Classification of Apples (Malus × domestica borkh.) According to Geographical Origin, Variety and Production Method Using Liquid Chromatography Mass Spectrometry and Random Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and LC-MS Data Acquisition
2.2. Data Used for the Different Authentication Issues
2.3. Data Processing and Analysis
3. Results and Discussion
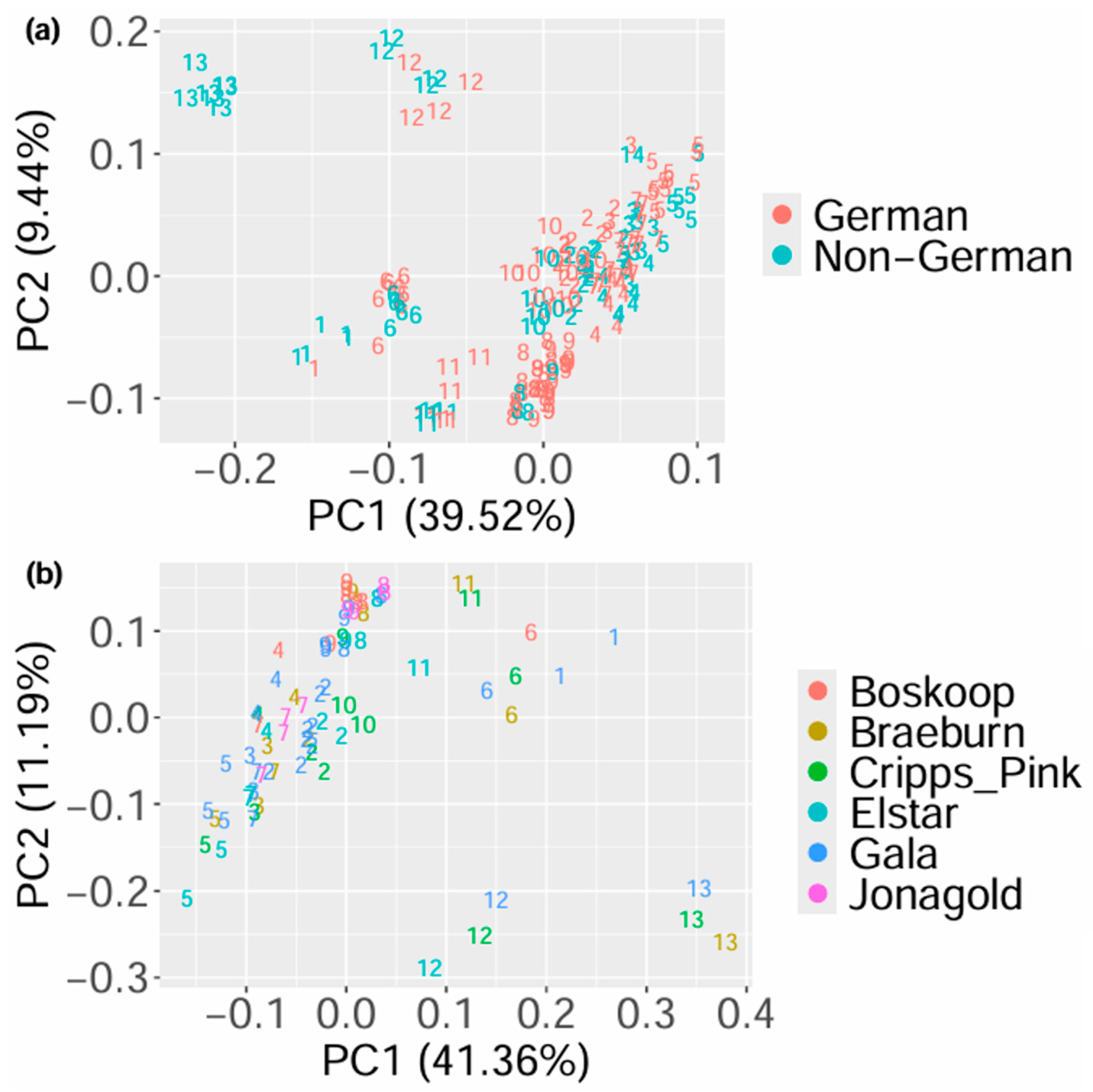
3.1. Principal Component Analysis
3.2. Differentiation Between German and Non-German Samples
3.3. Differentiation of the Regional Origin Within Germany
3.4. Differentiation Between Organically and Conventionally Produced Apples
3.5. Differentiation by Taxonomic Variety
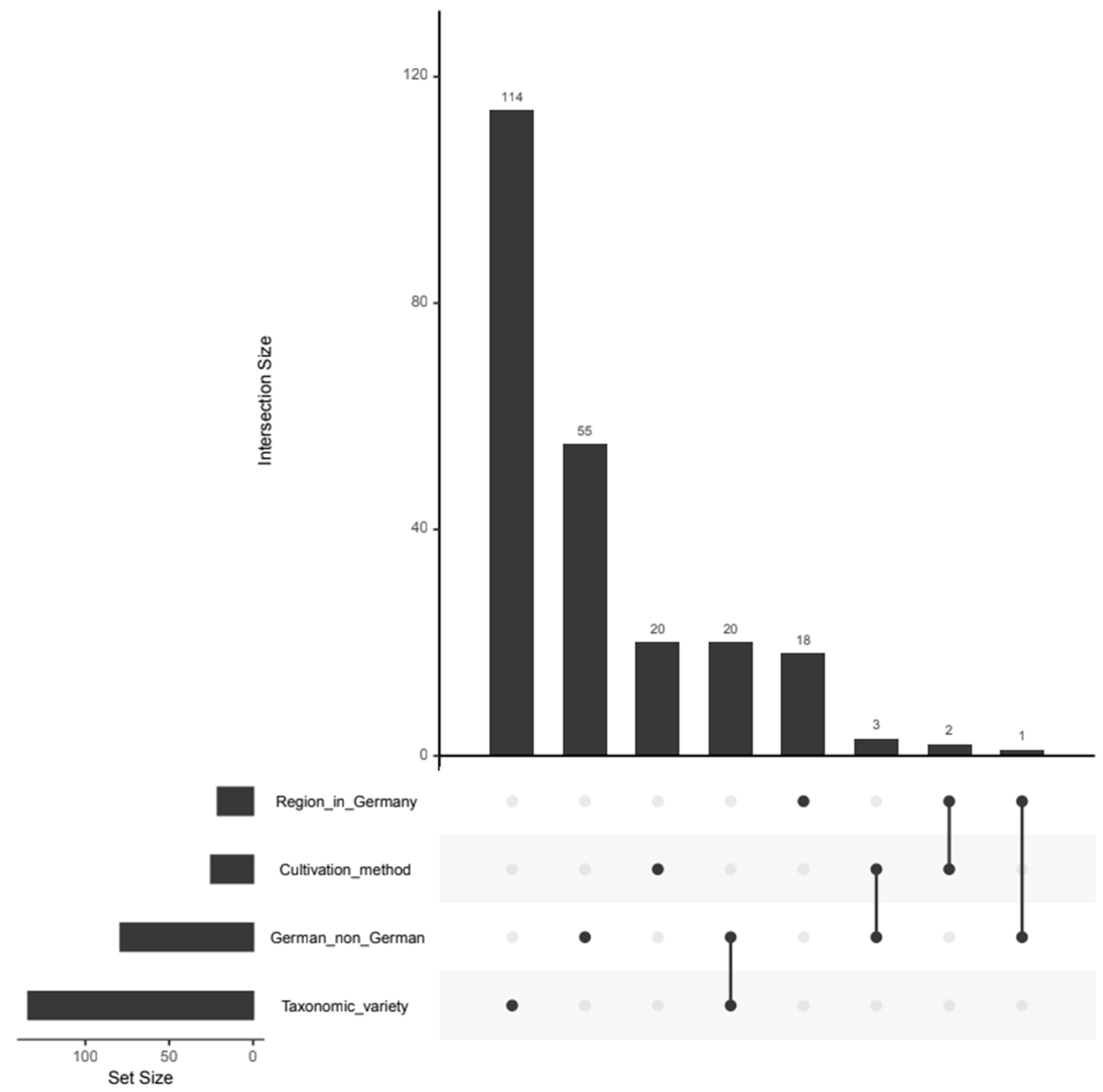
3.6. Analysis of the Intersection Between the Important Variables for Different Authentication Issues
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schaffner, D.; Demarmels, S.; Juettner, U. Promoting Biodiversity: Do Consumers Prefer Feelings, Facts, Advice or Appeals? J. Consum. Mark. 2015, 32, 266–277. [Google Scholar] [CrossRef]
- Tulloch, A.I.T.; Miller, A.; Dean, A.J. Does Scientific Interest in the Nature Impacts of Food Align with Consumer Information-Seeking Behavior? Sustain. Sci. 2021, 16, 1029–1043. [Google Scholar] [CrossRef]
- Denver, S.; Jensen, J.D. Consumer Preferences for Organically and Locally Produced Apples. Food Qual. Prefer. 2014, 31, 129–134. [Google Scholar] [CrossRef]
- SINUS-Instritut; YouGov. Studie zum Apfel: Die Hälfte Hat Schon Einmal Äpfel vom Nachbarsbaum Gepflückt; SINUS Markt- und Sozialforschung GmbH: Heidelberg, Germany, 2017. [Google Scholar]
