rukayadi, Y. Effect of Drying Methods on Physicochemical Characteristics of Boesenbergia rotunda (L.) Mansf. Powder. Int. J. Adv. Sci. Technol. 2020, 29, 3952–3962. [Google Scholar]
- Pao-la-or, P.; Posridee, K.; Buranakon, P.; Singthong, J.; Oonmetta-Aree, J.; Oonsivilai, R.; Oonsivilai, A. Beyond Traditional Methods: Deep-Learning Machines Empower Fingerroot (Boesenbergia rotunda)-Extract Production with Superior Antioxidant Activity. Foods 2024, 13, 2676. [Google Scholar] [CrossRef]
- Beristain, C.I.; García, H.S.; Vernon-Carter, E.J. Spray-Dried Encapsulation of Cardamom (Elettaria cardamomum) Essential Oil with Mesquite (Prosopis juliflora) Gum. LWT—Food Sci. Technol. 2001, 34, 398–401. [Google Scholar] [CrossRef]
- Arebo, M.A.; Feyisa, J.D.; Tafa, K.D.; Satheesh, N. Optimization of Spray-Drying Parameter for Production of Better Quality Orange Fleshed Sweet Potato (Ipomoea batatas L.) Powder: Selected Physiochemical, Morphological, and Structural Properties. Heliyon 2023, 9, e13078. [Google Scholar] [CrossRef] [PubMed]
- Bashir, O.; Hussain, S.Z.; Ameer, K.; Amin, T.; Beenish; Ahmed, I.A.M.; Aljobair, M.O.; Gani, G.; Mir, S.A.; Ayaz, Q.; et al. Influence of Anticaking Agents and Storage Conditions on Quality Characteristics of Spray Dried Apricot Powder: Shelf Life Prediction Studies Using Guggenheim-Anderson-de Boer (GAB) Model. Foods 2022, 12, 171. [Google Scholar] [CrossRef]
- Balci-Torun, F.; Ozdemir, F. Encapsulation of Strawberry Flavour and Physicochemical Characterization of the Encapsulated Powders. Powder Technol. 2021, 380, 602–612. [Google Scholar] [CrossRef]
- Caballero-Román, A.; Nardi-Ricart, A.; Vila, R.; Cañigueral, S.; Ticó, J.R.; Miñarro, M. Use of Natural Polymers for the Encapsulation of Eugenol by Spray Drying. Pharmaceutics 2024, 16, 1251. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Madison, WI, USA, 2000. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Gatti, N.; Maghrebi, M.; Serio, G.; Gentile, C.; Bunea, V.V.; Vigliante, I.; Boitte, C.; Garabello, C.; Contartese, V.; Bertea, C.M.; et al. Seaweed and Yeast Extracts as Sustainable Phytostimulant to Boost Secondary Metabolism of Apricot Fruits. Front. Plant Sci. 2025, 15, 1455156. [Google Scholar] [CrossRef]
- Velazquez-Martinez, V.; Valles-Rosales, D.; Rodriguez-Uribe, L.; Holguin, O.; Quintero-Quiroz, J.; Reyes-Jaquez, D.; Rodriguez-Borbon, M.I.; Villagrán-Villegas, L.Y.; Delgado, E. Antimicrobial, Shelf-Life Stability, and Effect of Maltodextrin and Gum Arabic on the Encapsulation Efficiency of Sugarcane Bagasse Bioactive Compounds. Foods 2021, 10, 116. [Google Scholar] [CrossRef]
