Dietary Interventions with Bletilla striata Polysaccharides and/or Composite Polysaccharides Remodel Liver Lipid Profiles and Ameliorate Gut Metabolic Disturbances in High-Fat Diet-Induced Obese Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. BSP Preparation
2.3. Experimental Design
2.4. Lipidomic Analysis
2.5. Metabolomic Analysis
2.6. Microbiome Analysis
2.7. Statistical Analysis
3. Results
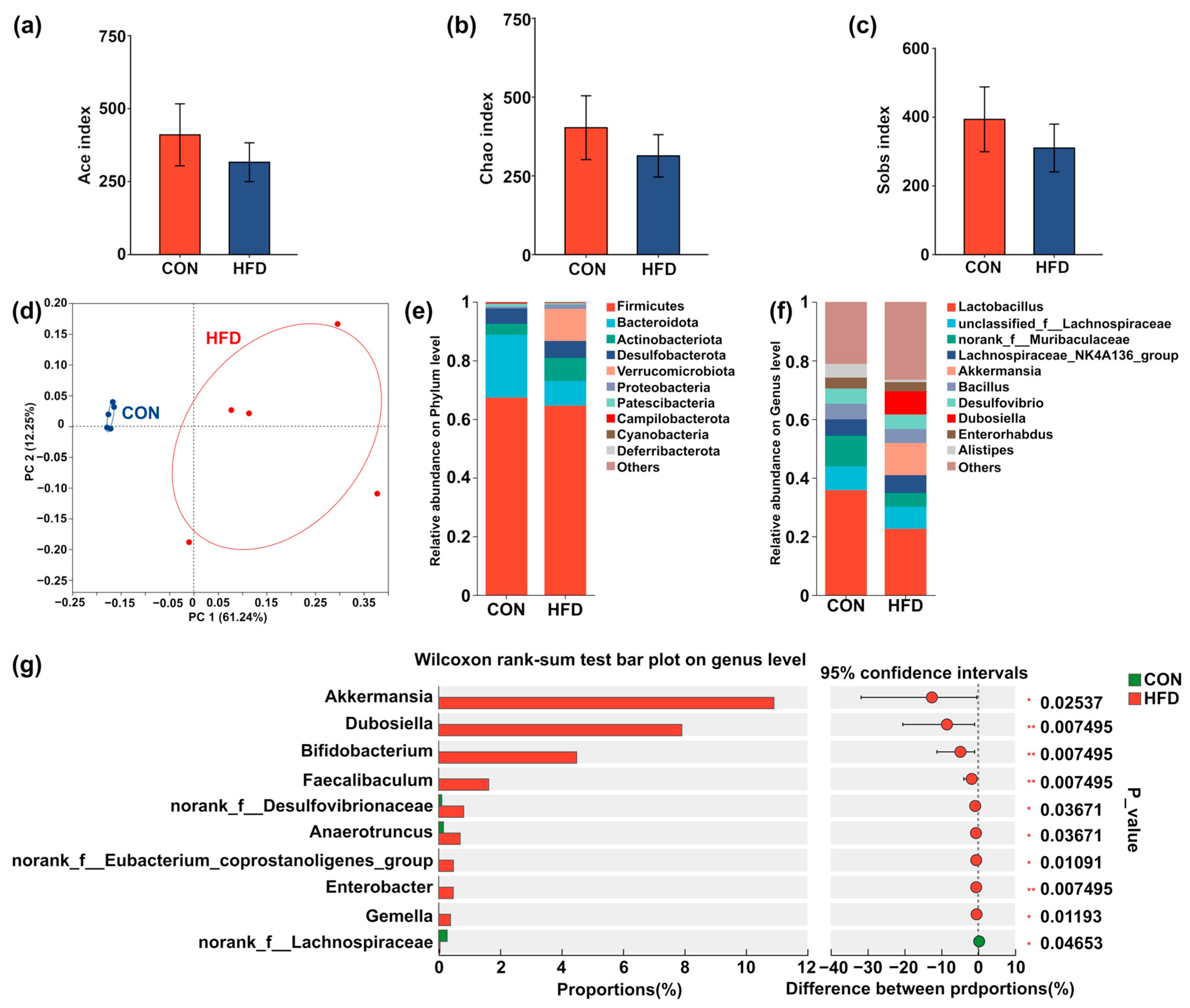
3.1. HFD Induces Disturbances in Liver Lipid Metabolism and Microbial Activity of Cecum Contents in Mice
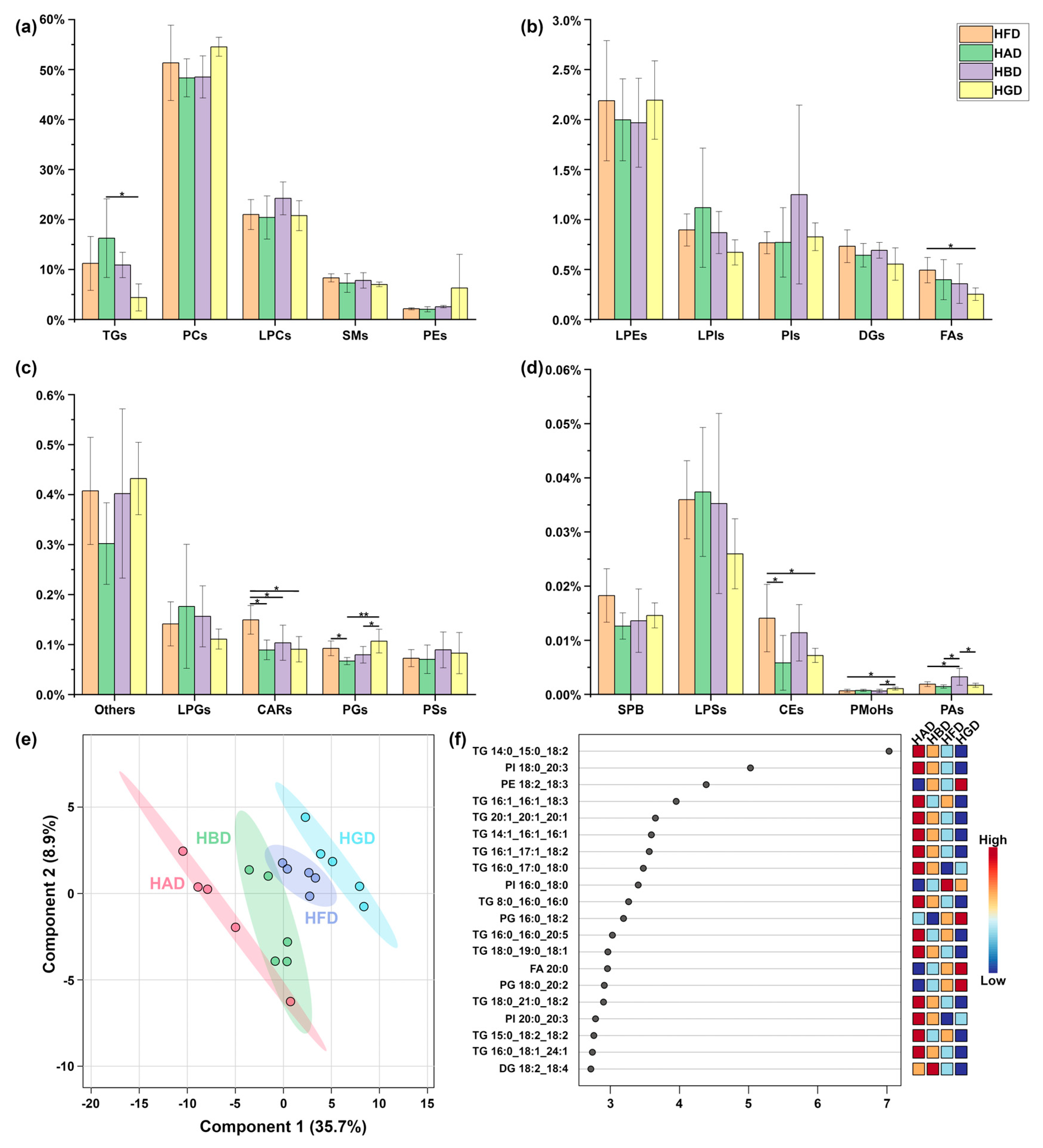
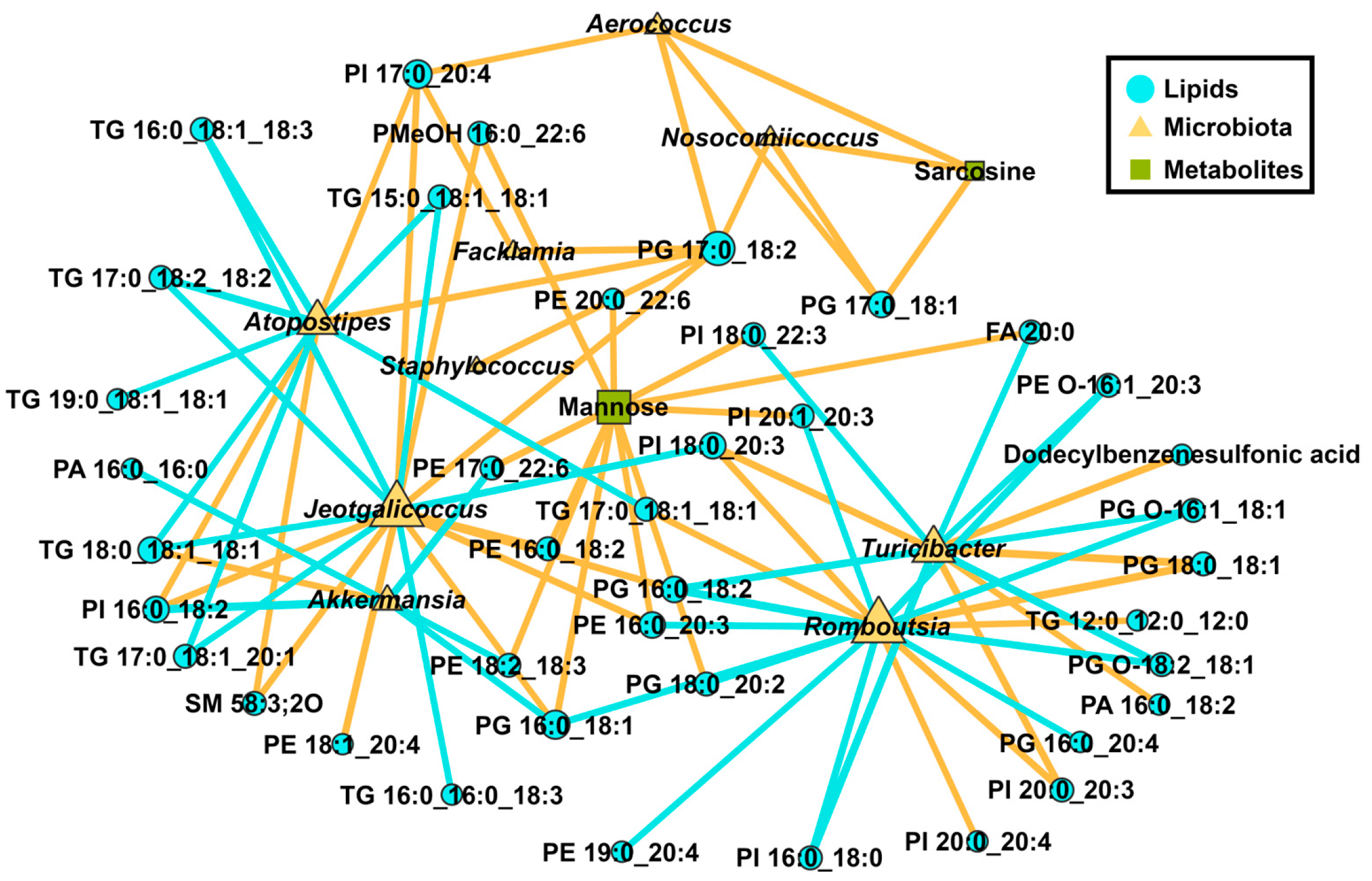
3.2. Polysaccharide Dietary Intervention Remodels Liver Lipid Profiles and Microbial Community Composition of Cecum Contents in Obese Mice on HFD
3.3. Formatting of Mathematical Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouyang, J.; Li, X.; Liu, C.; Lu, D.; Ouyang, J.; Zhou, F.; Liu, Q.; Huang, J.; Liu, Z. Junshanyinzhen Tea Extract Prevents Obesity by Regulating Gut Microbiota and Metabolic Endotoxemia in High-Fat Diet Fed Rats. Food Sci. Hum. Wellness 2024, 13, 2036–2047. [Google Scholar] [CrossRef]
- Song, X.; Luo, Z.; Li, M.; Li, J.; Hou, C. Pomegranate Juice Combined with Inulin Promote Their Mutual Metabolic Transformation by Gut Microbiota and Prevent HFD-Induced Metabolic Disorder in Rats. Food Sci. Hum. Wellness 2025, 14, 9250138. [Google Scholar] [CrossRef]
- Luo, H.-Y.; Fang, J.; Zhang, W.-H.; Chan, K.-C.; Chan, Y.-M.; Dong, C.-X.; Li, S.-L.; Lyu, A.-P.; Xu, J. Dissecting the Anti-Obesity Components of Ginseng: How Ginseng Polysaccharides and Ginsenosides Target Gut Microbiota to Suppress High-Fat Diet-Induced Obesity. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Ning, E.-J.; Sun, C.-W.; Wang, X.-F.; Chen, L.; Li, F.-F.; Zhang, L.-X.; Wang, L.-P.; Ma, Y.-N.; Zhu, J.; Li, X.; et al. Artemisia Argyi Polysaccharide Alleviates Intestinal Inflammation and Intestinal Flora Dysbiosis in Lipopolysaccharide-Treated Mice. Food Med. Homol. 2024, 1, 9420008. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Zhou, S.; Chen, D.; Qin, W.; Li, C.; Li, H.; Lin, D.; Zhang, Q.; Liu, Y.; et al. Arabinoxylan Combined with Different Glucans Improve Lipid Metabolism Disorder by Regulating Bile Acid and Gut Microbiota in Mice Fed with High-Fat Diet. Int. J. Biol. Macromol. 2021, 168, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Hu, J.; Chen, H.; Geng, F.; Nie, S. Arabinoxylan Ameliorates Type 2 Diabetes by Regulating the Gut Microbiota and Metabolites. Food Chem. 2022, 371, 131106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liang, T.; Dai, G.; Zheng, J.; Dong, J.; Xia, C.; Duan, B. Extraction, Structural-Activity Relationships, Bioactivities, and Application Prospects of Bletilla Striata Polysaccharides as Ingredients for Functional Products: A Review. Int. J. Biol. Macromol. 2023, 245, 125407. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yin, M.; Nie, H.; Liu, Y. A Review of Isolation, Chemical Properties, and Bioactivities of Polysaccharides from Bletilla Striata. BioMed Res. Int. 2020, 2020, 5391379. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Liu, Y.; Zhen, L.; Zhao, T.; Luo, L.; Zhang, J.; Deng, T.; Wu, M.; Cheng, J.; Hu, J. The structures of two glucomannans from Bletilla formosana and their protective effect on inflammation via inhibiting NF-κB pathway. Carbohydr. Polym. 2022, 292, 119694. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Structural Characterization and Antioxidant Activities of Bletilla Striata Polysaccharide Extracted by Different Methods. Carbohydr. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, H.; Yin, M.; Cheng, X.; Xia, H.; Hu, H.; Zheng, J.; Zhang, Z.; Liu, H. In Vitro Digestion and Human Gut Microbiota Fermentation of Bletilla Striata Polysaccharides and Oligosaccharides. Front. Cell. Infect. Microbiol. 2023, 13, 1105335. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ye, C.; Leung, E.L.H.; Zhu, L.; Hu, H.; Zhang, Z.; Zheng, J.; Liu, H. Bletilla Striata Oligosaccharides Improve Metabolic Syndrome through Modulation of Gut Microbiota and Intestinal Metabolites in High Fat Diet-Fed Mice. Pharmacol. Res. 2020, 159, 104942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zeng, R.; Tang, J.; Jiang, X.; Zhu, C. The “Crosstalk” between Microbiota and Metabolomic Profile in High-Fat-Diet-Induced Obese Mice Supplemented with Bletilla Striata Polysaccharides and Composite Polysaccharides. Int. J. Biol. Macromol. 