Therapeutic Potential of Kelp Fucoidan in Rebiosis of Gut Microflora and Immune Homeostasis in Cyclophosphamide-Induced Immunosuppressed Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Experimental Materials
2.1.2. Main Reagents
2.1.3. Main Instruments
2.2. Experimental Methods
2.2.1. Preparation of Kelp Fucoidan
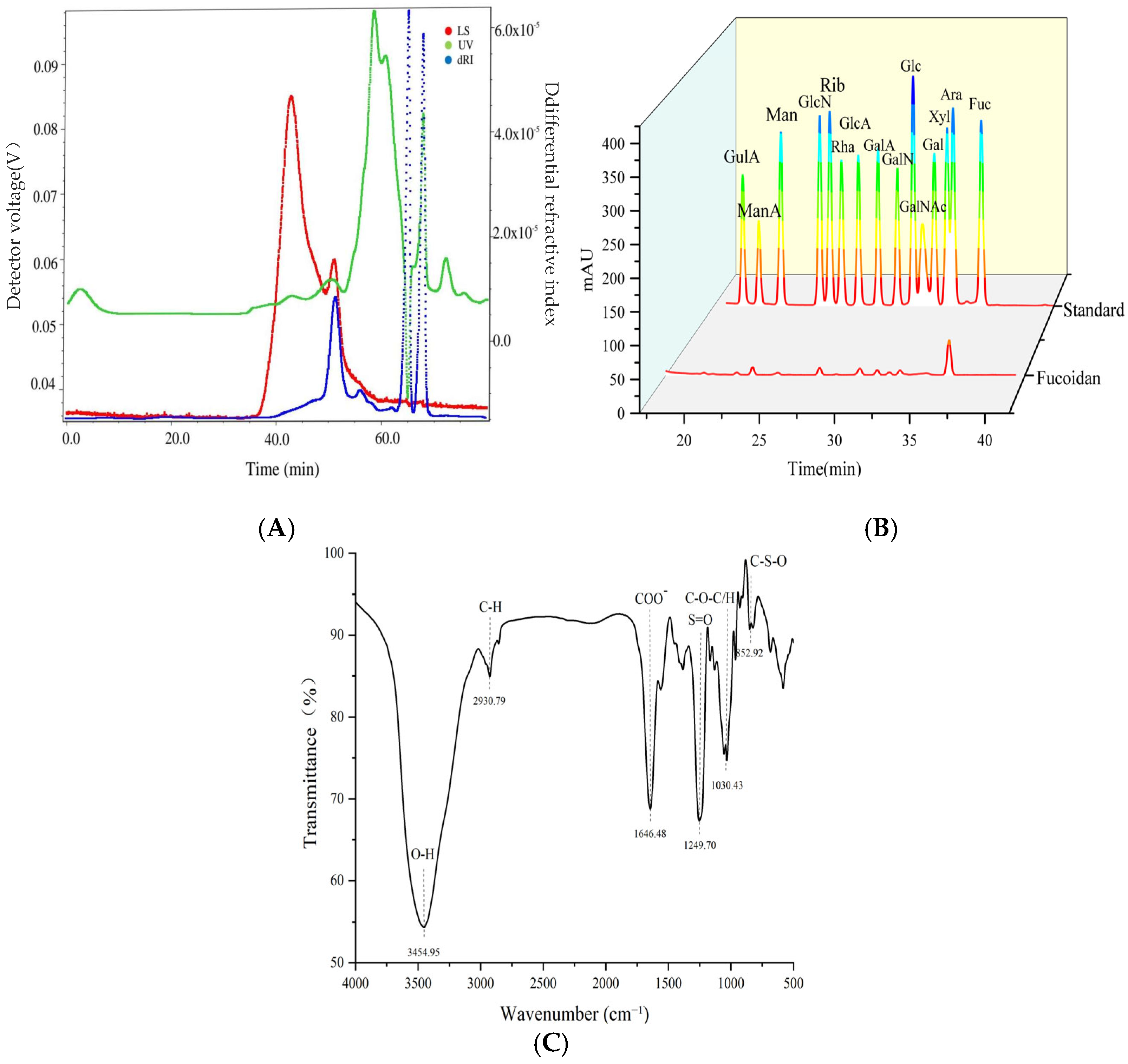
2.2.2. Structural Characterization of Kelp Fucoidan
2.2.3. Establishment and Administration of Immunocompromised Mouse Models
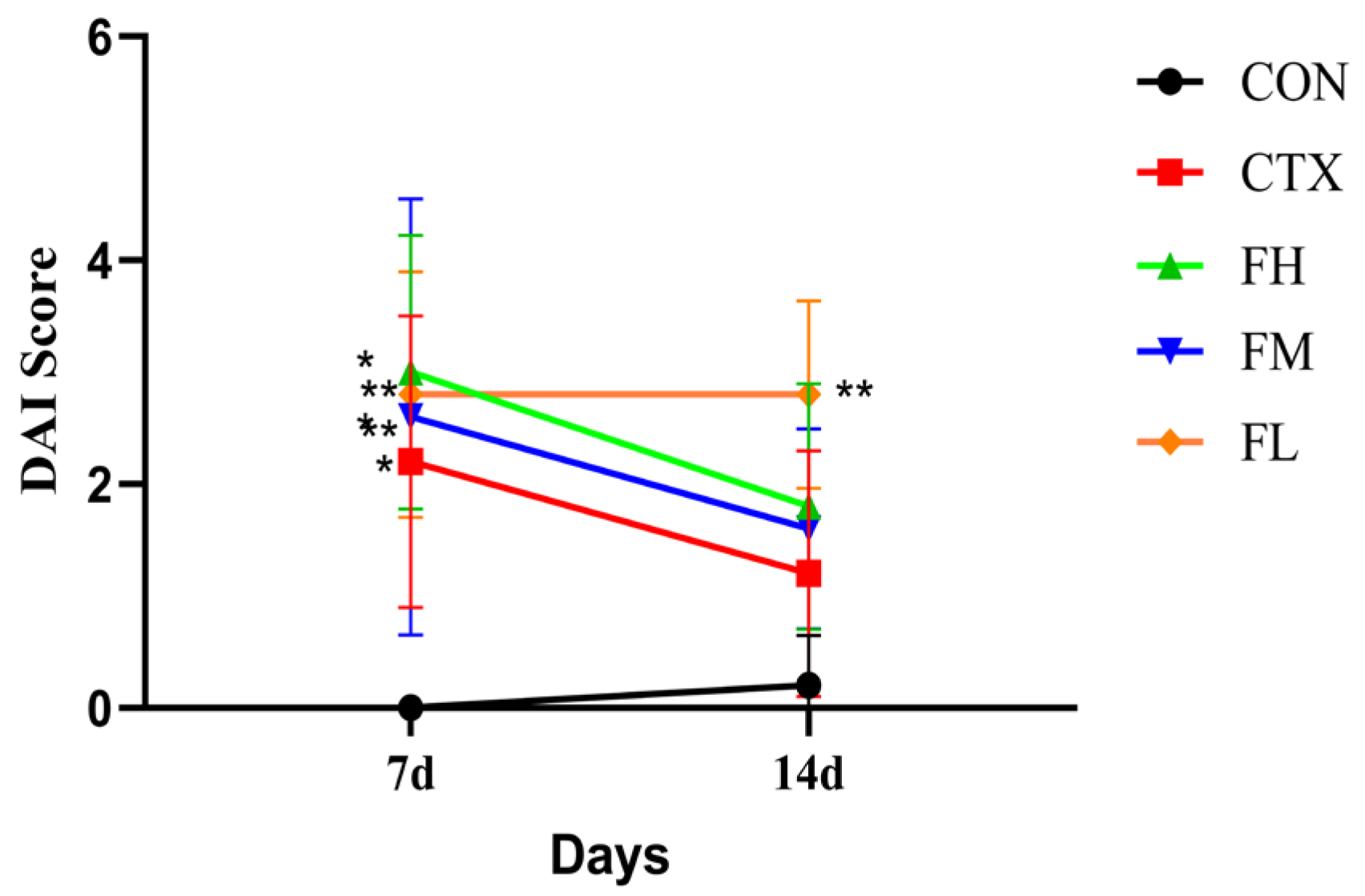
2.2.4. DAI Index of Immunosuppressed Mice
2.2.5. Determination of Immune Organ Indices in Immunosuppressed Mice
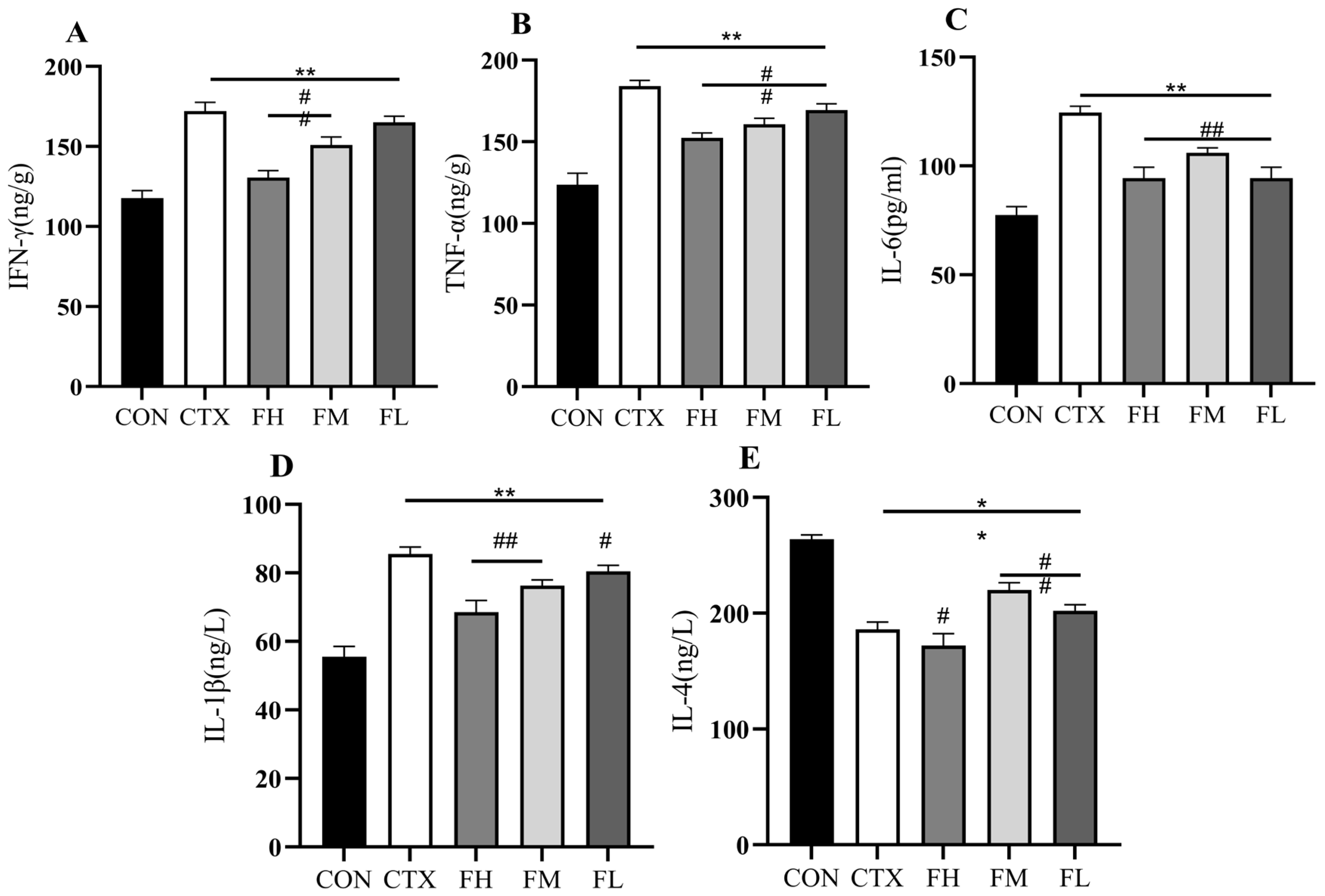
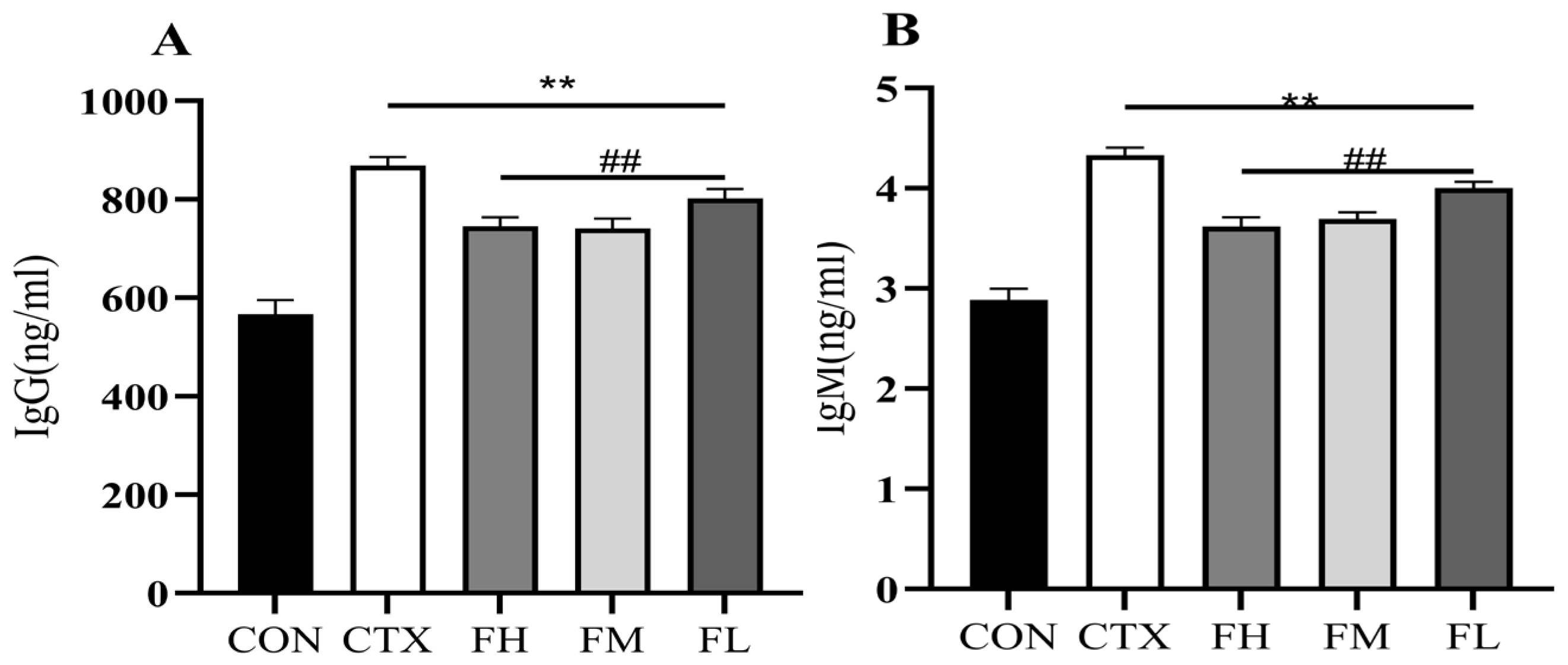
2.2.6. Determination of Serum Immune Indicators in Immunosuppressed Mice
2.2.7. Determination of 16S rRNA in Immunocompromised Mice
2.2.8. Determination of SCFAs in Feces and Colonic Contents of Immunosuppressed Mice
2.2.9. Data Statistical Processing
3. Results and Discussion
3.1. Structural Characterization of Kelp Fucoidan
3.2. Effects of Each Dose Group of Fucoidan on the Immune Indicators of Mice
3.2.1. Analysis of DAI Index and Body Weight in Immunosuppressed Mice
3.2.2. Analysis of Immune Organs in Immunosuppressed Mice
3.2.3. Analysis of Cytokine Levels in the Serum of Immunosuppressed Mice
3.2.4. Effect of Serum Immunoglobulin Content in Immunosuppressed Mice
3.3. Effects of Each Dose Group of Fucoidan on the Intestinal Flora of Immunocompromised Mice
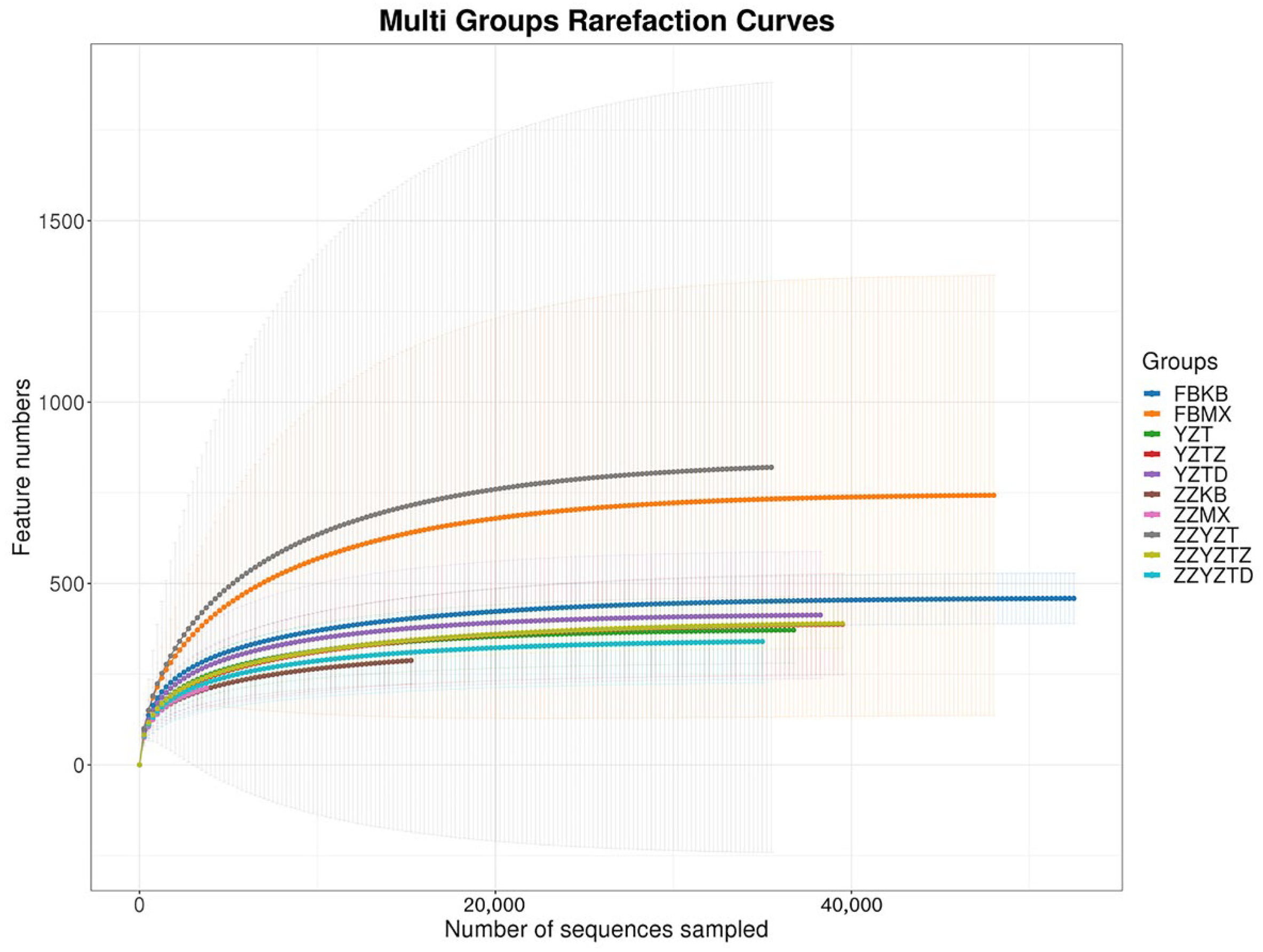
3.3.1. Analysis of Intestinal Flora Richness
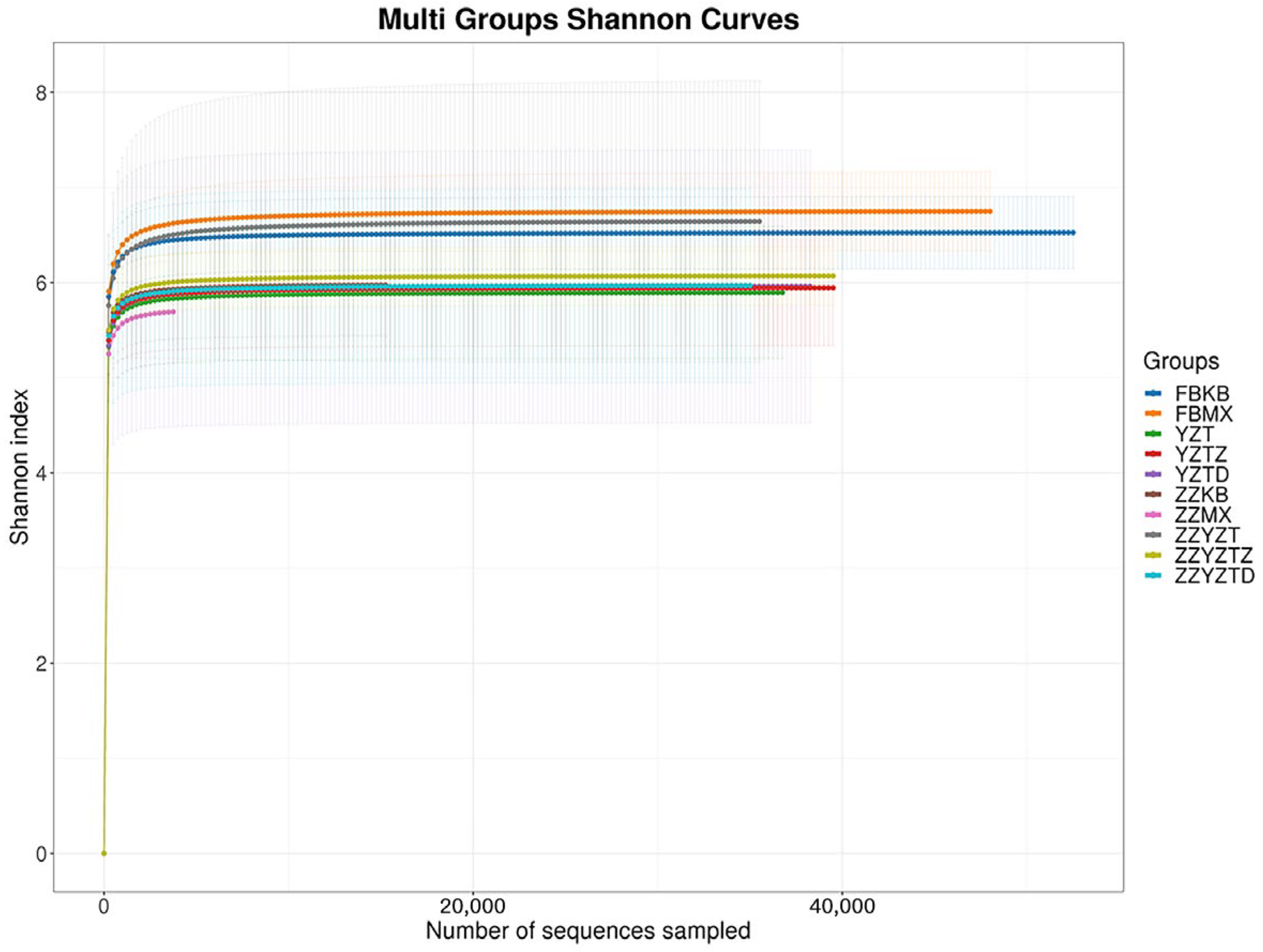
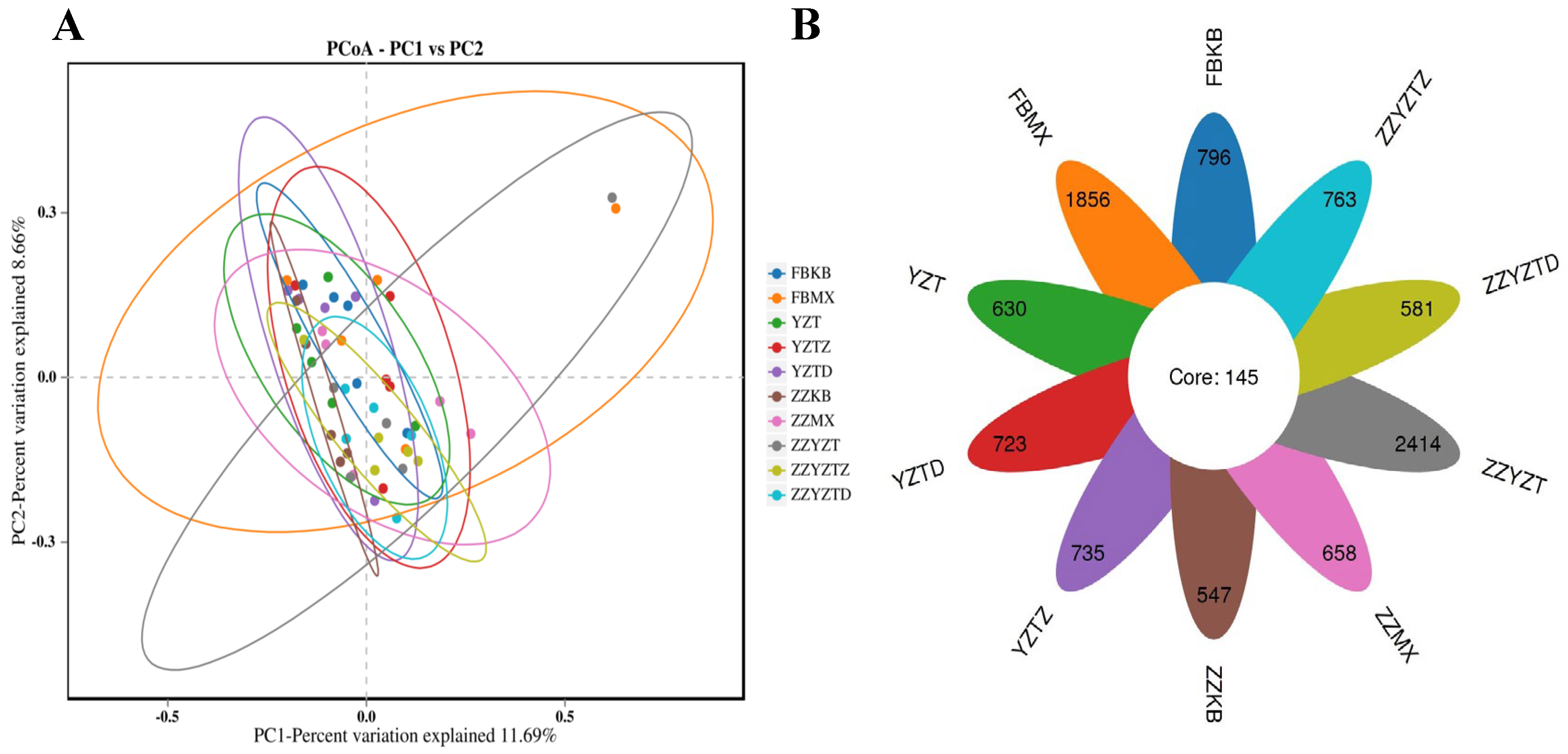
3.3.2. Analysis of the Diversity of Intestinal Flora in Mice
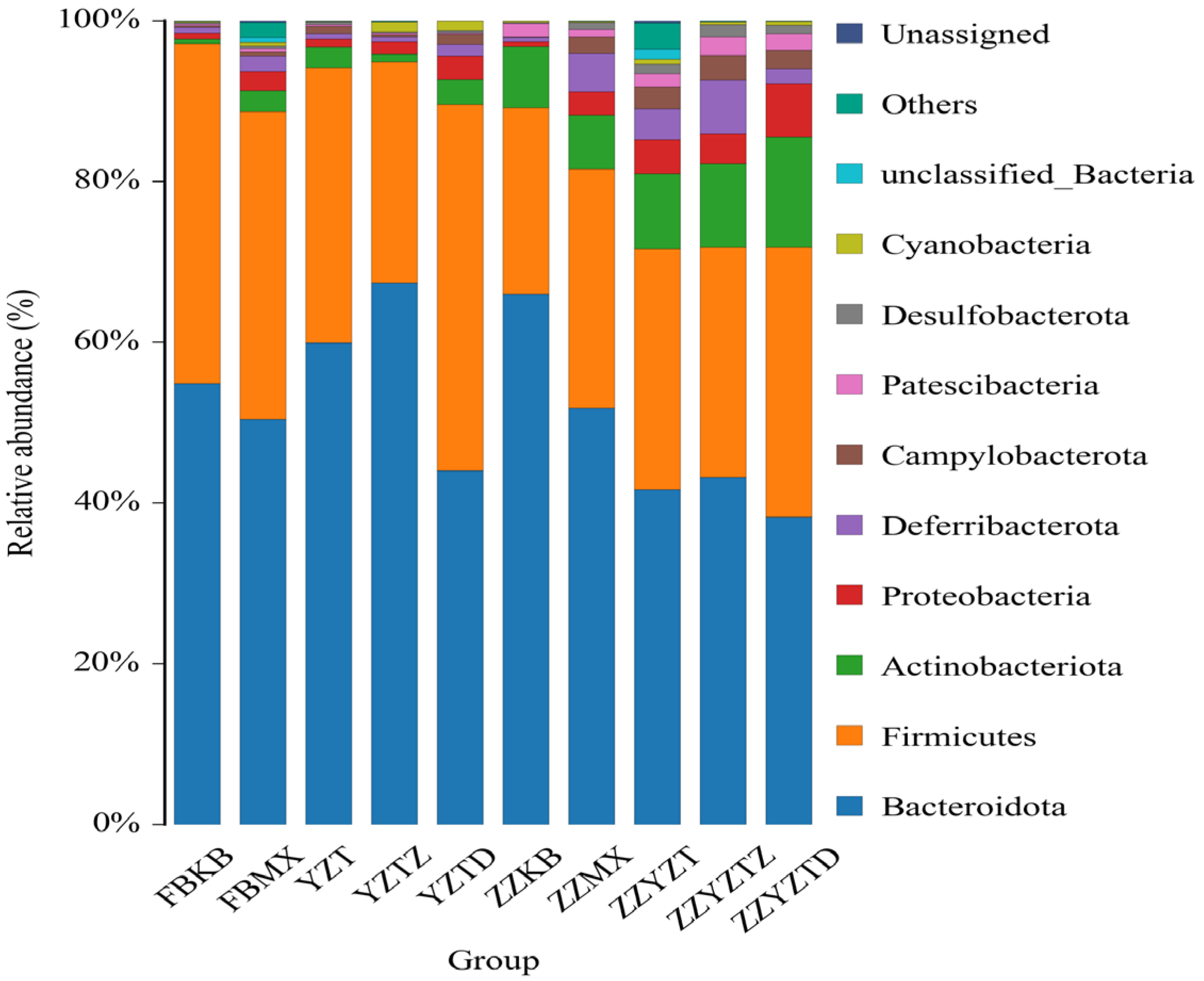
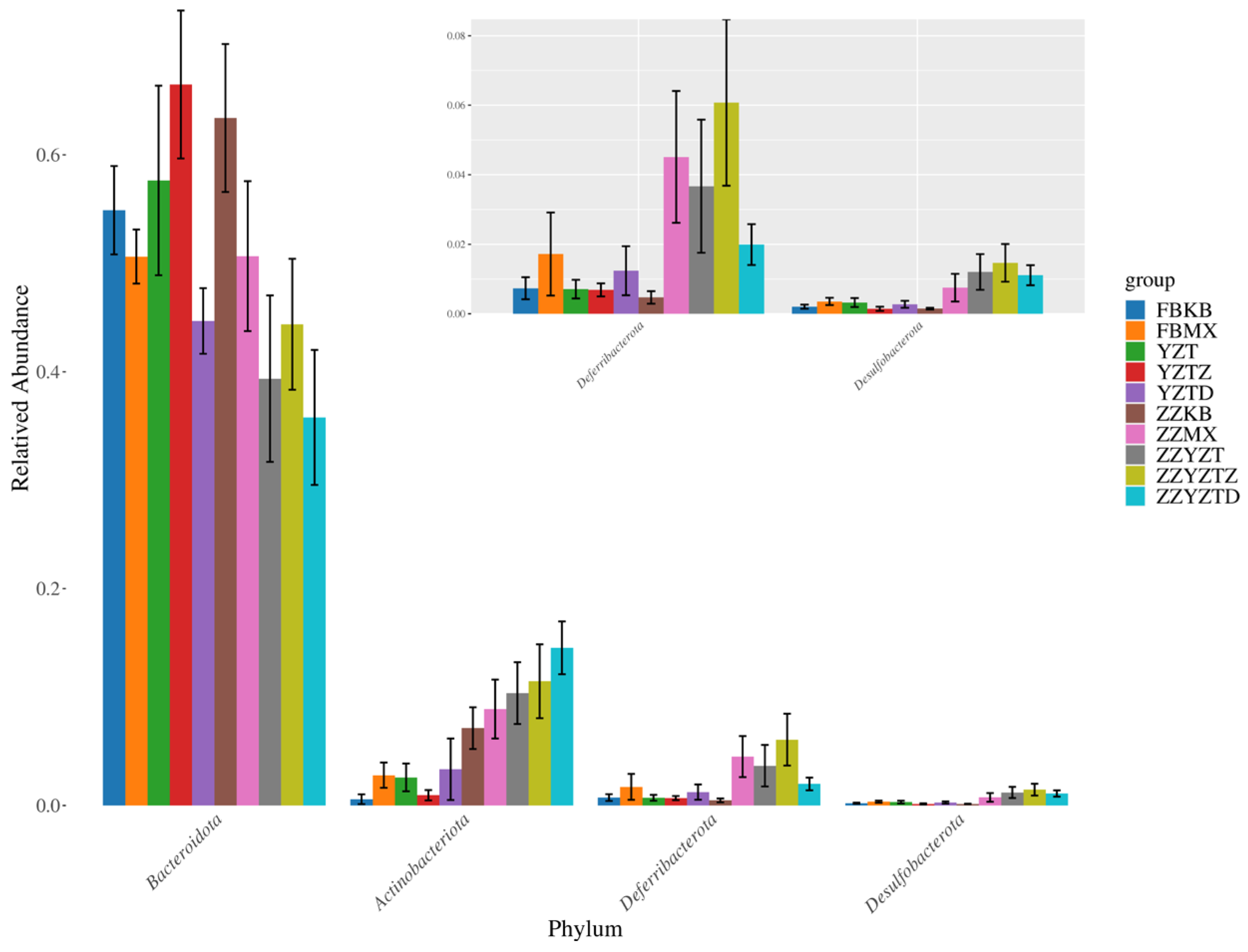
3.3.3. Analysis Based on the Gate Classification Level
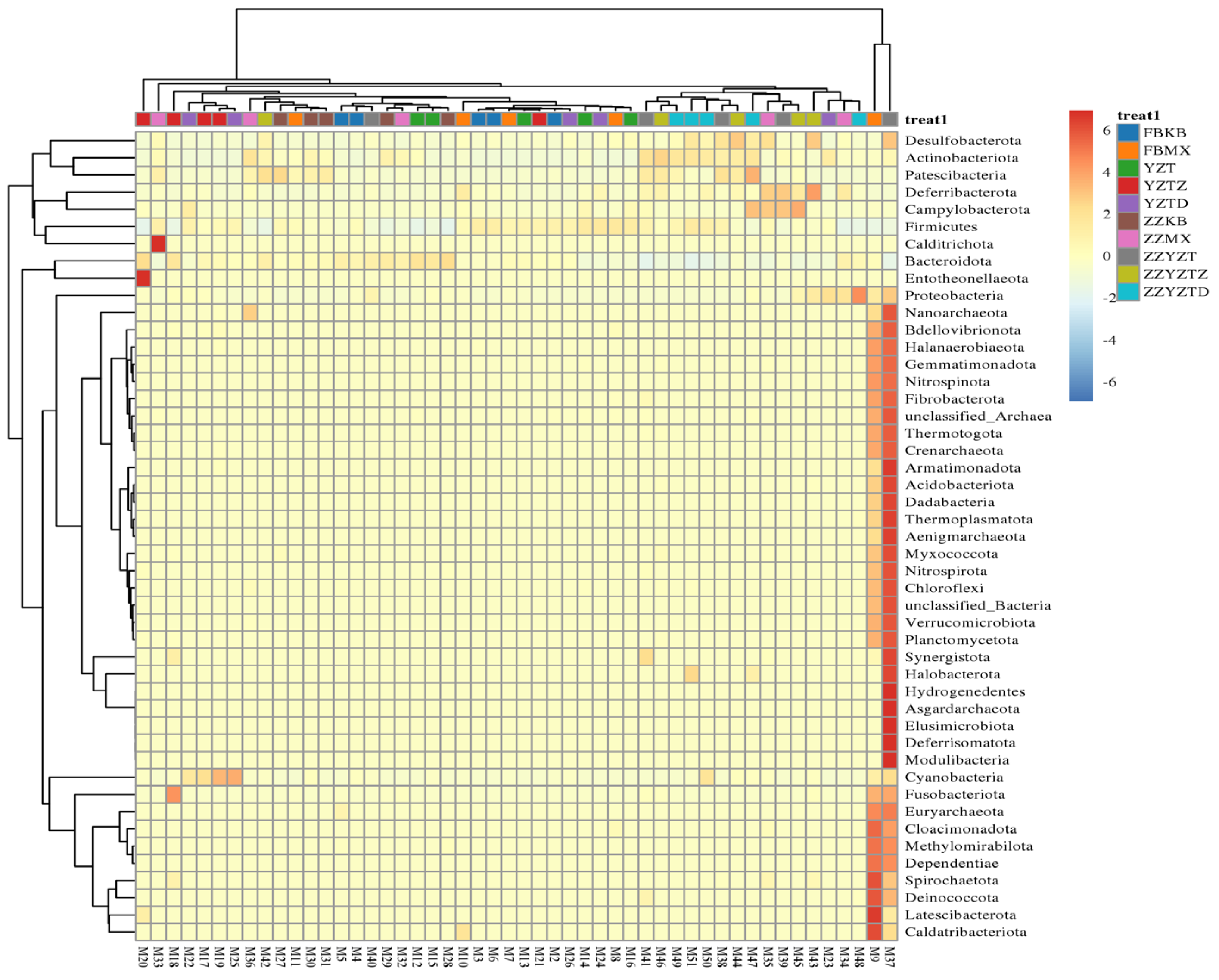
3.3.4. Analysis of Differences in Intestinal Flora
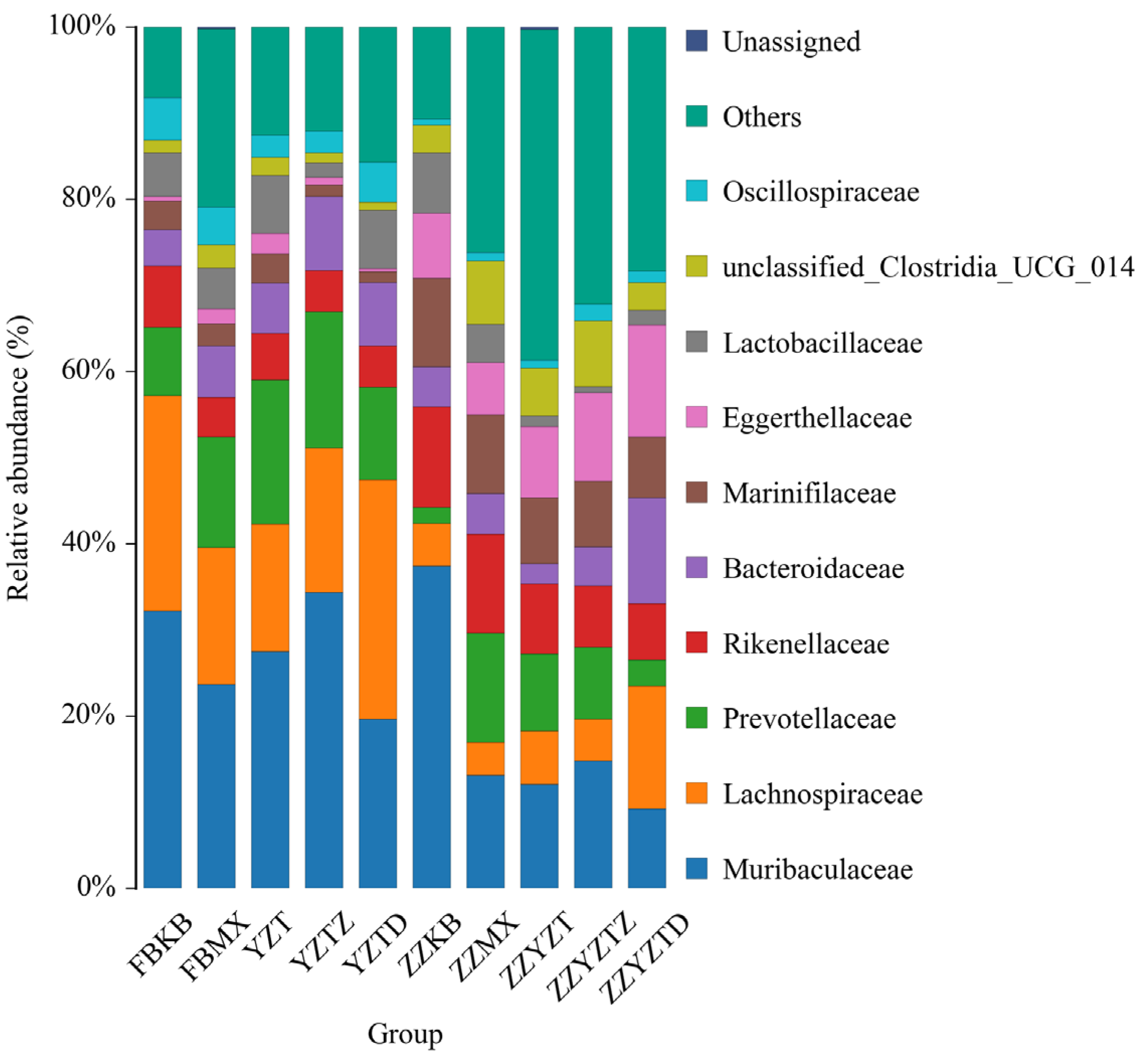
3.3.5. Analysis Based on the Classification Level of Families and Genera
3.4. Determination of SCFAs in Feces and Colonic Contents of Immunocompromised Mice
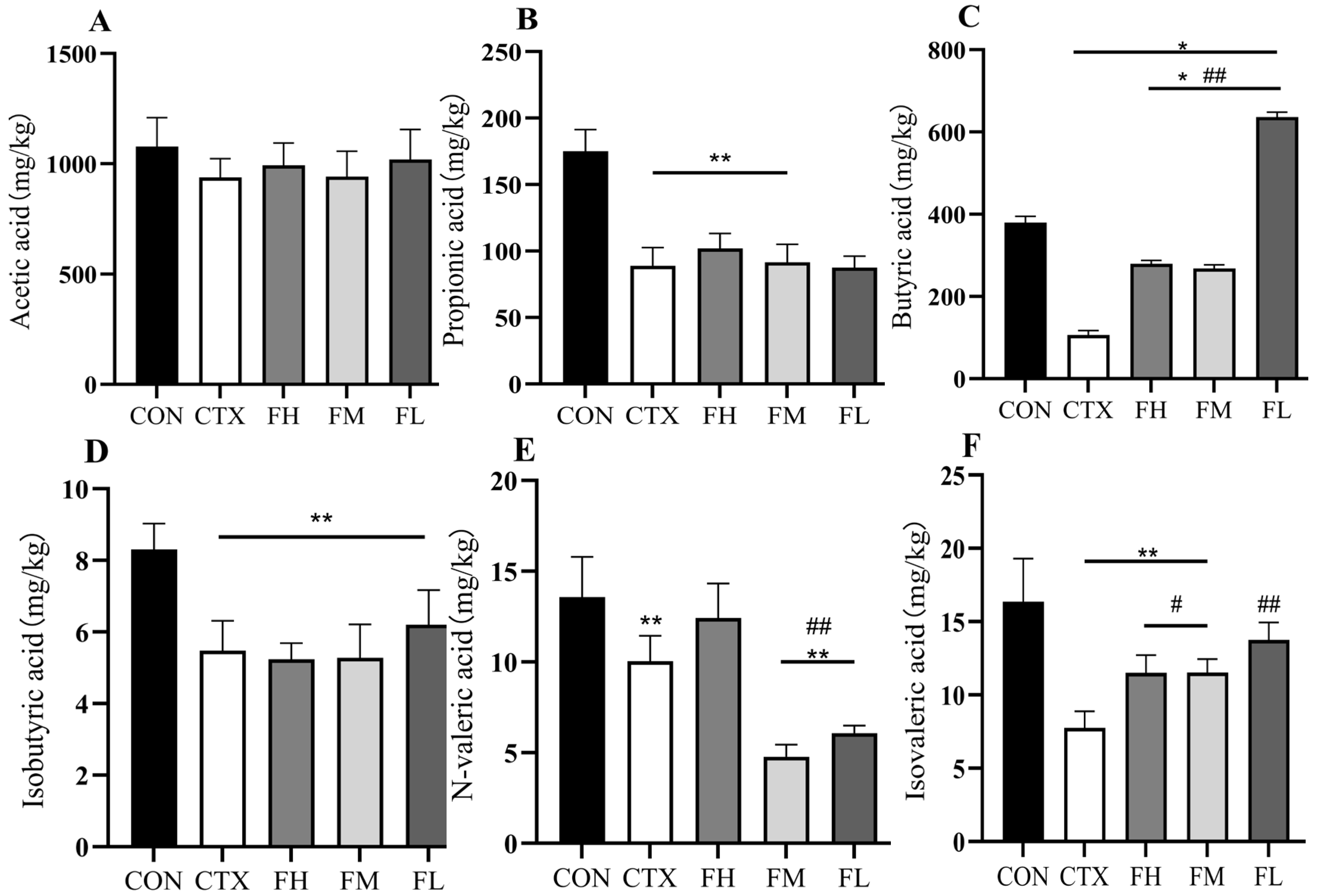
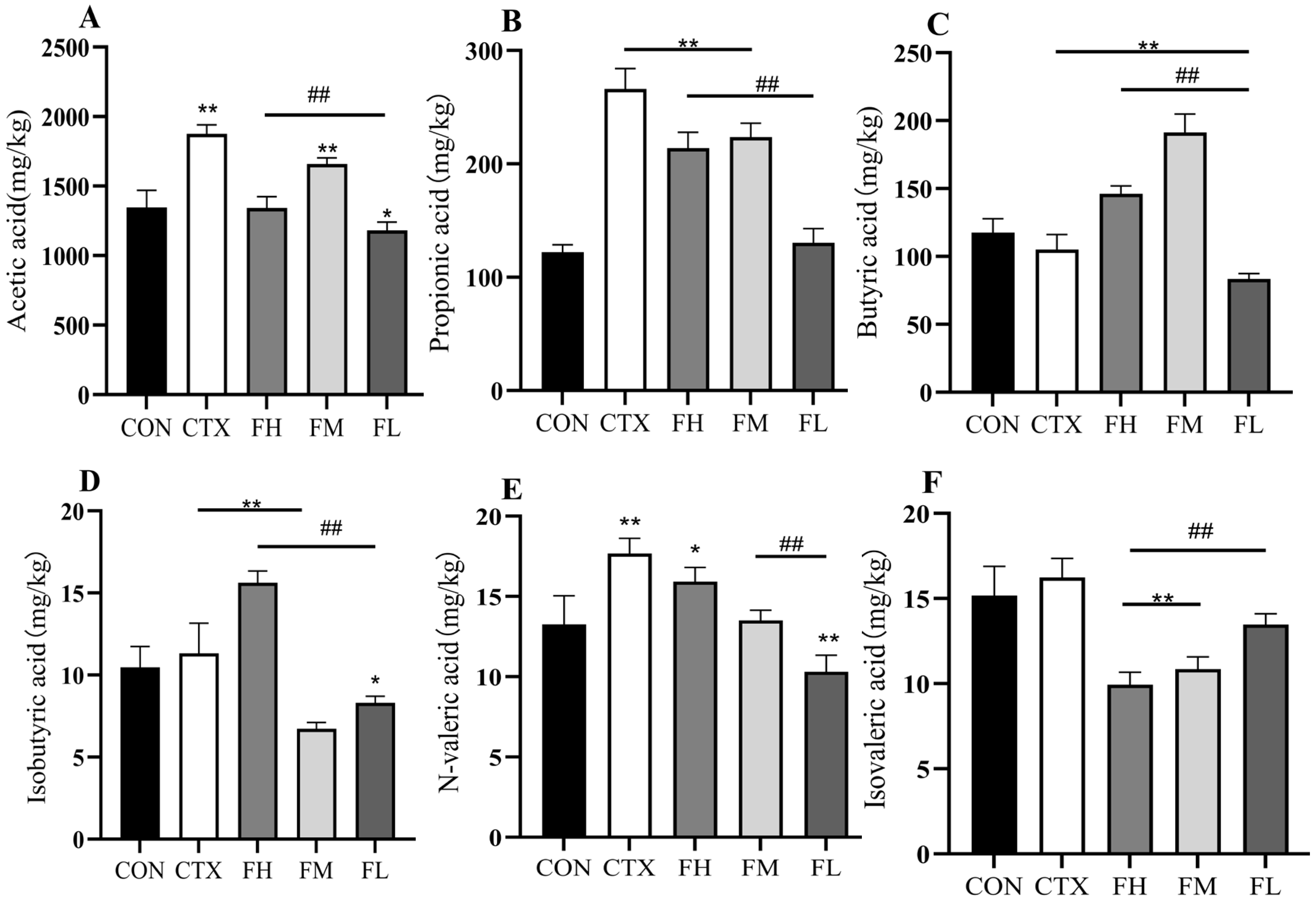
3.4.1. Effects of Each Dose Group of Fucoidan on SCFAs in the Colonic Contents of Mice
