Abstract
Pleurotus eryngii is an edible mushroom with previously characterized β-glucans. Its potential to ameliorate postprandial glycemia and regulate appetite at the postprandial state has been previously shown. However, its effect on protein digestion remains unexplored. We aimed to investigate the effect of baked P. eryngii with a known β-glucan content (4.5 g) on plasma free amino acids of patients with central obesity and metabolic abnormalities at a postprandial state. In this acute, randomized controlled cross-over study, thirteen healthy male volunteers consumed one meal that was prepared with P. eryngii and one control meal; each meal was separated by one month. Blood was collected, and plasma was isolated at different timepoints before and after the consumption. Gas chromatography–mass spectrometry was used to quantify 24 free amino acids in the plasma samples. The area under the curve with respect to increase (AUCi) was computed, and the AUCi for aromatic amino acids was found to be higher after the consumption of the control meal compared to the P. eryngii meal (p = 0.027 for phenylalanine, p = 0.008 for tyrosine, and p = 0.003 for tryptophan). The above novel findings suggest that the β-glucans present in P. eryngii mushrooms are potential modulators of AA release into the bloodstream.
1. Introduction
Metabolic syndrome (MetS) is a cluster of metabolic abnormalities, including dyslipidemia, insulin resistance, hypertension, and central obesity [1]. The prevalence of obesity has risen dramatically in the past 50 years, and obesity is an important health challenge since it is a major risk factor for type 2 diabetes (T2D), hypertension, and cardiovascular disease development [2]. Recent advances support the importance of dietary strategies in the prevention and treatment of central obesity and MetS. Specific foods and food components as well as some nutraceuticals have been suggested as promising alternatives for the management of MetS and its components [1].
Edible mushrooms, such as Pleurotus eryngii (P. eryngii), have an important nutritional value and contain a number of bioactive compounds, including phenolic acids, resveratrol, triterpenic compounds, ergosterol, and β glucans [3]. In particular, according to current findings based on NMR metabolomics, P. eryngii has a mixture of alkali-soluble (1➔3 ) and (1➔ 6)-β-d-glucans, the alkali extract from P. eryngii comprising at least three structurally distinct polymers, hypothesized to be structurally related [4]. In previous studies from our group conducted in metabolically unhealthy humans, we showed that consumption of P. eryngii (containing 4.5 g of b-glucans) as part of a meal with a known protein and carbohydrate composition could ameliorate postprandial glycemia and appetite and could regulate ghrelin at the postprandial level [5]. Likewise, the daily consumption of P. eryngii for three months (equal to 3.5 g b-glucans daily) was shown to regulate glucose levels and anthropometric measurements as well as decrease LDL, SGOT, IL-6, and ox-LDL levels in people with obesity [6].
Amino acids (AAs) play an important role in the insulin signaling pathway, and their profiles are different among obese and insulin-resistant patients [7]. Hence, a number of studies have reported that blood AAs are significantly increased in T2D and may lead to the development of obesity [8,9]. More specifically, branched-chain AAs (BCAAs), including leucine, isoleucine, and valine, are increased in patients with obesity and are related to abnormal glucose and lipid metabolism [10,11]. Aromatic amino acids (AAAs), especially phenylalanine and tyrosine, are associated with metabolic disorders [12]. In a large cohort study, nine AAs, including phenylalanine, tyrosine, tryptophan, alanine, isoleucine, leucine, and others, were associated with a decrease in insulin secretion and an increase in fasting and 2 h glucose levels [13]. In the same study, tyrosine, alanine, isoleucine, aspartate, and glutamate were significantly associated with an increased risk of T2D incidence [13].
Regarding protein digestion and release of free AAs, it has been shown that the in vitro protein digestibility of a plant protein (either lentil or pea protein) is limited by approximately 30% in the presence of barley β-glucans. This is probably attributed to the protein–barley β-glucan complex formation having decreased transmittance and surface charge and increased particle size [14]. On the contrary, in other studies, the presence of oat glucans in yogurt led to faster proteolysis and a higher percentage of free amino acids in an in vitro-simulated digestion [15,16]. Apart from the potential impact on protein physicochemical properties, β-glucans have been shown to alter gut microbiota and AA metabolism in inflammation-mediated diseases [17].