- Kaeswurm, J.A.H.; Neuwald, D.A.; Straub, L.V.; Buchweitz, M. Impact of Cultivation and Storage Conditions on Total Mal d 1 Content and Isoallergen Profile in Apples. J. Agric. Food Chem. 2023, 71, 12975–12985. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.H.; Straub, L.V.; Siegele, A.; Brockmeyer, J.; Buchweitz, M. Characterization and Quantification of Mal d 1 Isoallergen Profiles and Contents in Traditional and Commercial Apple Varieties by Mass Spectrometry. J. Agric. Food Chem. 2023, 71, 2554–2565. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.-S.; Oest, M.; Scheffler, S.; Horns, A.L.; Paasch, N.; Bachmann, R.; Fischer, M. Food Authentication Goes Green: Method Optimization for Origin Discrimination of Apples Using Apple Juice and ICP-MS. Foods 2024, 13, 3783. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Jiang, N.; Wang, H.; Guo, G. Evaluation of Machine Learning Methods for Organic Apple Authentication Based on Diffraction Grating and Image Processing. J. Food Compos. Anal. 2020, 88, 103437. [Google Scholar] [CrossRef]
- Barberis, E.; Amede, E.; Dondero, F.; Marengo, E.; Manfredi, M. New Non-Invasive Method for the Authentication of Apple Cultivars. Foods 2021, 11, 89. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass Spectrometry-Based Metabolomics: MASS SPECTROMETRY-BASED METABOLOMICS. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on Metabolomics for Food Authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Creydt, M.; Fischer, M. Food Phenotyping: Recording and Processing of Non-Targeted Liquid Chromatography Mass Spectrometry Data for Verifying Food Authenticity. Molecules 2020, 25, 3972. [Google Scholar] [CrossRef] [PubMed]