2024, 262, 130018. [Google Scholar] [CrossRef] [PubMed]
- Zandani, G.; Anavi-Cohen, S.; Yudelevich, T.; Nyska, A.; Dudai, N.; Madar, Z.; Gorelick, J. Chiliadenus Iphionoides Reduces Body Weight and Improves Parameters Related to Hepatic Lipid and Glucose Metabolism in a High-Fat-Diet-Induced Mice Model of NAFLD. Nutrients 2022, 14, 4552. [Google Scholar] [CrossRef] [PubMed]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular Mechanisms of Metabolic Associated Fatty Liver Disease (MAFLD): Functional Analysis of Lipid Metabolism Pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ding, Q.-Y.; Duan, W.-H.; Guan, Q.-J.; Ren, Y.-L.; Xue, Y.-Z.; Xu, Z.-H.; Geng, Y. Goji-Derived Exosomes-Like Nanoparticles Ameliorate Alcohol-Induced Acute Liver Injury by Modulating Gut Microbiota and Metabolites. Food Med. Homol. 2025, 3, 9420081. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, S.; Chen, Y.; Li, K.; Chen, W.; Liu, Y. Curcumin Anti-Diabetic Effect Mainly Correlates with Its Anti-Apoptotic Actions and PI3K/Akt Signal Pathway Regulation in the Liver. Food Chem. Toxicol. 2020, 146, 111803. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Lü, J.; Yao, T.; Zhang, P.; Chai, X.; Wang, Y.; Jiang, M. Verbascoside Exerts an Anti-Atherosclerotic Effect by Regulating Liver Glycerophospholipid Metabolism. Food Sci. Hum. Wellness 2023, 12, 2314–2323. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Xu, K.; Zhang, W.; Chen, F.; Liu, B.; Guo, B. Fucoxanthin Improves Serum Lipids, Liver Metabolism and Gut Microbiota in Hyperlipidemia Mice. Food Sci. Hum. Wellness 2025, 14, 9250017. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Xie, X.; Wang, X.; Luo, Y.; Xu, N.; Sa, Z.; Zhang, M.; Chen, Z.; Hu, X.; et al. Hepatic Lipidomics Analysis Reveals the Ameliorative Effects of Highland Barley β-Glucan on Western Diet-Induced Nonalcoholic Fatty Liver Disease Mice. J. Agric. Food Chem. 2021, 69, 9287–9298. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Xie, J.-B.; Xiao, M.-Y.; Guo, M.; Qi, Y.-S.; Li, F.-F.; Piao, X.-L. Liver Lipidomics Analysis Reveals the Anti-Obesity and Lipid-Lowering Effects of Gypnosides from Heat-Processed Gynostemma Pentaphyllum in High-Fat Diet Fed Mice. Phytomedicine 2023, 115, 154834. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zeng, R.; Hu, L.; Maffucci, K.G.; Qu, Y. Polysaccharides from Tubers of Bletilla Striata: Physicochemical Characterization, Formulation of Buccoadhesive Wafers and Preliminary Study on Treating Oral Ulcer. Int. J. Biol. Macromol. 2019, 122, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lei, Y.; Lu, C.; Liu, D.; Ma, W.; Lu, H.; Wang, Y. Punicic Acid Ameliorates Obesity-Related Hyperlipidemia and Fatty Liver in Mice via Regulation of Intestinal Flora and Lipopolysaccharide-Related Signaling Pathways. Food Funct. 2024, 15, 5012–5025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, J.; Jiang, X.; Tang, J.; Zhu, C.; Chen, H.; Laghi, L. Metabolomic Characteristics of Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Different Fibers. Foods 2023, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B Stat. Methodol. 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, X.; Lan, Q.; Wan, F.; Yang, Z.; Nie, X.; Cai, Z.; Hu, B.; Tang, J.; Zhu, C.; et al. Comparison of Aroma and Taste Profiles of Kiwi Wine Fermented with/without Peel by Combining Intelligent Sensory, Gas Chromatography-Mass Spectrometry, and Proton Nuclear Magnetic Resonance. Foods 2024, 13, 1729. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xia, P.; Deng, Z.; Hou, T.; Li, J.; Li, B. Effects of Konjac Glucomannan on Weight Management and Liver Health: Insights from Liver Lipidomics in Obese and Nonobese Mice. J. Agric. Food Chem. 2024, 72, 7906–7918. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yin, W.; Hao, P.; Chen, Y.; Yu, L.; Yu, X.; Wu, Y.; Li, X.; Wang, W.; Zhou, H.; et al. Polysaccharide from Panax Japonicus C.A. Mey Prevents Non-Alcoholic Fatty Liver Disease Development Based on Regulating Liver Metabolism and Gut Microbiota in Mice. Int. J. Biol. Macromol. 