While AA blood levels in obesity are linked to decreased insulin sensitivity and β-glucans may affect protein digestion and AA metabolism, data derived from food interventions aimed at controlling AA levels in people with obesity are absent. Thus, the main objective of this study is to evaluate the effects of P. eryngii mushrooms, rich in a mixture of (1➔ 3) and (1➔ 6)-β-d-glucans, on the plasma free AA profile of patients with metabolically unhealthy obesity at a postprandial state. As studies of postprandial responses of natural products rich in glucans usually focus solely on glycemic outcomes, our study intends to focus on other parameters associated with glucose regulation, such as free AAs.
2. Materials and Methods
2.1. Study Design
This study was performed in a subset of adults with central obesity and metabolic abnormalities who participated in an acute, randomized controlled cross-over study registered under the ID number NCT04444219 with the Clinicaltrials.gov platform [5]. The protocol was approved by the Institutional Review Board of Laiko General Hospital (ID protocol: 4931/22.03.2020). The Helsinki Declaration principles and the Data Protection Act 1998 were adhered to. All participants, after being informed about the objectives and procedures of the study, signed a written consent after agreeing to participate.
Eligible participants had to visit the premises of the “Diabetes Laboratory” of the First Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens (Greece) on two separate days for testing. This study comprises two days of testing after consuming one meal with P. eryngii and one control meal, respectively, separated by one month and in a randomized order. On each of the test days, participants were required to fast overnight. An intravenous catheter was placed into a peripheral vein, and blood samples were taken. Then, the participants consumed a breakfast consisting of 40 g commercial yellow cheese combined with white bread and 15 g of baked P. eryngii or the control breakfast consisting of the same quantity of cheese and bread, no mushrooms, and only 6–7 cherry tomatoes to achieve the same caloric content. A total of 15 g of baked mushrooms was selected to ensure an intake of approximately 4.5 g of glucans in the test meal, based on measurements from our previous study [5]. This dose was selected as most studies report health benefits in doses between 2 and 6 g, while doses exceeding this range have documented minor side effects. The time of the meal did not exceed 10 min. The nutritional composition of the two breakfasts is presented in Table 1. In detail, each meal was evaluated for its protein, fat, and available carbohydrate content, expressed in grams, as well as total energy, measured in kilocalories. Both meals were similar in nutritional profile. Protein content differed only slightly (23.0 g vs. 25.5 g), as did fat (12.7 g vs. 13.3 g) and available carbohydrates (55.4 g vs. 52.6 g). This minimal variation leads to nearly identical energy values—427.9 Kcal for the control and 432.1 Kcal for the P. eryngii meal. During the test, subjects could drink up to 500 mL of water if needed. More details on the preparation of the snack and on the study protocol and procedures have already been extensively described in the main publication of the study [5].

Table 1.
Nutritional composition of the two experiments.
Subjects were advised not to drink alcohol or exercise the day before the trial and to keep stable dietary habits and physical activity levels throughout the study. Experienced dietitians provided consulting on how to preserve their lifestyle habits and were monitoring protocol adherence through validated questionnaires before each intervention. More specifically, dietary intake was evaluated with a 24 h recall record (4 non-consecutive days) and was analyzed with Nutritionist Pro™ v 7.9 (Axxya Systems, Stafford, TX, USA) software. Physical activity was assessed with the International Physical Activity Questionnaire Short Form, a 7-day recall instrument that estimates physical activity in MET-min/week. No statistical differences were observed on energy intake and physical activity score between the two interventions; therefore, all subjects were included in the analysis.
2.2. Participants
Subjects with central obesity, as determined by greater than 94 cm waist circumference (WC) in males and greater than 80 cm in females, were recruited. Non-eligible subjects were pregnant or lactating females as well as subjects with thyroid disease, alcohol abuse, or with known psychiatric or mental disorders. All participants were advised to abstain from alcohol and vigorous exercise on the day before each trial. They were also instructed to keep their dietary habits and physical activity levels consistent until the completion of the study.
2.3. Clinical Assessment and Anthropometric Measurements
Detailed medical history (personal and family) was obtained. Body weight was measured to the nearest kg with a flat scale in light clothing without shoes. Height was measured to the nearest cm using a stadiometer (Seca Mode 220, Hamburg, Germany). Body composition was analyzed with bioelectrical impedance analysis (Tanita BC-418, Tokyo, Japan) in order to calculate body fat, fat-free mass, and visceral fat level. Hip circumferences (HC) and WC were measured with a flexible, non-stretch tape on minimal clothing.