3.4.2. Effects of Each Dose Group of Fucoidan on SCFAs in Mouse Feces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillusand and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Li, C.; Duan, S.; Li, Y.; Pan, X.; Han, L. Polysaccharides in natural products that repair the damage to intestinal mucosa caused by cyclophosphamide and their mechanisms: A review. Carbohydr. Polym. 2021, 261, 117876. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhong, Y.F.; Wu, Y.P.; Luo, Z.; Sun, Y.M.; Wang, G.E.; Kurihara, H.; Li, Y.F.; He, R.R. Carnosine attenuates cyclophosphamide-induced bone marrow suppression by reducing oxidative DNA damage. Redox Biol. 2018, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mo, S.; Shen, M.; Chen, Y.; Yu, Q.; Li, Z.; Xie, J. Sulfated modification enhances the immunomodulatory effect of Cyclocarya paliurus polysaccharide on cyclophosphamide-induced immunosuppressed mice through MyD88-dependent MAPK/NF-ΚB and PI3K-Akt signaling pathways. Food Res. Int. 2021, 150, 110756. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, Y.; Wang, L.; Pang, X.; Zhao, L.; Yuan, H.; Zhang, C. A More Robust Gut Microbiota in Calorie-Restricted Mice Is Associated with Attenuated Intestinal Injury Caused by the Chemotherapy Drug Cyclophosphamide. MBio 2019, 10, e02903–e02918. [Google Scholar] [CrossRef]
- Shi, H.; Chang, Y.; Gao, Y.; Wang, X.; Chen, X.; Wang, Y.; Xue, C.; Tang, Q. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 2017, 8, 3383–3393. [Google Scholar] [CrossRef]
- Huang, R.; Xie, J.; Liu, X.; Shen, M. Sulfated modification enhances the modulatory effect of yam polysaccharide on gut microbiota in cyclophosphamide-treated mice. Food Res. Int. 2021, 145, 829–836. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, Y.; Chen, W.; Jia, R.; Yu, X.; Wang, Y.; Li, Y.; Liu, Y.; Ye, X.; Yu, L.; et al. Apios americana Medik flowers polysaccharide (AFP) alleviate Cyclophosphamide-induced immunosuppression in ICR mice. Int. J. Biol. Macromol. 2020, 144, 829–836. [Google Scholar] [CrossRef]
- Jie, D.; Gao, T.; Shan, Z.; Song, J.; Zhang, M.; Kurskaya, O.; Sharshov, K.; We, L.; Bi, H. Immunostimulating effect of polysaccharides isolated from Ma-Nuo-Xi decoction in cyclophosphamide-immunosuppressed mice. Int. J. Biol. Macromol. 2020, 146, 45–52. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Zhao, J.; Yin, L.; Lin, J.; Fu, H.; Tang, H.; Fang, J.; Peng, X.; Zhao, X. Protective effect of Polygonatum sibiricum polysaccharide on cyclophosphamide-induced immunosuppression in chickens. Res. Vet. Sci. 2021, 135, 96–105. [Google Scholar] [CrossRef]