- Vasile, C.; Tudorachi, N.; Zaharescu, T.; Darie-Nita, R.N.; Cheaburu-Yilmaz, C.N. Study on Thermal Behavior of Some Biocompatible and Biodegradable Materials Based on Plasticized PLA, Chitosan, and Rosemary Ethanolic Extract. Int. J. Polym. Sci. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Ahmadi, F.; Suleria, H.A.R.; Dunshea, F.R. Physicochemical Characterization, Storage Stability Behavior, and Intestinal Bioaccessibility of Clove Extract Encapsulated Using Varying Combinations of Gum Arabic and Maltodextrin. Foods 2025, 14, 237. [Google Scholar] [CrossRef]
- Ng, L.H.; Ling, J.K.U.; Hadinoto, K. Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals. Pharmaceutics 2022, 14, 2015. [Google Scholar] [CrossRef]
- Yu, D.; Nie, H.; Hoag, S.W. Comprehensive Evaluation of Polymer Types and Ratios in Spray-Dried Dispersions: Compaction, Dissolution, and Physical Stability. Int. J. Pharm. 2024, 650, 123674. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Malekjani, N.; Baeghbali, V.; Ansarifar, E.; Hedayati, S.; Jafari, S.M. Optimization of Spray Drying Process Parameters for the Food Bioactive Ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 5631–5671. [Google Scholar] [CrossRef] [PubMed]
- Gouaou, I.; Shamaei, S.; Koutchoukali, M.S.; Bouhelassa, M.; Tsotsas, E.; Kharaghani, A. Impact of Operating Conditions on a Single Droplet and Spray Drying of Hydroxypropylated Pea Starch: Process Performance and Final Powder Properties. Asia-Pac. J. Chem. Eng. 2018, 14, e2268. [Google Scholar] [CrossRef]
- Farinha, S.; Sá, J.V.; Lino, P.R.; Galésio, M.; Pires, J.; Rodrigues, M.Â.; Henriques, J. Spray Freeze Drying of Biologics: A Review and Applications for Inhalation Delivery. Pharm. Res. 2023, 40, 1115–1140. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Suriyarak, S.; Devahastin, S.; Borompichaichartkul, C. A Novel Approach to Develop Spray-dried Encapsulated Curcumin Powder from Oil-in-water Emulsions Stabilized by Combined Surfactants and Chitosan. J. Food Sci. 2020, 85, 3874–3884. [Google Scholar] [CrossRef]
- Huynh, T.; Kha, T.C.; Duy Nguyen, V.A.; Nguyen, T.; Ha, T.M.; Ngo, H.H. Spray Drying Conditions of Lime Juice Prepared by Freeze-Concentration. IOP Conf. Ser. Earth Environ. Sci. 2023, 1155, 012017. [Google Scholar] [CrossRef]
- Muangrat, R.; Ravichai, K.; Jirarattanarangsri, W. Encapsulation of Polyphenols from Fermented Wastewater of Miang Processing by Freeze Drying Using a Maltodextrin/Gum Arabic Mixture as Coating Material. J. Food Process Preserv. 2019, 43, e13908. [Google Scholar] [CrossRef]
- Remígio, M.S.d.N.; Greco, T.; Silva Júnior, J.O.C.; Converti, A.; Ribeiro-Costa, R.M.; Rossi, A.; Barbosa, W.L.R. Spray-Drying Microencapsulation of Bauhinia ungulata L. var. obtusifolia Aqueous Extract Containing Phenolic Compounds: A Comparative Study Using Different Wall Materials. Pharmaceutics 2024, 16, 488. [Google Scholar] [CrossRef]