2.4. Biochemical Analyses
Standard blood withdrawal was performed (20 mL) at baseline and at 30 min, 60 min, 90 min, 120 min, 150 min, and 180 min after finishing each breakfast meal. Blood samples were centrifuged at 20 °C and 3000 rpm for 10 min for serum isolation. Biochemical markers were measured in serum with the use of a Cobas 8000 analyzer, an automatic biochemical analyzer (Roche Diagnostics GmbH, Mannheim, Germany): glucose; insulin; total cholesterol (TC); low-density lipoprotein (LDL); high-density lipoprotein (HDL); triglycerides (TG); alanine aminotransferase (ALT); aspartate aminotransferase (AST); glutamyltransferase (γ-GT); alkaline phospatase (ALP); lactate dehydrogenase (LDH); urea; uric acid; creatinine; iron (Fe); ferritin; albumin; and C-reactive protein (CRP). Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated as follows: Fasting Glucose (mg/dL) × Fasting Insulin (μU/mL))/405. Plasma was used for the quantitative analysis of free amino acids using gas chromatography–mass spectrometry (GC/MS).
2.5. Free Amino Acids Quantification
2.5.1. Derivatization of Free Amino Acids
Free AAs were derivatized, as described by Kaspar et al. (2008), with minor modifications [18]. In detail, 50 μL of plasma sample along with 20 μL of stabilization solution (aqueous solution of 10% iso-propanol, 0.1% phenol, and 2% thiodiglycol) were pipetted into glass test tubes. Then, 10 μL of norvaline solution 200 μΜ was added as an internal standard, followed by 120 μL of NaOH 0.33 M and 50 μL of picoline in propanol (20:80). Afterwards, the mixture was vortexed, and 50 μL of propyl chloroformate in chloroform (chloroform:propyl chloroformate:isooctane 60:20:20) was added. The mixture was emulsified by vortexing for 10 s and then left at room temperature for at least 1 min. The mixture was re-emulsified by vortexing again and then allowing the reaction to proceed for at least one additional minute. Finally, 250 μL of iso-octane was added. The mixture was vortexed for 10 s and then left to equilibrate for one minute or more until it was separated into two layers. Aliquots of 100 μL of the upper organic layer were transferred into GC vials, sealed, and analyzed immediately.
2.5.2. GC/MS Analysis of Free Amino Acids
A gas chromatograph (Agilent GC 6890N, Waldbronn, Germany) coupled with a mass selective detector (HP5973, Electron Impact, 70 eV), split–splitless injector, and an auto sampler (HP7683) was used for the free amino acids analysis. Two µL of derivatized samples was injected into the GC at a split ratio of 1:15. An amino acids analysis dedicated column (length = 10 m, internal diameter = 0.25 mm, film thickness = 25 µm) was used, allowing for separation (Phenomenex Zebron ZB-A, Phenomenex®, Torrance, CA, USA). The carrier gas, high purity helium, was at a constant flow of 1.1 mL/min. The oven program along with the temperatures used were as described in the EZ:faast manual instructions (Phenomenex®, Torrance, CA, USA). The temperatures of the transfer line and of the injector were kept a 250 °C and 340 °C, respectively. The initial temperature of the oven was set at 110 °C and then increased to 320 °C at 30 °C/min for 3 min. A selective ion monitoring GC–MS method was applied for detecting and quantifying free amino acids, based on the ±0.05 RT presence of target and qualifier ions at the predetermined ratios, together with the electronic library “Agilent.L”, which was provided with the Ez:faast (Phenomenex®, Torrance, CA, USA) (Table S1). Quantification was performed using norvaline as the internal standard, and five points’ reference curves were constructed for each AA by multistandard AAs solutions provided by Sigma-Aldrich (Darmstadt, Germany).
2.6. Statistical Analysis
A sample size calculation estimated that, for an effect size of 0.8, an alpha level of 0.05, and an estimated power of 80%, the required sample size for a paired sample t-test was 13 subjects. For the purposes of this study, we used a subsample from the initial population [5] to measure the levels of free amino acids in plasma after applying an additional inclusion criterion—presence of a sufficient biological sample and non-hemolytic plasma. Statistical analysis was conducted using SPSS statistical software (version 26.0, SPSS, Inc., ΙΒΜ, Chicago, IL, USA). Continuous variables are presented as mean and standard deviation (SD) and qualitative variables as counts (%). For all amino acids, measured in different timepoints, the area under the curve with respect to increase (AUCi) was computed. AUCi values were compared between the two meals via the paired t-test. All p-values reported are two-tailed. Statistical significance was set at p < 0.05.
