3.1. Identification and Determination of Fungi
The results of the incidence of fungi and mycotoxins in the highland and coastal areas of Ecuador are shown in
Figure 1. Only one maize sample of the total collected was free of fungi. In addition, in half (15/29) of the samples analyzed, all kernels were contaminated with fungi, with
Fusarium and
Aspergillus being the most frequently isolated fungal genera.
Several species of
Fusarium have the characteristic of infecting crops before harvest, especially under favorable climatic conditions for the development of the fungus, such as rainy periods and high temperatures [
56]. On the other hand, the genus
Aspergillus is frequently associated with samples in storage, although some species of this fungus can also infect maize in the field [
21].
In general, xerophilic Aspergillus section Aspergillus (syn. Eurotium) were predominant in all analyzed samples. However, Fusarium of the Liseola group (which includes F. verticillioides, F. proliferatum, and other related species) should be mentioned because they were isolated from all samples from the coast and only one sample from the highlands. In the samples from the coast, there was also a higher prevalence of the genus Penicillium compared to the highlands.
The relationship between fungi and maize has been extensively explained by the work of Pitt and Hocking [
21], where it is pointed out that maize ears are enveloped in a strong protective husk that manages to reduce the attack of several fungi. However, the genus
Fusarium is able to infect and invade the kernels.
F. graminearum is a species that causes reddening of the kernels and husk, while
F. verticillioides and
F. subglutinans cause less aggressive infections and often occur as commensal fungi. No less important than diseases caused by
Fusarium are infections caused by
A. flavus, which can even spread into maize ears from the field. Depending on the size of the maize kernels, which can be quite large, drying occurs more slowly, allowing the establishment of fungal species in the pre- and postharvest stages. Indeed, xerophilic species of
Aspergillus and
Penicillium generally prevail together. Similarly,
F. verticillioides,
F. semitectum, and
F. proliferatum are often persistent in stored maize kernels.
Species of toxigenic interest belonging to the genus
Aspergillus and
Fusarium are presented in
Table 3.
3.2. Mycotoxins
The presence of mycotoxins was detected in 93.1% of the samples analyzed (27/29 units). Five classes of mycotoxins were determined with a predominance of fumonisins (68.9%, 20/29 samples with maximum levels of 6777 µg/kg), zearalenone (31%, 9/29 samples with maximum levels of 573 µg/kg), and aflatoxins (17.2%, 5/29 samples with maximum levels of 130 µg/kg). On the other hand, group B trichothecenes were present in 13.8% of the samples (4/29 units), while ochratoxin A and group A trichothecenes were not detected in any of the analyzed samples (
Figure 2).
It should be noted that Miller [
57] considered aflatoxins, ochratoxin A, fumonisins, trichothecenes (deoxynivalenol and nivalenol), and zearalenones as the most important mycotoxins worldwide. Most of these compounds were detected in the samples of this study. Since maize is considered a staple food in several countries of Meso and South America: Mexico, Peru, and Ecuador, once it is contaminated with mycotoxins, this implies the risk of diseases caused by the ingestion of this cereal [
58].
The most prevalent mycotoxins in maize in Ecuador, according to this study, and which coincide with those most frequent in the United States and Nepal, are zearalenone, fumonisins, and aflatoxins (
Table 4). Fusilier [
59] reports these three mycotoxins as the most prevalent in samples from the state of Michigan. Similarly, Joshi [
60] indicates that the highest incidence of mycotoxins, in a study in Nepal, corresponded to aflatoxins present in 76% of the samples analyzed, followed by fumonisins and zearalenone, present in 76% of all samples. These percentages are higher than those found in maize samples from Ecuador. According to [
61], the limits for mycotoxins in maize (row grain) are total aflatoxins (B
1 + B
2 + G
1 + G
2) 15 µg/kg, fumonisin (B
1 + B
2) 4000 µg/kg, deoxinivalenol (DON) 2000 µg/kg for further processing and ochratoxin A 5 µg/kg. Currently, in Ecuador there is no regulation set for mycotoxin occurrence in maize, and the limits are variable in other South American countries [
62].