2024, 260, 129430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Ling, N.; Zhang, B.; Chen, C.; Mo, X.-N.; Cai, J.-Y.; Tan, X.-D.; Yu, Q.-M. Flavonoid-Rich Mulberry Leaf Extract Modulate Lipid Metabolism, Antioxidant Capacity, and Gut Microbiota in High-Fat Diet-Induced Obesity: Potential Roles of FGF21 and SOCS2. Food Med. Homol. 2024, 1, 9420016. [Google Scholar] [CrossRef]
- Du, S.; Chen, X.; Ren, R.; Li, L.; Zhang, B.; Wang, Q.; Meng, Y.; Qiu, Z.; Wang, G.; Zheng, G.; et al. Integration of Network Pharmacology, Lipidomics, and Transcriptomics Analysis to Reveal the Mechanisms Underlying the Amelioration of AKT-Induced Nonalcoholic Fatty Liver Disease by Total Flavonoids in Vine Tea. Food Funct. 2024, 15, 5158–5174. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Chen, S.; Yang, Y.; Tang, B.; Weng, M.; Shen, H.; Chen, J.; Lai, P. Widely Targeted Lipidomics and Microbiomics Perspectives Reveal the Mechanism of Auricularia Auricula Polysaccharide’s Effect of Regulating Glucolipid Metabolism in High-Fat-Diet Mice. Foods 2024, 13, 2743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mu, J.; Meng, J.; Su, W.; Li, J. Dietary Phospholipids Alleviate Diet-Induced Obesity in Mice: Which Fatty Acids and Which Polar Head. Mar. Drugs 2023, 21, 555. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wei, Y.; Wang, M.; Tao, J.; Ouyang, H.; Du, Z.; Li, S.; Jiang, H. Network Pharmacology Combined with Metabolomics and Lipidomics to Reveal the Hypolipidemic Mechanism of Alismatis Rhizoma in Hyperlipidemic Mice. Food Funct. 2022, 13, 4714–4733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huai, Q.; Zhang, X.; Dai, H.; Li, X.; Wang, H. Insights into the Roles and Pathomechanisms of Ceramide and Sphigosine-1-Phosphate in Nonalcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2023, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jiang, L.; Xue, L.; Wang, Y.; Sun, Y.; Fan, M.; Qian, H.; Wang, L.; Li, Y. The Enhancement of Acylcarnitine Metabolism by 5-Heptadecylresorcinol in Brown Adipose Tissue Contributes to Improving Glucose and Lipid Levels in Aging Male Mice. Nutrients 2023, 15, 4597. [Google Scholar] [CrossRef] [PubMed]