- Jafari, S.; Karami, Z.; Shiekh, K.A.; Kijpatanasilp, I.; Worobo, R.W.; Assatarakul, K. Ultrasound-Assisted Extraction of Bioactive Compounds from Cocoa Shell and Their Encapsulation in Gum Arabic and Maltodextrin: A Technology to Produce Functional Food Ingredients. Foods 2023, 12, 412. [Google Scholar] [CrossRef]
- Júnior, M.E.d.S.; da Silva, N.B.; Araújo, M.V.R.L.; Converti, A.; Lima, M.d.S.; Maciel, M.I.S. Effect of Coating Material on Microencapsulated Phenolic Compounds Extracted from Agroindustrial Ciriguela Peel Residue. J. Sci. Food Agric. 2024, 104, 1335–1346. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Van Chuyen, H.; Tran, M.D.; Nguyen, Q.-V. Microencapsulation of Syzygium zeylanicum (L.) DC. Extract Using Spray Drying: Effects of Wall Materials on Physicochemical Characteristics and Biological Activities of the Microcapsules. J. Food Process Preserv. 2022, 46, e16647. [Google Scholar] [CrossRef]
- de Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; de Sá, S.H.G.; Thomazini, M.; Favaro-Trindade, C.S. Combination of Spray-Chilling and Spray-Drying Techniques to Protect Carotenoid-Rich Extracts from Pumpkin (Cucurbita moschata) Byproducts, Aiming at the Production of a Powdered Natural Food Dye. Molecules 2022, 27, 7530. [Google Scholar] [CrossRef]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Mohamed Ahmed, I.A.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of Maltodextrin Combination with Gum Arabic and Whey Protein Isolate on the Microencapsulation of Gurum Seed Oil Using a Spray-Drying Method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; Alvim, I.D.; Vissotto, F.Z.; de Aguirre, J.M. Influence of Carrier Agents on the Physicochemical Properties of Blackberry Powder Produced by Spray Drying. Int. J. Food Sci. Technol. 2012, 47, 1237–1245. [Google Scholar] [CrossRef]
- Mutavski, Z.; Vidović, S.; Lazarević, Z.; Ambrus, R.; Motzwickler-Németh, A.; Aladić, K.; Nastić, N. Stabilization and Preservation of Bioactive Compounds in Black Elderberry By-Product Extracts Using Maltodextrin and Gum Arabic via Spray Drying. Foods 2025, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Gümüşay, Ö.A.; Cerit, İ.; Demirkol, O. Utilization of Yeast Cells as Alternative Carriers in the Microencapsulation of Black Chokeberry (Aronia melanocarpa) Phenolic Extract. Foods 2025, 14, 625. [Google Scholar] [CrossRef]
- Medfai, W.; Oueslati, I.; Dumas, E.; Harzalli, Z.; Viton, C.; Mhamdi, R.; Gharsallaoui, A. Physicochemical and Biological Characterization of Encapsulated Olive Leaf Extracts for Food Preservation. Antibiotics 2023, 12, 987. [Google Scholar] [CrossRef] [PubMed]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of Carrier Agents on the Physicochemical and Technofunctional Properties and Antioxidant Capacity of Freeze-Dried Pomegranate Juice (Punica granatum) Powder. Foods 2020, 9, 1388. [Google Scholar] [CrossRef]