Mallmann et al. [
63] analyzed maize samples from the southern region of Brazil between 2011 and 2014 and determined fumonisin contamination in 25% of the samples, with an average of 6 µg/kg and a maximum of 24 µg/kg. These levels are below those recorded in Ecuador in the present study. On the other hand, in Brazil, refs. [
62,
63,
64,
65] recorded the incidence of fumonisins, aflatoxins, and zearalenones. Indeed, in products derived from maize collected in the Pernambuco state, characterized by a warm climate, fumonisins and aflatoxins were found in all samples, but zearalenone was not detected.
According to Yli-Mattila [
66], in South Africa, considered one of the main maize producers on the African continent, grain produced by small-scale producers has a 100% fumonisin contamination rate, while the incidence of this mycotoxin in grain produced by large-scale producers is 98.6%. This confirms the relevance of this compound in maize.
On the other hand, B-type trichothecenes and zearalenone were the mycotoxins with the highest occurrence in temperate regions of northeastern China. In these regions, mycotoxin concentrations were highly variable from year to year, and this could be explained by major changes in precipitation or temperature during periods of maize crop susceptibility [
67]. The most frequently detected group B trichothecene in the samples from Ecuador was nivalenol, but deoxynivalenol was not detected.
Yang et al. [
68] also indicate that the most prevalent mycotoxin in maize samples in Taiwan was deoxynivalenol (group B trichothecene), followed by zearalenones, aflatoxins, and fumonisins. In addition, as in the present study, the samples were generally contaminated simultaneously by several mycotoxins.
3.3. Co-Occurrence of Several Mycotoxins
Up to three classes of mycotoxins were detected simultaneously in the same maize sample in Ecuador (
Table 5). Fumonisins, trichothecenes B, and zearalenone were present in two of the samples analyzed. On the other hand, aflatoxins, fumonisins, and zearalenones were found in another sample. Mycotoxins were absent in the soft and “morocho” maize produced in the highlands, while on the coast, where hard corn is grown, the presence of several mycotoxins were identified (
Table 5). This could have occurred due to the environmental conditions of the region, since the highlands are cold and with low relative humidity in comparison to the coast, where high temperature and relative humidity predominate and are favorable for fungal growth and mycotoxin synthesis [
8,
69].
Confirming the presence of multiple mycotoxins in food for human and animal consumption is of utmost importance, as they represent a health risk to the consumer. It should be noted that mycotoxins can have additive, synergistic, or antagonistic interactions. Joint occurrence of mycotoxins is frequent since toxigenic fungi are capable of synthesizing more than a single active compound, and likewise, several fungi can simultaneously infect maize kernels [
15,
67,
68].
A global monitoring work indicated that 72% of grain samples were contaminated with more than one mycotoxin and demonstrated that the co-occurrence of these toxins is high worldwide [
70]. In Europe, several studies describe that the joint incidence of mycotoxins is relatively high and that grains were found to be contaminated in a large percentage by trichothecenes, fumonisins, and zearalenone [
70]. Another study revealed that 75–100% of the analyzed grain samples were contaminated by more than one mycotoxin and that this could affect the health of consumers in Europe [
71].
Biscoto et al. [
72] analyzed samples of maize kernels in Brazil and observed that co-occurrence was also very common, with 87% of the samples containing one or more mycotoxins at the same time. The mycotoxins with the highest recurrence were fumonisins, zearalenone and, slightly, to a lesser extent, aflatoxins.
Topi et al. [
73] show in their studies on maize produced in Albania that two or more toxins were detected in all the samples evaluated, and the incidence was mainly of toxins associated with
Fusarium. They also point out that the five samples contaminated with aflatoxins also had fumonisins and that the only sample they found with ochratoxin A also presented zearalenone.
The isolated or concomitant presence of aflatoxins and fumonisins is of particular concern for maize-based products because both are thermally stable mycotoxins and can therefore prevail after the fungi have been killed. This is a real challenge for safe food production [
21].