- Wenck, S.; Bachmann, R.; Barmbold, S.-M.; Horns, A.L.; Paasch, N.; Seifert, S. Authentication of Apples (Malus × Domestica Borkh.) According to Geographical Origin, Variety and Production Method Using 1H NMR Spectroscopy and Random Forest. Food Control 2025, 167, 110817. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Stella, R.; Baggio, A.; Biancotto, G.; Mutinelli, F. LC-HRMS-Based Non-Targeted Metabolomics for the Assessment of Honey Adulteration with Sugar Syrups: A Preliminary Study. Metabolites 2022, 12, 985. [Google Scholar] [CrossRef]
- Hansen, J.; Kunert, C.; Raezke, K.-P.; Seifert, S. Detection of Sugar Syrups in Honey Using Untargeted Liquid Chromatography–Mass Spectrometry and Chemometrics. Metabolites 2024, 14, 633. [Google Scholar] [CrossRef]
- Schütz, D.; Achten, E.; Creydt, M.; Riedl, J.; Fischer, M. Non-Targeted LC-MS Metabolomics Approach towards an Authentication of the Geographical Origin of Grain Maize (Zea mays L.) Samples. Foods 2021, 10, 2160. [Google Scholar] [CrossRef]
- Creydt, M.; Hudzik, D.; Rurik, M.; Kohlbacher, O.; Fischer, M. Food Authentication: Small-Molecule Profiling as a Tool for the Geographic Discrimination of German White Asparagus. J. Agric. Food Chem. 2018, 66, 13328–13339. [Google Scholar] [CrossRef]
- Klockmann, S.; Reiner, E.; Bachmann, R.; Hackl, T.; Fischer, M. Food Fingerprinting: Metabolomic Approaches for Geographical Origin Discrimination of Hazelnuts (Corylus Avellana) by UPLC-QTOF-MS. J. Agric. Food Chem. 2016, 64, 9253–9262. [Google Scholar] [CrossRef]
- Lösel, H.; Brockelt, J.; Gärber, F.; Teipel, J.; Kuballa, T.; Seifert, S.; Fischer, M. Comparative Analysis of LC-ESI-IM-qToF-MS and FT-NIR Spectroscopy Approaches for the Authentication of Organic and Conventional Eggs. Metabolites 2023, 13, 882. [Google Scholar] [CrossRef]