- Jamdar, F.; Ali Mortazavi, S.; Reza Saiedi Asl, M.; Sharifi, A. Physicochemical Properties and Enzymatic Activity of Wheat Germ Extract Microencapsulated with Spray and Freeze Drying. Food Sci. Nutr. 2021, 9, 1192–1201. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Meor Hussin, A.S. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Bleive, U.; Kaldmäe, H.; Aluvee, A.; Rätsep, R.; Sats, A.; Pap, N.; Järvenpää, E.; Rinken, T. Characterization of Plant Based Spray Dried Powders Using Oil Seed Proteins and Chokeberry Extract from Wine Byproduct. Sci. Rep. 2024, 14, 27409. [Google Scholar] [CrossRef]
- Song, F.; Li, Y.; Wang, B.; Shen, X.; Wang, H.; Li, R.; Xia, Q. Effect of Drying Method and Wall Material Composition on the Characteristics of Camellia Seed Oil Microcapsule Powder. J. Am. Oil Chem. Soc. 2021, 99, 353–364. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review. Polymers 2023, 15, 2659. [Google Scholar] [CrossRef] [PubMed]
| Sample | Maltodextrin (% w/v) | Gum Arabic (% w/v) | Wall Material Ratio (MD:GA) |
|---|---|---|---|
| MD20 | 20 | 0 | 1:0 |
| GA20 | 0 | 20 | 0:1 |
| MD1GA1 | 10 | 10 | 1:1 |
| MD1GA0.5 | 13.33 | 6.66 | 2:1 |
| MD0.5GA1 | 6.66 | 13.33 | 1:2 |
| Carr Index | Flow Property | Hausner Ratio |
|---|---|---|
| ≤10 | Excellent | 1.00–1.11 |
| 11–15 | Good | 1.12–1.18 |
| 16–20 | Fair | 1.19–1.125 |
| 21–25 | Passable | 1.26–1.34 |
| 26–31 | Poor | 1.35–1.45 |
| 32–37 | Very poor | 1.46–1.59 |
| >38 | Very, very poor | >1.60 |
| Samples | Loose Bulk Density (g/mL) | Tapped Bulk Density (g/mL) | Hausner Ratio | Carr Index | Flowability |
|---|---|---|---|---|---|
| MD20 | 0.37 ± 0.00 a | 0.54 ± 0.03 bc | 1.45 ± 0.06 c | 31.15 ± 2.83 c | Poor |
| GA20 | 0.34 ± 0.00 b | 0.52 ± 0.01 bc | 1.55 ± 0.04 b | 35.39 ± 1.82 b | Very poor |
| MD1GA1 | 0.34 ± 0.01 b | 0.51 ± 0.01 c | 1.52 ± 0.01 bc | 34.28 ± 0.58 bc | Very poor |
| MD1GA0.5 | 0.36 ± 0.00 a | 0.53 ± 0.01 bc | 1.49 ± 0.06 bc | 33.01 ± 2.63 bc | Very poor |
| MD0.5GA1 | 0.35 ± 0.00 b | 0.58 ± 0.01 a | 1.64 ± 0.04 a | 39.01 ± 1.49 a | Very, very poor |
| Samples | Mean Particle Size (µm) | PDI | D[4,3] (μm) | Hygroscopicity (%) | Powder Caking (%) | Solubility (%) |
|---|---|---|---|---|---|---|
| MD20 | 6.76 ± 0.39 b | 0.43 ± 0.10 c | 4.13 ± 0.18 a | 4.17 ± 0.13 c | 49.35 ± 0.96 c | 98.62 ± 0.20 b |
| GA20 | 5.24 ± 0.12 c | 0.19 ± 0.11 d | 4.45 ± 0.12 a | 5.52 ± 0.21 a | 70.12 ± 2.28 a | 98.33 ± 1.44 b |
| MD1GA1 | 6.50 ± 0.00 b | 0.42 ± 0.04 c | 3.15 ± 2.33 b | 5.08 ± 0.06 b | 59.99 ± 0.66 b | 98.70 ± 0.65 b |
| MD1GA0.5 | 10.94 ± 2.10 a | 0.53 ± 0.39 b | 1.08 ± 0.58 c | 4.92 ± 0.05 b | 39.79 ± 0.51 d | 99.08 ± 0.32 a |
| MD0.5GA1 | 3.11 ± 0.13 d | 0.84 ± 0.22 a | 1.42 ± 0.04 c | 4.99 ± 0.06 b | 56.10 ± 0.49 b | 97.80 ± 0.37 b |