3.4. Place of Origin and Fungal-Mycotoxin Interaction
A closer analysis of results presented in
Table 5 shows significant differences in the incidence profile of fungi and mycotoxins in the two regions.
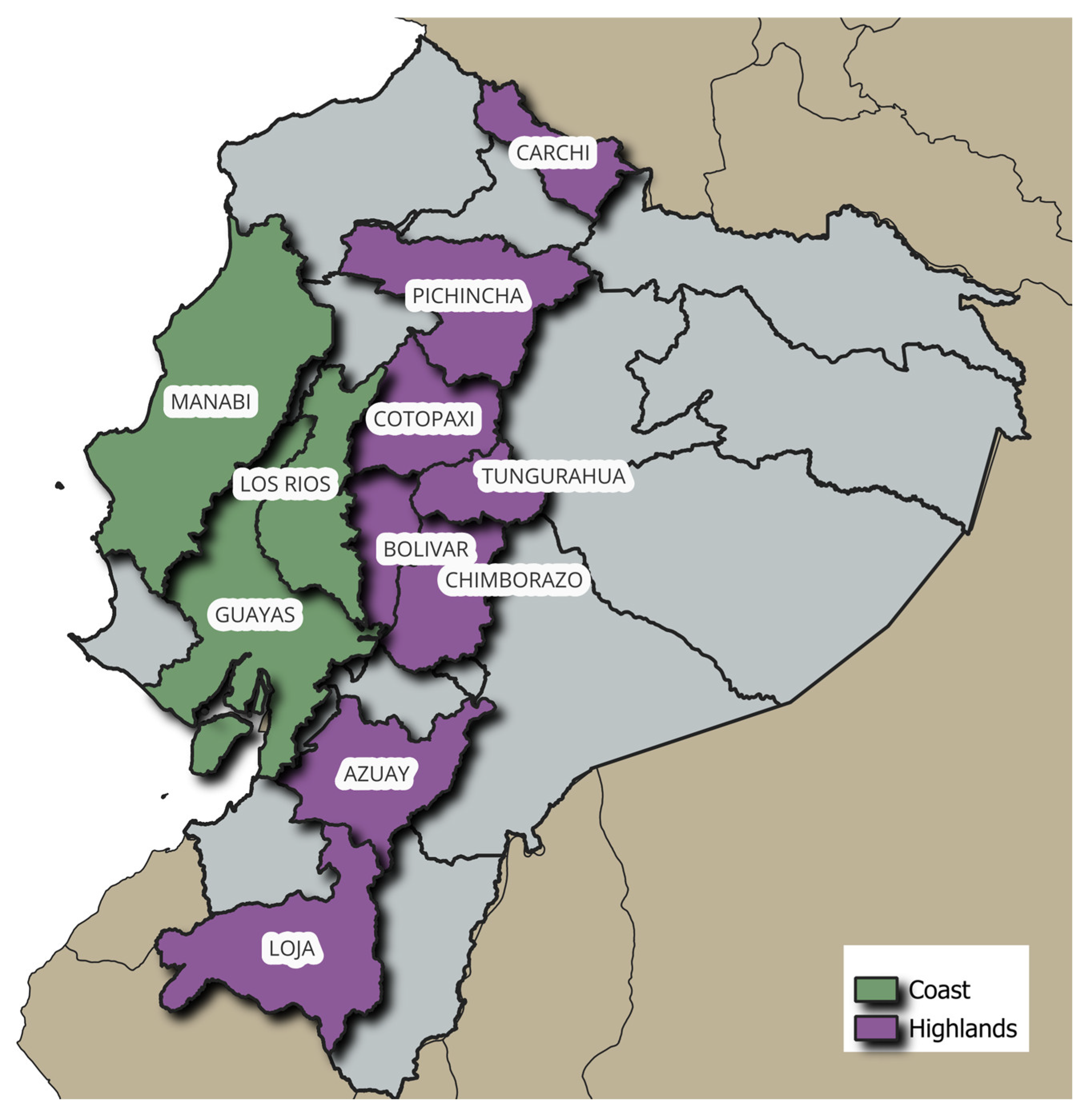
Due to its geographic position, Ecuador suffers little variation in temperature and sunshine throughout the year in the same region; however, the fluctuation in altitude, temperature, and humidity is wide between regions [
8]. On the coast, the temperature ranges between 23 and 36 °C all year long: the rainy season is between December and May, while the dry season is between June and November. The highlands, on the other hand, have a rainy climate between November and April and a dry season from May to October. The temperature in this area varies between temperate and cold depending on the altitude. Thus, the incidence of fungi and mycotoxins is not only influenced by the variety of maize grown but also by the microclimates present in each region.