3. Results
Table 2 and Figure 1 present the descriptive characteristics of the subjects. As shown in Table 2, 61.5% of the subjects were males, the mean age was 56.3 ± 11.1 years, and all subjects had central obesity with BMI 35.6 ± 6.6 kg/m2. Mean glucose levels were above normal limits (105.9 ± 32.9 mg/dL), and HOMA-IR (4.36 ± 4.01) indicated significant insulin resistance. Mean TC (195.7 ± 33.2 mg/dL) and LDL (119.8 ± 31.1 mg/dL) were abnormal, and HDL was below the protective limit. Other biochemical indices that reflect hepatic, renal, and overall health exhibited normal levels. Free AAs (Figure 1) depict the baseline plasma levels of all amino acids, expressed in nmol/mL.

Table 2.
Anthropometric and biochemical characteristics of the study sample at baseline.
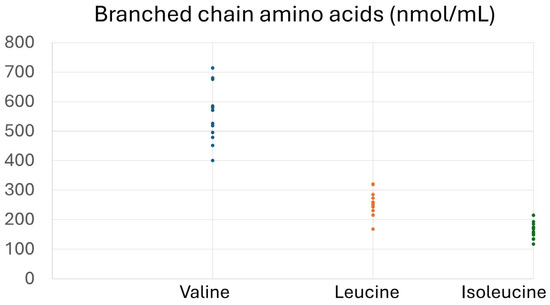
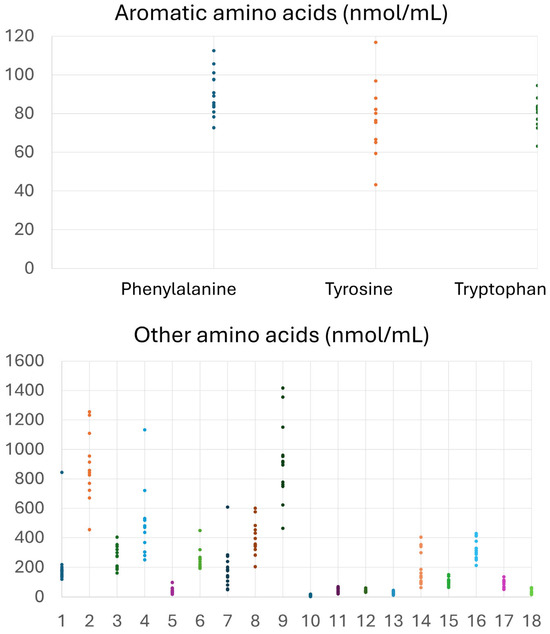
Figure 1.
Concentrations (nmol/mL) of free amino acids in plasma samples of the participants at baseline. Other amino acids include the following: 1. Alo isoleucine, 2. Alanine, 3. Glutamic acid, 4. Glycine, 5. a-Aminobutyric acid, 6. Threonine, 7. Serine, 8. Proline, 9. Asparagine, 10. Thioproline, 11. Aspartic acid, 12. Methionine, 13. Hydroxyproline, 14. Glutamine, 15. Ornithine, 16. Lysine, 17. Hystidine, 18. Cystine.
Table 3 presents the comparisons of postprandial AUCi of all AAs, whereas Figure 2 depicts postprandial levels of AAs with differences in AUCi. As shown in Table 3, consumption of the P. eryngii meal significantly affected AUCi for AAAs, namely phenylalanine, tyrosine, and tryptophan. More specifically, AUCi for all three AAAs was significantly higher after the consumption of the control meal compared to the P. eryngii meal (p = 0.027 for phenylalanine, p = 0.008 for tyrosine, and p = 0.003 for tryptophan), although no differences were observed between the two meals (p > 0.05) at any timepoint (Figure 2). No significant differences were observed between the two meals regarding the AUCi of all other amino acids (Table 3).

Table 3.
Postprandial AUCi of amino acids after the consumption of the two meals.