- Chen, X.; Cai, B.; Wang, J.; Sheng, Z.; Yang, H.; Wang, D.; Chen, J.; Ning, Q. Mulberry leaf-derived polysaccharide modulates the immune response and gut microbiota composition in immunosuppressed mice. J. Funct. Foods 2021, 83, 104545. [Google Scholar] [CrossRef]
- Chen, X.; Sun, W.; Xu, B.; Wu, E.; Cui, Y.; Hao, K.; Zhang, G.; Zhou, C.; Xu, Y.; Li, J.; et al. Polysaccharides From the Roots of Millettia Speciosa Champ Modulate Gut Health and Ameliorate Cyclophosphamide-Induced Intestinal Injury and Immunosuppression. Front. Immunol. 2021, 12, 766296. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tan, H.; Nie, S. Beneficial effects of seaweed-derived dietary fiber: Highlights of the sulfated polysaccharides. Food Chem. 2022, 373, 131608. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Yi, Y.-L.; Guo, S.; Zhang, F.; Yan, H.; Zhan, Z.-L.; Zhu, Y.; Duan, J.-A. Isolation, structural characterization and bioactivities of polysaccharides from Laminaria japonica: A review. Food Chem. 2022, 370, 131010. [Google Scholar] [CrossRef]
- Peng, Y.; Song, Y.; Wang, Q.; Hu, Y.; He, Y.; Ren, D.; Wu, L.; Liu, S.; Cong, H.; Zhou, H. In vitro and in vivo immunomodulatory effects of fucoidan compound agents. Int. J. Biol. Macromol. 2019, 127, 48–56. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Lin, H.-T.V.; Lin, H.-Y.; Lo, S.-K. Dietary Supplementation with Low-Molecular-Weight Fucoidan Enhances Innate and Adaptive Immune Responses and Protects against Mycoplasma pneumoniae Antigen Stimulation. Mar. Drugs 2019, 17, 175. [Google Scholar] [CrossRef]
- Shang, Q.; Song, G.; Zhang, M.; Shi, J.; Xu, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Wu, X.; Guo, Y.X.; Dai, C.J.; Zhao, C. The Pharmacological Potential of Algal Polysaccharides in Food Applications and Chronic Disease Management. Future Pharmacol. 2025, 5, 29. [Google Scholar] [CrossRef]
- Yao, W.Z.; Kong, Q.H.; You, L.J.; Zhong, S.Y.; Hileuskaya, K. Polysaccharides from brown seaweed: Physicochemical properties, absorption in the intestine, and beneficial effects on intestinal barrier. Food Front. 2023, 4, 1547–1560. [Google Scholar] [CrossRef]