Regarding the fungi that have the potential to produce mycotoxins in maize, all the samples analyzed that had Fusarium section Liseola were also contaminated with fumonisins and vice versa.
Goertz et al. [
74] ] report that
F. verticilloides (section
Liseola) is a species associated with maize in warmer and drier regions such as Spain or Italy. In terms of dispersal, the genus
Fusarium was predominantly isolated from maize in Germany in 2006. It should be emphasized that high levels of fumonisins tend to occur when maize plants are subjected to water stress or suffer significant damage by insect pests [
21,
26].
In other areas, such as Africa, Dutton [
75] describes the predominance of
F. verticilloides in commercial maize throughout the continent, including South Africa, which is considered the major producer of fumonisins together with
F. proliferatum. Yoshizawa [
76] analyzed samples from Thailand and observed that most samples were contaminated with fumonisins produced by
Fusarium sp. It should be noted that among the known fumonisin compounds, those of the B series (B1, B2, B3) are of most concern in relation to the incidence of toxicity. Fumonisin B1 is the most relevant since it has the highest prevalence and toxicity; it is also suspected to be carcinogenic to humans [
77].
On the other hand, 44.4% (8/18) of the samples from the Ecuadorian coast that showed the presence of
Aspergillus section
Flavi had no aflatoxins. This
Aspergillus section, as well as aflatoxins, was not detected in samples from the highlands. Aflatoxins were present in 27.8% (5/18) of the coastal samples, with varying levels between 1.68 and 132.3 µg/kg.
A. flavus is considered the main source of aflatoxins, the most important mycotoxin worldwide in food production and in maize and its derivatives; it constitutes a problem of specific importance [
15].
Magnoli et al. [
78] found that maize samples from the Ecuadorian highlands’ region had higher infestations of
A. flavus, and it was among the most prevalent fungi after
F. graminearum,
F. verticillioides, and
A. parasiticus, fungi that were also described. These results disagree with those found in the present study, where potentially aflatoxin-producing fungi such as
Aspergillus section
Flavi were not detected in samples from the highlands, nor
Fusarium section
Discolor. Koletsi et al. [
79], through the analysis of maize samples, observed that aflatoxins were present mainly in cereals produced in warm regions such as southern Europe, Africa, and south and southwest Asia.
The mycotoxin that predominated in the Ecuadorian highlands was zearalenone, present in 41.7% of the samples, while in the coastal region, fumonisins were present in 100% of the samples from that area and in only one sample from the highlands. It should be considered that the joint incidence was present in 22% of the samples from the coast, and this index was considerably lower in the highlands.
Surprisingly, no Fusarium section Discolor was detected in the samples from the highlands; however, the zearalenone-positive samples from this region were contaminated with Fusarium section Arthrosporiella. Moreover, the four zearalenone-positive samples were infected by Fusarium section Discolor and vice versa: only two of these four samples had Fusarium section Arthrosporiella.
Pitt and Hocking [
21] report
F. graminearum and
F. culmorum, both belonging to section Discolor, as the main zearalenone-producing species, although some other isolates such as
F. equiseti (section
Gibbosum) and
Fusarium semitectum (section
Arthrosporiella) also possess this characteristic. This suggests that different species of Fusarium produce zearalenone in Ecuadorian maize:
Fusarium section
Discolor in the samples from the highlands and
Fusarium section
Arthrosporiella in the coastal region.