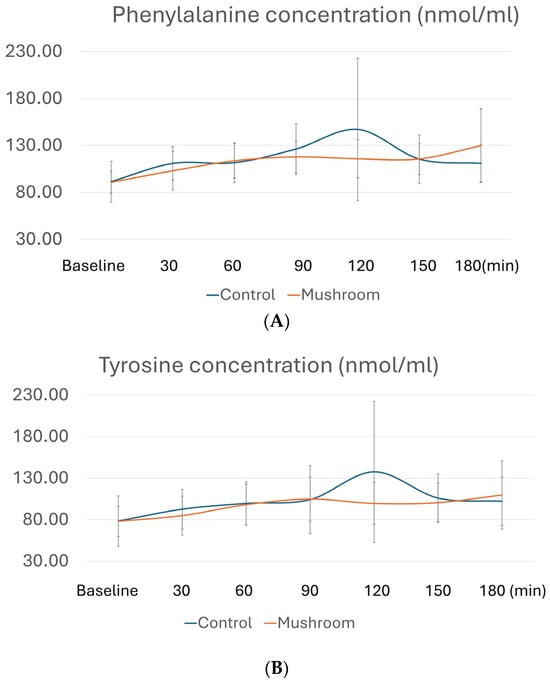
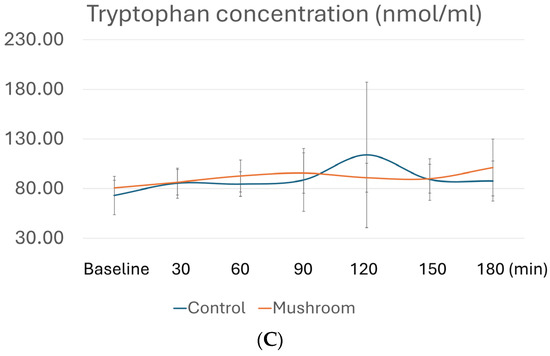
Figure 2.
Postprandial amino acid profiles for (A) phenylalanine, (B) tyrosine, and (C) tryptophan at different timepoints. Concentrations of AAs in plasma after the control meal are depicted with blue, whereas after the meal with P. eryngii mushrooms, they are depicted in orange. For amino acid quantification, GC/MS analysis was conducted at baseline, 30 min, 60 min, 90 min, 120 min, 150 min, and 180 min after consumption of the two test meals, i.e., the mushroom meal and the control meal.
4. Discussion
Β-glucans in Pleurotus species exert several immunoregulatory activities due to their complex interaction with the immune system, which depends on the complexity of their molecular weight, branching degree, and presence of functional groups [19,20]. Also, several studies have shown the glucose and lipid regulatory effects of fungal β-glucans, mostly in animal models [21]. However, human studies show inconsistent results, most possibly due to their variability and the fact that β-glucans’ structure and branching with protein/peptide affect their conformation and may influence water solubility and biological activities [22]. Therefore, more research for the exploitation of the mechanisms under which β-glucans exert their metabolic regulatory properties is needed.
The complex profile of β-glucans in P. eryngii was most recently analyzed by Ellefsen and co-workers [4]. In detail, the alkali soluble Fucp-(1➔ , Xylp-(1➔ , ➔ 2)-Xylp-(1➔ , ➔ 3)-Galp-(1➔ , ➔ 6)-Galp-(1➔ , ➔ 2,6)-Galp-(1➔ , ➔ 3)-Manp-(1➔ , Glcp-(1➔ , ➔ 3)-Glcp-(1➔ , ➔ 6)-Glcp-(1➔ , and ➔ 3,6)-Glcp-(1➔ were identified. In our study, we showed that, owing to the b-glucans, postprandial AUCi of aromatic amino acids (AAAs: tyrosine, tryptophan, and phenylalanine) were significantly lower after consumption of a P. eryngii meal compared to a control meal. To the best of our knowledge, our study is the first that examines the in vivo potential of β-glucans in mushrooms to control the postprandial release of AAs into the bloodstream in people with central obesity and metabolic abnormalities. Overall, the results herein are similar to those in a cross-over study with a Mexican rural diet rich in dietary fibers, which induced a lower increase in phenylalanine, proline, and BCAAs compared to an urban diet in women from a Mexican rural population [23].
Recent research has shown that an altered plasma AAAs profile is observed in patients with metabolic abnormalities and cardiometabolic risk factors, such as BMI, blood pressure, lipids, and insulin [24,25,26]. Additionally, AAAs are higher in diabetic and obese patients compared to healthy individuals, with their levels being strongly associated with HOMA-IR and insulin [27]. In a Greek population of 100 middle-aged men, a BCAA/AAA-related pattern was associated with metabolic syndrome as well as with glucose and insulin levels, confirming the above associations in Greek individuals as well [28]. In our population, the postprandial decrease in AAA and the possible effect of the P. eryngii meal on their absorption coincide with the lower iAUC of glucose, ghrelin, hunger, and fullness shown previously in the same cross-over study [5], suggesting that postprandial alteration of AAAs is associated with glycemic and appetite regulation.