- Wenck, S.; Creydt, M.; Hansen, J.; Gärber, F.; Fischer, M.; Seifert, S. Opening the Random Forest Black Box of the Metabolome by the Application of Surrogate Minimal Depth. Metabolites 2022, 12, 5. [Google Scholar] [CrossRef]
- Dinis, K.; Tsamba, L.; Thomas, F.; Jamin, E.; Camel, V. Preliminary Authentication of Apple Juices Using Untargeted UHPLC-HRMS Analysis Combined to Chemometrics. Food Control 2022, 139, 109098. [Google Scholar] [CrossRef]
- Wang, J.; Chow, W. Study of Ultrahigh-Performance Liquid Chromatography Electrospray Ionization Q-Orbitrap Mass Spectrometry and Various Extraction Methods for Fingerprinting and Identification of Molecular Authenticity Markers in Apple and Grape Juices. ACS Food Sci. Technol. 2022, 2, 1326–1338. [Google Scholar] [CrossRef]
- Hansen, J.; Kunert, C.; Münstermann, H.; Raezke, K.-P.; Seifert, S. Application of Untargeted Liquid Chromatography-Mass Spectrometry to Routine Analysis of Food Using Three-Dimensional Bucketing and Machine Learning. Sci. Rep. 2024, 14, 16594. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, L. Evaluating and Minimizing Batch Effects in Metabolomics. Mass Spectrom. Rev. 2022, 41, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y. (Eds.) Ensemble Machine Learning: Methods and Applications; Springer: New York, NY, USA, 2012; ISBN 978-1-4419-9325-0. [Google Scholar]
- Lim, D.K.; Long, N.P.; Mo, C.; Dong, Z.; Lim, J.; Kwon, S.W. Optimized Mass Spectrometry-Based Metabolite Extraction and Analysis for the Geographical Discrimination of White Rice (Oryza sativa L.): A Method Comparison Study. J. AOAC Int. 2018, 101, 498–506. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Rainer, J. Metabolomics Data Pre-Processing Using Xcms. 2020. Available online: https://jorainer.github.io/metabolomics2018/xcms-preprocessing.html (accessed on 9 January 2025).