- Villar, M.M.-D.; Pérez-Rubio, K.G.; Hernández-Corona, D.M.; Cortez-Navarrete, M. Therapeutic Effect of Fucoidan on Metabolic Diseases: Experimental Data and Clinical Evidence. J. Med. Food 2022, 25, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sibusiso, L.; Hou, L.; Jiang, H.; Chen, P.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lin, Y.; Xie, H.; Farag, M.A.; Feng, S.; Li, J.; Shao, P. Dendrobium officinale leaf polysaccharides ameliorated hyperglycemia and promoted gut bacterial associated SCFAs to alleviate type 2 diabetes in adult mice. Food Chem.-X 2022, 13, 100207. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, X.; Miao, Y.; Zhou, Y.; Shi, J.; Yan, M.; Chen, A. Studies on Antiviral and Immuno-Regulation Activity of Low Molecular Weight Fucoidan from Laminaria japonica. J. Ocean Univ. China 2018, 17, 705–711. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Li, S.; Hao, L.; Du, J.; Gao, D.; Kang, Q.; Lu, J. Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Dictyopteris divaricata. Int. J. Biol. Macromol. 2018, 117, 256–263. [Google Scholar] [CrossRef]
- Lin, Q.; Zheng, L.; Pan, W.; Zhou, L.; Lai, R.; Huang, Y.; Zhang, N.; Yang, Y.; Xiao, M.; Ye, J. Fucoidan derived from Saccharina japonica: Structural characterization and amelioration of high-fat-diet induced obesity in male C57BL/6J mice via modulating the gut microbiota and lipid metabolites. Int. J. Biol. Macromol. 2025, 310, 143026. [Google Scholar] [CrossRef]
- Yue, Q.; Liu, Y.; Li, F.; Hong, T.; Guo, S.; Cai, M.; Zhao, L.; Su, L.; Zhang, S.; Zhao, C.; et al. Antioxidant and anticancer properties of fucoidan isolated from Saccharina Japonica brown algae. Sci. Rep. 2025, 15, 8962. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, Y.; Zhang, T.; Yin, C.; Qiao, J.; Guo, W.; Lu, B. TrkB agonist antibody ameliorates fertility deficits in aged and cyclophosphamide-induced premature ovarian failure model mice. Nat. Commun. 2022, 13, 914. [Google Scholar] [CrossRef]
- Ding, C.Y.; Zhu, L.P.; Shen, H.; Lu, J.F.; Zou, Q.Y.; Huang, C.; Li, H.; Huang, B.X. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells 2020, 38, 1137–1148. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zhang, D.; Zhou, X.W.; Duan, J.R.; Hu, Y.Q.; Zhang, W.J.; Liu, Q.; Xu, B.F.; Zhang, A.J. Cell-free fat extract improves ovarian function and fertility in mice with premature ovarian insufficiency. Stem Cell Res. Ther. 2022, 13, 320. [Google Scholar] [CrossRef]
- Sanchez, K.K.; Chen, G.Y.; Schieber, A.M.P.; Redford, S.E.; Shokhirev, M.N.; Leblanc, M.; Lee, Y.M.; Ayres, J.S. Cooperative Metabolic Adaptations in the Host Can Favor Asymptomatic Infection and Select for Attenuated Virulence in an Enteric Pathogen. Cell 2018, 175, 146–158.e15. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ju, H.X.; Li, J.S. Extraction and Determination of Short-Chain Fatty Acids in Biological Samples. Chin. J. Chromatogr. 2006, 24, 81–87. [Google Scholar]