Protein source seems to affect postprandial AA profile, as a dairy meal induced higher postprandial isoleucine, tyrosine, and phenylalanine than fish, meat, or plant protein meals in a cross-over study with healthy individuals and four different isocaloric test meals [29]. Our findings support the proposed mechanism that protein digestibility is impaired by the presence of β-glucans due to their interaction with proteins. It is well known that AAs kinetics are affected by protein quality and quantity, and the presence of other dietary factors, such as fibers, that influence digesta passage kinetics via affecting viscosity [30,31]. In pigs that followed diets with increased concentrations of β-glucans, an effect on digesta physichochemical properties was observed, with increased mean retention time of digesta liquids and decreased digesta viscosity and protein digestibility in the stomach [30]. Studies investigating β-glucan release from fungal and plant cell walls showed that cooking before digestion increases β-glucan release from mycoproteins and barley bran, maybe through influencing their physicochemical properties, and protein release in white button mushrooms, although lower protein release was seen in digested oak flakes [31,32]. Interestingly, the gelatinized network of starch and β-glucans, presented in scanning electron microscopy in cooked barley, could explain the increased viscosity due to cooking and the blocking in protein release. Release of β-glucans from cell walls could be different in mushrooms and plants due to a different origin and cell wall structure [33].
The main strengths of this study include its cross-over design, where samples serve as their own controls, as well as the randomization in the order of meals that were consumed by the participants. Also, the incorporation of GC–MS analysis for the measurement of plasma free amino acids offers a very reliable quantitation.
Regarding the study limitations, we acknowledge the relatively small sample size and the sex imbalance that may have influenced our results. Although an effect on other amino acids, such as the BCAAs, might be expected based on their roles in obesity and abnormal metabolism, our results revealed significant findings exclusively in AAAs. This may be due to various factors such as the relatively low sample size or other biological implications, such as the different genetic backgrounds and intervariability of metabolic pathway regulations between subjects, which could have affected the detection of changes in the BCAAs.
5. Conclusions
Our results demonstrate the good performance of β-glucans present in P. eryngii mushrooms as potential modulators of AA release into the bloodstream. To the best of our knowledge, this is the first human study showing that β-glucans present in mushrooms affect protein digestion and the release of free AAs into the blood in central obesity. However, the small sample size could be considered as a limitation in generalizing the results, and in the future, similar studies in larger samples should be conducted. From a public health perspective, a dietary recommendation could be to add mushrooms to the meal of people with obesity and metabolic disorders to control the postprandial increase in free AAs in the blood and, further, to prevent insulin resistance. Beyond that, from a technological point of view, we consider the present results to be the starting point of research for the development of products aimed at influencing proteolysis through mushroom β-glucan enrichment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods14152649/s1, Table S1: Retention times (Rt) and m/z of ions used for the selective ion monitoring of amino acids.
Author Contributions
Conceptualization, A.C.K.; methodology, N.K., A.K. (Alexander Kokkinos), and A.C.K.; formal analysis, C.T.; investigation, C.A., S.-A.K., A.K. (Alexandra Kasoura), D.T., S.S., and A.G.; writing—original draft preparation, C.A.; writing—review and editing, all authors; supervision, A.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Union and Greek national funds (European Social Fund—ESF) through the Operational Program Competitiveness, Entrepreneurship, and Innovation, under the call RESEARCH CREATE-INNOVATE (project code: T1EDK-02560). The implementation of the doctoral thesis of S.-A.K. was co-financed by Greece and the European Union (European Social Fund) through the Operational Program “Human Resource Development, Education and Lifelong Learning”, 2014–2020, within the framework of the Action “Strengthening human resources through the implementation of doctoral research-Sub-Action 2: IKY grant program for doctoral candidates of Greek universities”.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to the patients for participating in this study. Also, we thank Lefteris Lachouvaris from Dirfis Mushrooms for providing P. eryngii.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALT | Alanine transaminase |