Weaver et al. [
80] analyzed samples from the United States and established that 100% of the samples were infested with one or more mycotoxins, most of which were produced by fungi of the genus
Fusarium, and especially represented was zearalenone.
Fumonisins and trichothecenes B were also detected in samples from the highlands in low frequency (7.69% in 1/13 samples).
In the samples from the coast, the most important mycotoxins were fumonisins, trichothecenes, and zearalenone. These mycotoxins come from fungal species that are highly prevalent in the samples, such as
Fusarium section
Liseola and
Arthroporiella. In the southern region of Brazil, which includes the states of Rio Grande do Sul, Santa Catarina, and Paraná, which have a temperate climate combined with hot summers with the particularity of high humidity, rainfall, and relatively low temperatures are characteristics that favor the contamination of maize grains with fumonisins [
81]. These results coincide with those found in the present study in the coastal region, where similar climatic conditions are found.
Ducos et al. [
82] observed results similar to the present study when analyzing maize samples in Peru, where they visualized high levels of fumonisins associated with the presence of
Fusarium spp. Although
Aspergillus niger is a fungus that could infect maize and has the potential to produce fumonisins, the level of importance of this fungus in fumonisin contamination in this product is still unclear [
21].
Studies by Fusilier et al. [
59] indicate that the main mycotoxins prevalent in maize are deoxynivalenol, fumonisins B1 + B2, and zearalenone. This shows that mycotoxins produced by the same fungal species or species of the same genus are frequently found together in maize samples.
3.6. Distribution of Variability (Inertia)
In order to determine the optimum number of Principal Components (dimensions) that explain the variability in the maize samples, the Elbow analysis was performed (
Figure 3A), where it can be seen that the first four groupings/dimensions have a significant contribution to the variability. However, the first two dimensions (components) of the PCA explain 55.7% of the total variability. This percentage is relatively high, so dimensions 1 and 2 are considered the most relevant in this study.
Considering the 11 variables studied in the 29 localities belonging to the coast and the highlands, the grouping of maize types according to production region can be clearly observed. Quadrants 2 and 3 are dominated by maize samples collected in the Sierra (highlands: red), while quadrants 1 and 4 are dominated by samples from the Costa (coast) (
Figure 3B). In the highlands, groups of very similar maize samples (3, 7) and others very different (5, 2, and 11) are observed. In the coast, maize samples from localities 15 and 17 are very similar, while those from localities 13, 24, and 26 had a very different behavior.
In
Figure 3B,C, individuals 13, 17, 22, and 19 (quadrant 4) are characterized by high positive values in component 1, which contrast with individuals 5, 8, 7, 3, and 1 (quadrant 3). The group formed by the observations of localities 13, 17, 22, and 19, is characterized by sharing high values for the variables temp_, Fusarium_X, Aw, Grain_moisture, Total_infection, and B1pB2. While the group of observations from localities 5, 8, 7, 3, and 1 share low values for the variables Fusarium_X, Total_infection, temp_, Grain_moisture, Aw, and B1pB2. It is important to highlight that the region factor is highly correlated with the water activity parameter in dimension 1.
Based on the number of groupings (4) shown in
Figure 3D, it can be indicated that group 1 is formed by the maize samples from locations 1, 3, 5, 7, and 8. This group is characterized by component 1, which has negative values for all variables, and with respect to component 2, it has negative values for the variables total_infection, temp_, and MO, and positive values for Grain_moisture, AW, FusariumX, and B1pB2. Group 2 is only formed by the sample from locality 2. Group 3 is composed of the maize samples obtained at locations 11, 24, 26, 27, and 28, which are characterized by high values for the variables MO and A. niger (%) and low values for water activity (Aw). Finally, group 4, composed of maize samples from locations 13, 15, 16, 17, 19, and 22, is characterized mainly by high values for the variables: Grain_moisture, temp_, Aw, Fusarium_X, B1pB2, and Total_infection.