- Birgersson, P.S.; Chahal, A.S.; Klau, L.J.; Holte, H.B.; Arlov, Ø.; Aachmann, F.L. Structural characterization and immunomodulating assessment of ultra-purified water extracted fucoidans from Saccharina latissima, Alaria esculenta and Laminaria hyperborea. Carbohydr. Polym. 2024, 343, 122448. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Usoltseva, R.V.; Mikkelsen, M.D.; Tran, V.H.N.; Ermakova, S.P.; Van, T.T.T.; Meyer, A.S.; Pham, T.D.; Hieu, V.M.N.; Cao, H.T.T. Structural characteristics and inhibition of tumor cell growth in vitro of enzymatically and chemically extracted fucoidans from Saccharina latissimia. J. Appl. Phycol. 2025, 1–9. [Google Scholar] [CrossRef]
- Zheng, W.Q.; Zhou, L.T.; Lin, L.S.; Cai, Y.; Sun, H.F.; Zhao, L.Y.; Gao, N.; Yin, R.H.; Zhao, J.H. Physicochemical Characteristics and Anticoagulant Activities of the Polysaccharides from Sea Cucumber Pattalus mollis. Mar. Drugs 2019, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Mao, F. Experimental Study on the Immunomodulating Effects of Warming the Kidney and Strengthening Spleen on Immuno Deficiency and Spleen Deficiency Mice. J. Zhejiang Chin. Med. Univ. 2012, 36, 1112–1116. [Google Scholar]
- Xu, Y. The influence of experimental diabetes on rat thymus:the polys accharide, lipids and enzyme-histochemical studies. J. Xuzhou Med. Univ. 2003, 6, 499–503. [Google Scholar]
- Littman, D.R.; Rudensky, A.Y. Th17 and Regulatory T Cells in Mediating and Restraining Inflammation. Cell 2010, 140, 845–858. [Google Scholar] [CrossRef]
- Akao, C.; Tanaka, T.; Onodera, R.; Ohyama, A.; Sato, N.; Motoyama, K.; Higashi, T.; Arima, H. Potential use of fucose-appended dendrimer/α-cyclodextrin conjugates as NF-κB decoy carriers for the treatment of lipopolysaccharide-induced fulminant hepatitis in mice. J. Control. Release 2014, 193, 35–41. [Google Scholar] [CrossRef]
- Santaolalla, A.; Sollie, S.; Rislan, A.; Josephs, D.H.; Hammar, N.; Walldius, G.; Garmo, H.; Karagiannis, S.N.; Van Hemelrijck, M. Association between serum markers of the humoral immune system and inflammation in the Swedish AMORIS study. BMC Immunol. 2021, 22, 61. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, G.; Hu, C. Role of immunoglobulin glycosylation in autoimmune diseases. Chin. J. Lab. Med. 2021, 44, 868–872. [Google Scholar]