- Al-Bulish, M.S.M.; Cao, W.; Yang, R.; Wang, Y.; Xue, C.; Tang, Q. Docosahexaenoic Acid-Rich Fish Oil Alleviates Hepatic Steatosis in Association with Regulation of Gut Microbiome in Ob/Ob Mice. Food Res. Int. 2022, 157, 111373. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Gu, M.; Werlinger, P.; Cho, J.-H.; Cheng, J.; Suh, J.-W. Lactobacillus Sakei MJM60958 as a Potential Probiotic Alleviated Non-Alcoholic Fatty Liver Disease in Mice Fed a High-Fat Diet by Modulating Lipid Metabolism, Inflammation, and Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 13436. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wen, Z.; Meng, K.; Yang, P. Metabolomic Signatures for Liver Tissue and Cecum Contents in High-Fat Diet-Induced Obese Mice Based on UHPLC-Q-TOF/MS. Nutr. Metab. 2021, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wen, Z.; Zhao, L.; Yu, D.; Meng, K.; Yang, P. Lactobacillus plantarum FRT4 Alleviated Obesity by Modulating Gut Microbiota and Liver Metabolome in High-Fat Diet-Induced Obese Mice. Food Nutr. Res. 2022, 66, 7974. [Google Scholar] [CrossRef] [PubMed]
- Bletsa, E.; Filippas-Dekouan, S.; Kostara, C.; Dafopoulos, P.; Dimou, A.; Pappa, E.; Chasapi, S.; Spyroulias, G.; Koutsovasilis, A.; Bairaktari, E.; et al. Effect of Dapagliflozin on Urine Metabolome in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; McEntyre, C.J.; George, P.M.; Chambers, S.T. Is N,N-Dimethylglycine N-Oxide a Choline and Betaine Metabolite? Biol. Chem. 2017, 398, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, Q.; Zhao, X.; Yang, Z.; Yang, F.; Yang, Y.; Tang, J.; Laghi, L. Metabolomic analysis of multiple biological specimens (feces, serum, and urine) by 1H-NMR spectroscopy from dairy cows with clinical mastitis. Animals 2023, 13, 741. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, Y.; Su, J.; Zhu, B.; Wang, S.; Liu, K.; Wang, H.; Shi, S.; Zhang, Q.; Qin, L.; et al. Roles of Gut Microbiota and Metabolites in a Homogalacturonan-Type Pectic Polysaccharide from Ficus Pumila Linn. Fruits Mediated Amelioration of Obesity. Carbohydr. Polym. 2020, 248, 116780. [Google Scholar] [CrossRef] [PubMed]