| AAs | Amino acids |
| ALP | Alkaline phosphatase |
| AUCi | Area under the curve with respect to increase |
| AAAs | Aromatic amino acids |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| BCAAs | Branched-chain amino acids |
| CRP | C-reactive protein |
| γ-GT | γ-glutamyl transferase |
| GC/MS | Gas chromatography–mass spectrometry |
| HDL | High-density lipoprotein |
| HC | Hip circumference |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| LDH | Lactate dehydrogenase |
| LDL | Low-density lipoprotein |
| P. eryngii | Pleurotus eryngii |
| TC | Total cholesterol |
| TG | Triglycerides |
| T2D | Type 2 diabetes |
| WC | Waist circumference |
References
- Ambroselli, D.; Masciulli, F.; Romano, E.; Catanzaro, G.; Besharat, Z.M.; Massari, M.C.; Ferretti, E.; Migliaccio, S.; Izzo, L.; Ritieni, A.; et al. New advances in metabolic syndrome, from prevention to treatment: The role of diet and food. Nutrients 2023, 15, 640. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2018, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.; Zervakis, G. Toward an increased functionality in oyster (Pleurotus) mushrooms produced on grape marc or olive mill wastes serving as sources of bioactive compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef] [PubMed]
- Ellefsen, C.; Lindstad, L.; Klau, L.; Aachmann, F.; Hiorth, M.; Samuelsen, A. Investigation of the structural and immunomodulatory properties of alkali-soluble β-glucans from Pleurotus eryngii fruiting bodies. Carbohydr. Polym. 2023, 322, 121367. [Google Scholar] [CrossRef]
- Kleftaki, S.; Simati, S.; Amerikanou, C.; Gioxari, A.; Tzavara, C.; Zervakis, G. Pleurotus eryngii improves postprandial glycaemia, hunger and fullness perception, and enhances ghrelin suppression in people with metabolically unhealthy obesity. Pharmacol. Res. 2022, 175, 105979. [Google Scholar] [CrossRef]
- Kleftaki, S.-A.; Amerikanou, C.; Gioxari, A.; Lantzouraki, D.Z.; Sotiroudis, G.; Tsiantas, K.; Tsiaka, T.; Tagkouli, D.; Tzavara, C.; Lachouvaris, L.; et al. A randomized controlled trial on Pleurotus eryngii mushrooms with antioxidant compounds and vitamin D2 in managing metabolic disorders. Antioxidants 2022, 11, 2113. [Google Scholar] [CrossRef]
- Simonson, M.; Boirie, Y.; Guillet, C. Protein, amino acids and obesity treatment. Rev. Endocr. Metab. Disord. 2020, 21, 341–353. [Google Scholar] [CrossRef]
- Wiklund, P.; Zhang, X.; Pekkala, S.; Autio, R.; Kong, L.; Yang, Y.; Keinänen-Kiukaanniemi, S.; Alen, M.; Cheng, S. Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Sci. Rep. 2016, 6, 24540. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- De Bandt, J.-P.; Coumoul, X.; Barouki, R. Branched-chain amino acids and insulin resistance, from protein supply to diet-induced obesity. Nutrients 2022, 15, 68. [Google Scholar] [CrossRef]
- Vanweert, F.; De Ligt, M.; Hoeks, J.; Hesselink, M.K.C.; Schrauwen, P.; Phielix, E. Elevated plasma branched-chain amino acid levels correlate with type 2 diabetes–related metabolic disturbances. J. Clin. Endocrinol. Metab. 2020, 106, e1827–e1836. [Google Scholar] [CrossRef]
- Shi, M.; Han, S.; Klier, K.; Fobo, G.; Montrone, C.; Yu, S.; Harada, M.; Henning, A.-K.; Friedrich, N.; Bahls, M.; et al. Identification of candidate metabolite biomarkers for metabolic syndrome and its five components in population-based human cohorts. Cardiovasc. Diabetol. 2023, 22, 18. [Google Scholar] [CrossRef]
- Vangipurapu, J.; Stancáková, A.; Smith, U.; Kuusisto, J.; Laakso, M. Nine amino acids are associated with decreased insulin secretion and elevated glucose levels in a 7.4-year follow-up study of 5,181 Finnish men. Diabetes 2019, 68, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Boachie, R.T.; Commandeur, M.M.B.; Abioye, R.O.; Capuano, E.; Oliviero, T.; Fogliano, V.; Udenigwe, C.C. β-Glucan interaction with lentil (Lens culinaris) and yellow pea (Pisum sativum) proteins suppresses their in vitro digestibility. J. Agric. Food Chem. 2021, 69, 10630–10637. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, B.; Yang, W.; Nazarenko, Y. Effect of oat β-glucan on in vitro digestion characteristics of set-type yogurt. Acta Innov. 2022, 43, 5–14. [Google Scholar] [CrossRef]