| Samples | Moisture Content (% db) | Water Activity |
|---|---|---|
| MD20 | 10.57 ± 0.40 b | 0.55 ± 0.02 ab |
| GA20 | 11.99 ± 0.84 a | 0.56 ± 0.00 a |
| MD1GA1 | 8.69 ± 0.94 c | 0.55 ± 0.01 ab |
| MD1GA0.5 | 8.46 ± 0.66 c | 0.54 ± 0.01 ab |
| MD0.5GA1 | 11.26 ± 0.54 ab | 0.54 ± 0.00 b |
| Samples | EE (%) | TPC (mg GAE/g Powder) | SPC (mg GAE/g Powder) | TFC (mg QE/g Powder) |
|---|---|---|---|---|
| MD20 | 74.23 ± 2.06 c | 163.02 ± 15.51 b | 41.80 ± 2.35 bc | 167.91 ± 14.35 a |
| GA20 | 67.86 ± 2.08 d | 138.34 ± 4.83 c | 44.49 ± 3.72 ab | 143.07 ± 3.31 b |
| MD1GA1 | 75.06 ± 1.11 bc | 185.51 ± 8.79 a | 46.18 ± 0.88 a | 157.44 ± 1.87 ab |
| MD1GA0.5 | 76.92 ± 1.03 ab | 190.21 ± 6.59 a | 43.87 ± 1.30 ab | 148.83 ± 12.82 b |
| MD0.5GA1 | 78.68 ± 0.65 a | 188.52 ± 5.12 a | 40.17 ± 1.03 c | 146.84 ± 3.09 b |
| Samples | Pinostrobin (mg/g Powder) | Pinocembrin (mg/g Powder) |
|---|---|---|
| MD20 | 33.21 ± 0.12 c | 81.34 ± 0.05 b |
| GA20 | 41.47 ± 0.08 b | 87.29 ± 0.18 b |
| MD1GA1 | 45.89 ± 0.12 b | 92.16 ± 0.37 a |
| MD1GA0.5 | 53.65 ± 1.03 a | 98.45 ± 0.20 a |
| MD0.5GA1 | 50.72 ± 0.65 a | 97.08 ± 0.14 a |
| Samples | Tg-half Cp (°C) | Onset (°C) | Tpeak (°C) | ΔH (J/g) | TEnd (°C) |
|---|---|---|---|---|---|
| MD20 | 91.9 ± 0.21 b | 127.12 ± 1.90 a | 127.92 ± 1.42 a | 25.48 ± 6.43 ab | 128.69 ± 1.57 a |
| GA20 | 81.57 ± 0.87 d | 127.51 ± 3.97 a | 128.53 ± 3.80 a | 14.52 ± 7.57 c | 128.94 ± 3.71 a |
| MD1GA1 | 85.98 ± 2.53 c | 127.51 ± 1.34 a | 128.56 ± 1.34 a | 27.35 ± 1.37 a | 129.39 ± 1.40 a |
| MD1GA0.5 | 74.82 ± 1.58 e | 128.52 ± 1.34 a | 129.36 ± 1.03 a | 16.72 ± 4.13 bc | 129.71 ± 0.95 a |
| MD0.5GA1 | 95.29 ± 2.74 a | 129.11 ± 5.00 a | 130.53 ± 4.57 a | 29.12 ± 1.50 a | 129.80 ± 2.56 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahrudin, F.I.; Phongthai, S.; Intipunya, P. Enhancing Stability of Boesenbergia rotunda Bioactive Compounds: Microencapsulation via Spray-Drying and Its Physicochemical Evaluation. Foods 2025, 14, 2699. https://doi.org/10.3390/foods14152699
Fahrudin FI, Phongthai S, Intipunya P. Enhancing Stability of Boesenbergia rotunda Bioactive Compounds: Microencapsulation via Spray-Drying and Its Physicochemical Evaluation. Foods. 2025; 14(15):2699. https://doi.org/10.3390/foods14152699
Chicago/Turabian StyleFahrudin, Fahmi Ilman, Suphat Phongthai, and Pilairuk Intipunya. 2025. "Enhancing Stability of Boesenbergia rotunda Bioactive Compounds: Microencapsulation via Spray-Drying and Its Physicochemical Evaluation" Foods 14, no. 15: 2699. https://doi.org/10.3390/foods14152699
APA StyleFahrudin, F. I., Phongthai, S., & Intipunya, P. (2025). Enhancing Stability of Boesenbergia rotunda Bioactive Compounds: Microencapsulation via Spray-Drying and Its Physicochemical Evaluation. Foods, 14(15), 2699. https://doi.org/10.3390/foods14152699