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Martens, L.; Chambers, M.; Sturm, M.; Kessner, D.; Levander, F.; Shofstahl, J.; Tang, W.H.; Römpp, A.; Neumann, S.; Pizarro, A.D.; et al. mzML—A Community Standard for Mass Spectrometry Data. Mol. Cell. Proteom. 2011, 10, R110.000133. [Google Scholar] [CrossRef]
- Pedrioli, P.G.A.; Eng, J.K.; Hubley, R.; Vogelzang, M.; Deutsch, E.W.; Raught, B.; Pratt, B.; Nilsson, E.; Angeletti, R.H.; Apweiler, R.; et al. A Common Open Representation of Mass Spectrometry Data and Its Application to Proteomics Research. Nat. Biotechnol. 2004, 22, 1459–1466. [Google Scholar] [CrossRef]
- Keller, A.; Eng, J.; Zhang, N.; Li, X.; Aebersold, R. A Uniform Proteomics MS/MS Analysis Platform Utilizing Open XML File Formats. Mol. Syst. Biol. 2005, 1, 1-8. [Google Scholar] [CrossRef]
- Gatto, L.; Lilley, K.S. MSnbase-an R/Bioconductor Package for Isobaric Tagged Mass Spectrometry Data Visualization, Processing and Quantitation. Bioinformatics 2012, 28, 288–289. [Google Scholar] [CrossRef]
- Gatto, L.; Gibb, S.; Rainer, J. MSnbase, Efficient and Elegant R-Based Processing and Visualization of Raw Mass Spectrometry Data. J. Proteome Res. 2021, 20, 1063–1069. [Google Scholar] [CrossRef]
- Benton, H.P.; Want, E.J.; Ebbels, T.M.D. Correction of Mass Calibration Gaps in Liquid Chromatography–Mass Spectrometry Metabolomics Data. Bioinformatics 2010, 26, 2488–2489. [Google Scholar] [CrossRef]
- Prince, J.T.; Marcotte, E.M. Chromatographic Alignment of ESI-LC-MS Proteomics Data Sets by Ordered Bijective Interpolated Warping. Anal. Chem. 2006, 78, 6140–6152. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Böttcher, C.; Neumann, S. Highly Sensitive Feature Detection for High Resolution LC/MS. BMC Bioinform. 2008, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Mardia, K.V.; Kent, J.M. BIBBY: Multivariate Analysis. Academic Press, London-New York-Toronto-Sydney-San Francisco 1979. xv, 518 pp., $ 61.00. Biom. J 1982, 24, 502. [Google Scholar] [CrossRef]
- Becker, R.M.; Chambers, J.M.; Wilks, A.R. The New S Language Data Analysis: A Programming Environment for Data Analysis and Graphics; The Wadsworth & Brooks/Cole statistics, probability series; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1988; ISBN 978-0-534-09193-4. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Wickham, H. Ggplot2; Use R! Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Wright, M.N.; Ziegler, A. Ranger: A Fast Implementation of Random Forests for High Dimensional Data in C++ and R. J. Stat. Soft. 2017, 77, 1–17. [Google Scholar] [CrossRef]
- Degenhardt, F.; Seifert, S.; Szymczak, S. Evaluation of Variable Selection Methods for Random Forests and Omics Data Sets. Brief. Bioinform. 2019, 20, 492–503. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Soft. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; HUSERMET Consortium; et al. Development of a Robust and Repeatable UPLC−MS Method for the Long-Term Metabolomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef]
- UN Comtrade. UN Comtrade Database. Available online: https://comtradeplus.un.org/TradeFlow?Frequency=A&Flows=M&CommodityCodes=080810&Partners=276&Reporters=all&period=2024&AggregateBy=none&BreakdownMode=plus (accessed on 6 April 2025).