- Huang, X.; Nie, S.; Xie, M. Interaction between gut immunity and polysaccharides. Crit. Rev. Food Sci. Nutr. 2015, 57, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, N.P.; Vongsa, R.A.; Wendt, M.K.; Dwinell, M.B. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 1000–1011. [Google Scholar] [CrossRef]
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of Elderly Japanese Men and Women with Fucoidan from Seaweed Increases Immune Responses to Seasonal Influenza Vaccination. J. Nutr. 2013, 143, 1794–1798. [Google Scholar] [CrossRef]
- Song, X.; Li, C.-Y.; Zeng, Y.; Wu, H.-Q.; Huang, Z.; Zhang, J.; Hong, R.-S.; Chen, X.-X.; Wang, L.-Y.; Hu, X.-P.; et al. Immunomodulatory effects of crude phenylethanoid glycosides from Ligustrum purpurascens. J. Ethnopharmacol. 2012, 144, 584–591. [Google Scholar] [CrossRef]
- Xie, S.-Z.; Liu, B.; Ye, H.-Y.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Liu, J.; Duan, J.; Luo, J.-P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Zhang, P.; Fan, S.; Huang, J.; Yu, S.; Zhang, C.; Li, H. Fucoidan and galactooligosaccharides ameliorate high-fat diet–induced dyslipidemia in rats by modulating the gut microbiota and bile acid metabolism. Nutrition 2019, 65, 50–59. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Li, Y.; Wang, Y.; Wang, F.; Qiu, X.; Liu, X.; Li, H. Sodium Alginate Modulates Immunity, Intestinal Mucosal Barrier Function, and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice. J. Agric. Food Chem. 2021, 69, 7064–7073. [Google Scholar] [CrossRef]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.R.; Greer, R.L.; Xiaoxi, D.; Dsouza, K.N.; Manoj, G.; Wu, J.Y.; Andrey, M.; Natalia, S. Antibiotic-Induced Alterations in Gut Microbiota Are Associated with Changes in Glucose Metabolism in Healthy Mice. Front. Microbiol. 2017, 8, 2306. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, M.; Zheng, P.; Liu, R.; Wang, D.; Zhao, D.; Wang, M. Effects of sulfated polysaccharides from Laminaria japonica on regularating the gut microbiotan and alleviating intestinal inflammation in obese mice. Food Chem. Toxicol. 2022, 168, 113401. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Q.; Li, T.; Nakatsu, G.; Chen, Y.-X.; Yau, T.O.; Chu, E.; Wong, S.; Szeto, C.H.; Ng, S.C.; Chan, F.K.L.; et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 2020, 69, 1248–1257. [Google Scholar] [CrossRef]