Developing ears of maize are encased in a strong, protective husk, which reduces invasion by fungi.
Fusarium is the principal pathogenic fungal genus causing spoilage of the ear in maize, the most commonly occurring species being
F. graminearum, F. verticillioides (=
F. moniliforme), and
F. subglutinans [
21].
Of no less importance than the
Fusarium diseases is the fact that the mycotoxigenic fungus
Aspergillus flavus also invades maize, although it is not considered to be a true pathogen. In the early literature,
A. flavus was regarded only as a storage fungus, but by 1970 the realization came that freshly harvested maize in the southeastern United States was sometimes infected by
A. flavus with the consequent production of aflatoxins [
21].
Maize cobs and kernels are relatively large. Moist conditions at harvest often result in slow drying. In consequence, both preharvest and postharvest fungi may become well established. Lichtwardt and Barron Refs. [
83,
84] carried out a very thorough study of the mycoflora of dried and stored maize in Iowa. Lichtwardt [
83] identified the internal flora of surface-disinfected maize grains both in sterile moist chambers and on malt salt agar (6% NaCl). In addition, samples were ground and dilution plated. Approximately 50 genera were recognized. A combination of isolation methods enabled Barron [
84] to estimate the relative importance of the isolated genera in the spoilage of stored maize.
Eurotium species, especially
E. rubrum,
E. amstelodami, and
E. chevalieri, were the most significant, together with
Aspergillus restrictus and
Penicillium spp., especially
P. aurantiogriseum and
P. viridicatum and closely related species. In maize samples from Thailand, the most encountered storage fungi were again
Eurotium species (
E. chevalieri,
E. rubrum, and
E. amstelodami) but
Wallemia sebi,
A. flavus,
A. wentii,
A. tamarii, and
A. niger were also present in a significant number of samples [
21].
Penicillium species occurring in maize both preharvest and in storage, and the factors that influenced their role as spoilage fungi, were investigated by [
85]. Some common preharvest species, such as
P. funiculosum, were rarely isolated later; species such as
P. citrinum and
P. oxalicum were always commonly present. Others again, such as
P. aurantiogriseum and
P. viridicatum, were almost exclusively associated with the stored grain.
Fungi acquired in the field, particularly
F. verticillioides,
F. proliferatum,
F. oxysporum, and
A. flavus, can persist in maize, and the molds, and their toxins may be carried through to maize products such as flour, grits, maize chips, tortillas, breakfast cereals, etc. [
21]. In Central America, the process of nixtamalization is commonly used in preparation of meals based on maize. Nixtamalization is a centuries old process in which maize is soaked and then cooked with ash or lime high in alkali. It removes almost all fumonisins (and aflatoxins), resulting in tortillas and other maize-based foods being substantially free of these mycotoxins [
86].
3.7. Selection of Explanatory Variables by Correlation Analysis to Determine Redundant Information
The influence of the explanatory (independent) variables on the amount of mycotoxins present in maize kernels harvested in the different locations was determined through the degree of linear association between the quantitative explanatory variables. The correlation coefficients (>0.5) between all quantitative explanatory variables are shown in
Table 6.
The most significant correlation coefficients are presented in
Table 7. The information presented in
Table 6 shows that there is a potential overlap of information between the percentage of grain moisture, the amount of Fusarium_X, and the average temperature of the collection site. There is evidence that environmental factors such as temperature, humidity, and precipitation, aong others (intensity and distribution), influence the presence of fungi in cereals such as maize, which produce mycotoxins, affecting food safety [
87,
88], and people’s and animal’s health if consumed [
89].
In all the cases indicated in the table, a significance test was applied to the value of the correlation coefficient, and in each case the value was found to be statistically non-zero with 95% confidence. Subsequently, the stepwise multiple regression algorithm was applied, in its two possible variants or directions, thus considering the Akaike information criterion (AIC) as a comparison parameter and obtaining the statistical models.