- Rinaldi, L.; Rioux, L.-E.; Britten, M.; Turgeon, S.L. In vitro bioaccessibility of peptides and amino acids from yogurt made with starch, pectin, or β-glucan. Int. Dairy J. 2014, 46, 39–45. [Google Scholar] [CrossRef]
- Liu, C.; Sun, C.; Cheng, Y. β-Glucan alleviates mice with ulcerative colitis through interactions between gut microbes and amino acids metabolism. J. Sci. Food Agric. 2023, 103, 4006–4016. [Google Scholar] [CrossRef]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Automated GC–MS analysis of free amino acids in biological fluids. J. Chromatogr. B 2008, 870, 222–232. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Vetvicka, V.; Gover, O.; Karpovsky, M.; Hayby, H.; Danay, O.; Ezov, N.; Hadar, Y.; Schwartz, B. Immune-modulating activities of glucans extracted from Pleurotus ostreatus and Pleurotus eryngii. J. Funct. Foods 2019, 54, 81–91. [Google Scholar] [CrossRef]
- Wouk, J.; Dekker, R.F.H.; Queiroz, E.A.I.F.; Barbosa-Dekker, A.M. β-Glucans as a panacea for a healthy heart? Their roles in preventing and treating cardiovascular diseases. Int. J. Biol. Macromol. 2021, 177, 176–203. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible mushrooms and beta-glucans: Impact on human health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef] [PubMed]
- López, A.M.; Noriega, L.G.; Diaz, M.; Torres, N.; Tovar, A.R. Plasma branched-chain and aromatic amino acid concentration after ingestion of an urban or rural diet in rural Mexican women. BMC Obes. 2015, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; He, D.; Luo, C.; Lin, X.; Wu, J.; Yin, X.; Jia, C.; Pan, Q.; Dong, X.; Zheng, F.; et al. Metabolic syndrome and its components are associated with altered amino acid profile in Chinese Han population. Front. Endocrinol. 2022, 12, 795044. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Miki, T.; Fukunaga, A.; Eguchi, M.; Kochi, T.; Nanri, A.; Kabe, I.; Mizoue, T. Associations of serum amino acids with insulin resistance among people with and without overweight or obesity: A prospective study in Japan. Clin. Nutr. 2022, 41, 1827–1833. [Google Scholar] [CrossRef]
- Weng, L.; Quinlivan, E.; Gong, Y.; Beitelshees, A.L.; Shahin, M.H.; Turner, S.T.; Chapman, A.B.; Gums, J.G.; Johnson, J.A.; Frye, R.F.; et al. Association of branched and aromatic amino acids levels with metabolic syndrome and impaired fasting glucose in hypertensive patients. Metab. Syndr. Relat. Disord. 2015, 13, 195–202. [Google Scholar] [CrossRef]
- Chen, T.; Ni, Y.; Ma, X.; Bao, Y.; Liu, J.; Huang, F.; Hu, C.; Xie, G.; Zhao, A.; Jia, W.; et al. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci. Rep. 2016, 6, 20594. [Google Scholar] [CrossRef]
- Ntzouvani, A.; Nomikos, T.; Panagiotakos, D.; Fragopoulou, E.; Pitsavos, C.; McCann, A.; Ueland, P.M.; Antonopoulou, S. Amino acid profile and metabolic syndrome in a male Mediterranean population: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1021–1030. [Google Scholar] [CrossRef]
- Ottosson, F.; Ericson, U.; Almgren, P.; Nilsson, J.; Magnusson, M.; Fernandez, C.; Melander, O. Postprandial levels of branched-chain and aromatic amino acids associate with fasting glycaemia. J. Amino Acids 2016, 2016, 8576730. [Google Scholar] [CrossRef]
- Schop, M.; Jansman, A.J.M.; De Vries, S.; Gerrits, W.J.J. Increased diet viscosity by oat β-glucans decreases the passage rate of liquids in the stomach and affects digesta physicochemical properties in growing pigs. Animal 2020, 14, 269–276. [Google Scholar] [CrossRef]
- Have, G.A.M.T.; Engelen, M.P.K.J.; Luiking, Y.C.; Deutz, N.E.P. Absorption kinetics of amino acids, peptides, and intact proteins. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S23–S36. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Quint, J.; Rieder, A.; Ballance, S.; Dreiss, C.A.; Cross, K.L.; Gray, R.; Bajka, B.H.; Butterworth, P.J.; Ellis, P.R.; et al. The impact of oat structure and β-glucan on in vitro lipid digestion. J. Funct. Foods 2017, 38, 378–388. [Google Scholar] [CrossRef]
- Colosimo, R.; Mulet-Cabero, A.-I.; Cross, K.L.; Haider, K.; Edwards, C.H.; Warren, F.J.; Finnigan, T.J.A.; Wilde, P.J. β-Glucan release from fungal and plant cell walls after simulated gastrointestinal digestion. J. Funct. Foods 2021, 83, 104543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).