- He, C.; Kong, F.; Chai, X.; Zou, C.; Zhu, X.; Zhao, D. Effect of Probiotic-Assisted Eradication of cagA+/vacA s1m1 Helicobacter pylori on Intestinal Flora. BioMed Res. Int. 2022, 2022, 8607671. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, C.-p.; Zhang, Y.; Li, Y.; Sun, J.-r. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int. J. Biol. Macromol. 2019, 137, 504–511. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.C.; Wang, Z.L.; Ang, K.Y.; Huang, S.; Hou, Q.C.; Su, X.Q.; Qiao, J.M.; Zheng, Y.; Wang, L.F.; et al. Intestinal Microbiota Distinguish Gout Patients from Healthy Humans. Sci. Rep. 2016, 6, 20602. [Google Scholar] [CrossRef]
- Yamanaka, H.; Taniguchi, A.; Tsuboi, H.; Kano, H.; Asami, Y. Hypouricaemic effects of yoghurt containing Lactobacillus gasseri PA-3 in patients with hyperuricaemia and/or gout: A randomised, double-blind, placebo-controlled study. Mod. Rheumatol. 2019, 29, 146–150. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, S.; Huang, Y.; Gao, Q.; Xie, X.; Wang, P.; Li, J.; Liang, L.; He, X.; Jiang, Y.; et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. Npj Biofilms Microbiomes 2021, 7, 66. [Google Scholar] [CrossRef]
- Méndez-Salazar, E.O.; Vázquez-Mellado, J.; Casimiro-Soriguer, C.S.; Dopazo, J.; Çubuk, C.; Zamudio-Cuevas, Y.; Francisco-Balderas, A.; Martínez-Flores, K.; Fernández-Torres, J.; Lozada-Pérez, C. Taxonomic variations in the gut microbiome of gout patients with and without tophi might have a functional impact on urate metabolism. Mol. Med. 2021, 27, 50. [Google Scholar] [CrossRef]
- Cavaglieri, C.R.; Nishiyama, A.; Fernandes, L.C.; Curi, R.; Miles, E.A.; Calder, P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003, 73, 1683–1690. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Al-Lahham, S.a.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2010, 1801, 1175–1183. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, H.; Liu, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. In-depth investigation of the mechanisms of Echinacea purpurea polysaccharide mitigating alcoholic liver injury in mice via gut microbiota informatics and liver metabolomics. Int. J. Biol. Macromol. 2022, 209, 1327–1338. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Zhang, B.; Yin, J.; Liuqi, S.; Wang, J.; Peng, B.; Wang, S. Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2022, 70, 14864–14876. [Google Scholar] [CrossRef]

| Experimental Group | Number of Animals | Gavage Sample | Daily Gavage Dose | Duration of Administration |
|---|---|---|---|---|
| Blank control (CON) | 5 | - | Natural diet | 14 days |