- de Moura e Dias, M.; da Silva Duarte, V.; Mota, L.; de Cássia Ávila Alpino, G.; dos Reis Louzano, S.; da Conceição, L.; Mantovanie, H.; Pereira, S.; Oliveira, L.; de Oliveira Mendes, T.; et al. Lactobacillus Gasseri LG-G12 Restores Gut Microbiota and Intestinal Health in Obesity Mice on Ceftriaxone Therapy. Foods 2023, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Feng, R.; Li, M.; Liu, L.; Xiao, Y.; Liu, Y.; Yin, C. Causal Relationship between Gut Microbiota and Childhood Obesity: A Mendelian Randomization Study and Case–Control Study. Clin. Nutr. ESPEN 2024, 63, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Liu, W.; Liu, L.; Wang, X.; Jiang, L.; Chen, Z.; Wang, Q.; Wang, P.; Xu, H. Morel (Morchella Spp.) Intake Alters Gut Microbial Community and Short-Chain Fatty Acid Profiles in Mice. Front. Nutr. 2023, 10, 1237237. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, Y.; Huang, Q.; Tan, W.; Li, B.; Zhou, H.; Wang, Z.; Zou, J.; Ding, C.; Jiang, B.; et al. Altered Fecal Microbiome and Metabolome in a Mouse Model of Choroidal Neovascularization. Front. Microbiol. 2021, 12, 738796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, X.; Ye, R.; Hu, Y.; Zheng, T.; Shi, R.; Cheng, W.; Lv, X.; Chen, L.; Liang, P. The Effect of Simvastatin on Gut Microbiota and Lipid Metabolism in Hyperlipidemic Rats Induced by a High-Fat Diet. Front. Pharmacol. 2020, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lin, Z.; Zhao, S.; Zhang, B.; Luo, L.; Zeng, L. Pu-Erh Tea Partly Improved Liver Cholesterol Metabolism Disorders in Colitis Mice via the Gut-Liver Axis. Food Sci. Hum. Wellness 2025, 14, 9250045. [Google Scholar] [CrossRef]
- Hu, J.-P.; Zheng, T.-T.; Zeng, B.-F.; Wu, M.-L.; Shi, R.; Zhang, Y.; Chen, L.-J.; Cheng, W.-J.; Liang, P. Effects of Lactobacillus Plantarum FZU3013-Fermented Laminaria Japonica on Lipid Metabolism and Gut Microbiota in Hyperlipidaemic Rats. Front. Nutr. 2021, 8, 786571. [Google Scholar] [CrossRef] [PubMed]