- Schimmel, J.; Gentsch, N.; Boy, J.; Uteau, D.; Rohr, A.-D.; Winkelmann, T.; Busnena, B.; Liu, B.; Krueger, J.; Kaufhold, S.; et al. Alleviation of Apple Replant Disease in Sandy Soils by Clay Amendments. Silicon 2024, 16, 4343–4360. [Google Scholar] [CrossRef]
- Creydt, M.; Fischer, M. Omics Approaches for Food Authentication. Electrophoresis 2018, 39, 1569–1581. [Google Scholar] [CrossRef]
- Richter, B.; Gurk, S.; Wagner, D.; Bockmayr, M.; Fischer, M. Food Authentication: Multi-Elemental Analysis of White Asparagus for Provenance Discrimination. Food Chem. 2019, 286, 475–482. [Google Scholar] [CrossRef]
- Von Wuthenau, K.; Segelke, T.; Müller, M.-S.; Behlok, H.; Fischer, M. Food Authentication of Almonds (Prunus Dulcis Mill.). Origin Analysis with Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Chemometrics. Food Control 2022, 134, 108689. [Google Scholar] [CrossRef]
- Von Wuthenau, K.; Müller, M.-S.; Cvancar, L.; Oest, M.; Fischer, M. Food Authentication of Almonds (Prunus Dulcis Mill.). Fast Origin Analysis with Laser Ablation Inductively Coupled Plasma Mass Spectrometry and Chemometrics. J. Agric. Food Chem. 2022, 70, 5237–5244. [Google Scholar] [CrossRef]
- Segelke, T.; Von Wuthenau, K.; Kuschnereit, A.; Müller, M.-S.; Fischer, M. Origin Determination of Walnuts (Juglans regia L.) on a Worldwide and Regional Level by Inductively Coupled Plasma Mass Spectrometry and Chemometrics. Foods 2020, 9, 1708. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.-S.; Springer, C.; Middendorf, E.; Cvancar, L.; Oest, M.; Fischer, M. Food Authentication Goes Green: Development of a Fast and Resource-Saving Method for Determining the Geographical Origin of Hazelnuts Using ICP-MS and Laser Ablation. J. Food Compos. Anal. 2025, 139, 107168. [Google Scholar] [CrossRef]
- Segelke, T.; Von Wuthenau, K.; Neitzke, G.; Müller, M.-S.; Fischer, M. Food Authentication: Species and Origin Determination of Truffles (Tuber spp.) by Inductively Coupled Plasma Mass Spectrometry and Chemometrics. J. Agric. Food Chem. 2020, 68, 14374–14385. [Google Scholar] [CrossRef] [PubMed]
- Bannier, H.-J. Moderne Apfelzüchtung: Genetische Verarmung und Tendenzen zur Inzucht: Vitalitätsverluste erst bei Verzicht auf Fungizideinsatz sichtbar. Erwerbs-Obstbau 2011, 52, 85–110. [Google Scholar] [CrossRef]
| True | |||
| Predicted | German | Non-German | |
| German | 112 | 8 | |
| Non-German | 5 | 68 | |
| True | |||
| Predicted | North | South | |
| North | 74 | 12 | |
| South | 5 | 26 | |
| True | |||
| Predicted | Conventional | Organic | |
| Conventional | 110 | 19 | |
| Organic | 3 | 21 | |
| True | |||||||
| Predicted | Boskoop | Braeburn | Cripps Pink | Elstar | Gala | Jonagold | |
| Boskoop | 8 | 1 | |||||
| Braeburn | 8 | 3 | |||||
| Cripps Pink | 12 | ||||||
| Elstar | 1 | 10 | 1 | ||||
| Gala | 1 | 27 | |||||
| Jonagold | 1 | 7 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, J.; Fransson, I.; Schrieck, R.; Kunert, C.; Seifert, S. Classification of Apples (Malus × domestica borkh.) According to Geographical Origin, Variety and Production Method Using Liquid Chromatography Mass Spectrometry and Random Forest. Foods 2025, 14, 2655. https://doi.org/10.3390/foods14152655
Hansen J, Fransson I, Schrieck R, Kunert C, Seifert S. Classification of Apples (Malus × domestica borkh.) According to Geographical Origin, Variety and Production Method Using Liquid Chromatography Mass Spectrometry and Random Forest. Foods. 2025; 14(15):2655. https://doi.org/10.3390/foods14152655
Chicago/Turabian StyleHansen, Jule, Iris Fransson, Robbin Schrieck, Christof Kunert, and Stephan Seifert. 2025. "Classification of Apples (Malus × domestica borkh.) According to Geographical Origin, Variety and Production Method Using Liquid Chromatography Mass Spectrometry and Random Forest" Foods 14, no. 15: 2655. https://doi.org/10.3390/foods14152655
APA StyleHansen, J., Fransson, I., Schrieck, R., Kunert, C., & Seifert, S. (2025). Classification of Apples (Malus × domestica borkh.) According to Geographical Origin, Variety and Production Method Using Liquid Chromatography Mass Spectrometry and Random Forest. Foods, 14(15), 2655. https://doi.org/10.3390/foods14152655