| Model group (CTX) | 5 | Normal saline | 0.1 mL/10 g | 14 days |
| Low-dose fucoidan (FL) | 5 | Fucoidan | 50 mg/(kg·bw) | 14 days |
| Medium-dose fucoidan (FM) | 5 | Fucoidan | 100 mg/(kg·bw) | 14 days |
| High-dose fucoidan (FH) | 5 | Fucoidan | 150 mg/(kg·bw) | 14 days |
| Grade | Weight Loss (%) | Fecal Traits | Bloody Stool Condition |
|---|---|---|---|
| 0 | Lossless | Regular | No occult blood |
| 1 | 1–5 | Feces are soft but shaped | Positive occult blood |
| 2 | 5–10 | Very soft | Blood in the stool |
| 3 | 10–15 | Diarrhea | Hemafecia |
| 4 | >15 | - | - |
| CON | CTX | FH | FM | FL | |
|---|---|---|---|---|---|
| 0 d | 17.72 ± 0.70 | 17.98 ± 0.30 | 18.44 ± 0.92 | 18.2 ± 1.07 | 18.48 ± 1.02 |
| 7 d | 19.9 ± 1.18 | 17.32 ± 0.19 * | 17.16 ± 0.99 ** | 17.22 ± 1.36 ** | 17.46 ± 1.21 * |
| 14 d | 20.36 ± 1.19 | 17.62 ± 0.84 ** | 17.56 ± 2.20 ** | 17.22 ± 2.06 ** | 16.34 ± 1.54 ** |
| CON | CTX | FH | FM | FL | |
|---|---|---|---|---|---|
| Pancreatic organ coefficient | 0.47 ± 0.06 | 1.25 ± 0.18 ** | 1.01 ± 0.28 ** | 1.02 ± 0.28 ** | 1.00 ± 0.25 ** |
| Thymus organ co- efficient | 0.19 ± 0.01 | 0.11 ± 0.04 * | 0.10 ± 0.05 * | 0.11 ± 0.06 * | 0.09 ± 0.03 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Kang, R.; Zhao, Y.; Zhang, H.; Rong, Q.; Yu, S.; Chang, Y.; Wei, Z.; Zhu, L. Therapeutic Potential of Kelp Fucoidan in Rebiosis of Gut Microflora and Immune Homeostasis in Cyclophosphamide-Induced Immunosuppressed Mice. Foods 2025, 14, 2662. https://doi.org/10.3390/foods14152662
Liu Y, Kang R, Zhao Y, Zhang H, Rong Q, Yu S, Chang Y, Wei Z, Zhu L. Therapeutic Potential of Kelp Fucoidan in Rebiosis of Gut Microflora and Immune Homeostasis in Cyclophosphamide-Induced Immunosuppressed Mice. Foods. 2025; 14(15):2662. https://doi.org/10.3390/foods14152662
Chicago/Turabian StyleLiu, Yaqing, Ruining Kang, Yanfei Zhao, Heng Zhang, Qingfeng Rong, Shaoxuan Yu, Yaoguang Chang, Zhengpeng Wei, and Lanlan Zhu. 2025. "Therapeutic Potential of Kelp Fucoidan in Rebiosis of Gut Microflora and Immune Homeostasis in Cyclophosphamide-Induced Immunosuppressed Mice" Foods 14, no. 15: 2662. https://doi.org/10.3390/foods14152662
APA StyleLiu, Y., Kang, R., Zhao, Y., Zhang, H., Rong, Q., Yu, S., Chang, Y., Wei, Z., & Zhu, L. (2025). Therapeutic Potential of Kelp Fucoidan in Rebiosis of Gut Microflora and Immune Homeostasis in Cyclophosphamide-Induced Immunosuppressed Mice. Foods, 14(15), 2662. https://doi.org/10.3390/foods14152662