| Time (min) | Aqueous Phase % | Organic Phase % |
|---|---|---|
| 0.0 | 40 | 60 |
| 2 | 43 | 57 |
| 2.1 | 50 | 50 |
| 12 | 60 | 40 |
| 12.1 | 75 | 25 |
| 18 | 99 | 1 |
| 19 | 99 | 1 |
| 20 | 40 | 60 |
| 25 | 40 | 60 |
| Compound Name | Molecular Concentration | |||
|---|---|---|---|---|
| HFD | HAD | HBD | HGD | |
| Mannose | 9.78×10−5 ± 2.24 × 10−5 ab | 5.00×10−5 ± 3.80 × 10−5 b | 1.10×10−4 ± 3.12 × 10−5 ab | 1.69×10−4 ± 8.41 × 10−5 a |
| Sarcosine | 1.09×10−4 ± 3.47 × 10−5 b | 4.40×10−4 ± 1.88 × 10−4 a | 2.32×10−4 ± 1.27 × 10−4 ab | 3.92×10−4 ± 2.68 × 10−4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Dong, J.; Lu, J.; Cai, Z.; Zhou, B.; Zhang, Q.; Zhu, C.; Laghi, L. Dietary Interventions with Bletilla striata Polysaccharides and/or Composite Polysaccharides Remodel Liver Lipid Profiles and Ameliorate Gut Metabolic Disturbances in High-Fat Diet-Induced Obese Mice. Foods 2025, 14, 2653. https://doi.org/10.3390/foods14152653
Zhang P, Dong J, Lu J, Cai Z, Zhou B, Zhang Q, Zhu C, Laghi L. Dietary Interventions with Bletilla striata Polysaccharides and/or Composite Polysaccharides Remodel Liver Lipid Profiles and Ameliorate Gut Metabolic Disturbances in High-Fat Diet-Induced Obese Mice. Foods. 2025; 14(15):2653. https://doi.org/10.3390/foods14152653
Chicago/Turabian StyleZhang, Peiting, Jinjin Dong, Jiamin Lu, Zijian Cai, Bingde Zhou, Qian Zhang, Chenglin Zhu, and Luca Laghi. 2025. "Dietary Interventions with Bletilla striata Polysaccharides and/or Composite Polysaccharides Remodel Liver Lipid Profiles and Ameliorate Gut Metabolic Disturbances in High-Fat Diet-Induced Obese Mice" Foods 14, no. 15: 2653. https://doi.org/10.3390/foods14152653
APA StyleZhang, P., Dong, J., Lu, J., Cai, Z., Zhou, B., Zhang, Q., Zhu, C., & Laghi, L. (2025). Dietary Interventions with Bletilla striata Polysaccharides and/or Composite Polysaccharides Remodel Liver Lipid Profiles and Ameliorate Gut Metabolic Disturbances in High-Fat Diet-Induced Obese Mice. Foods, 14(15), 2653. https://doi.org/10.3390/foods14152653






