Mycotoxin Assessment in Minimally Processed Traditional Ecuadorian Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sampling Frame
2.3. Sample Treatment
2.4. HPLC Conditions and Analysis
3. Results and Discussion
3.1. HPLC Method Performance
3.2. Maximum Permitted Levels in Minimally Processed Foods
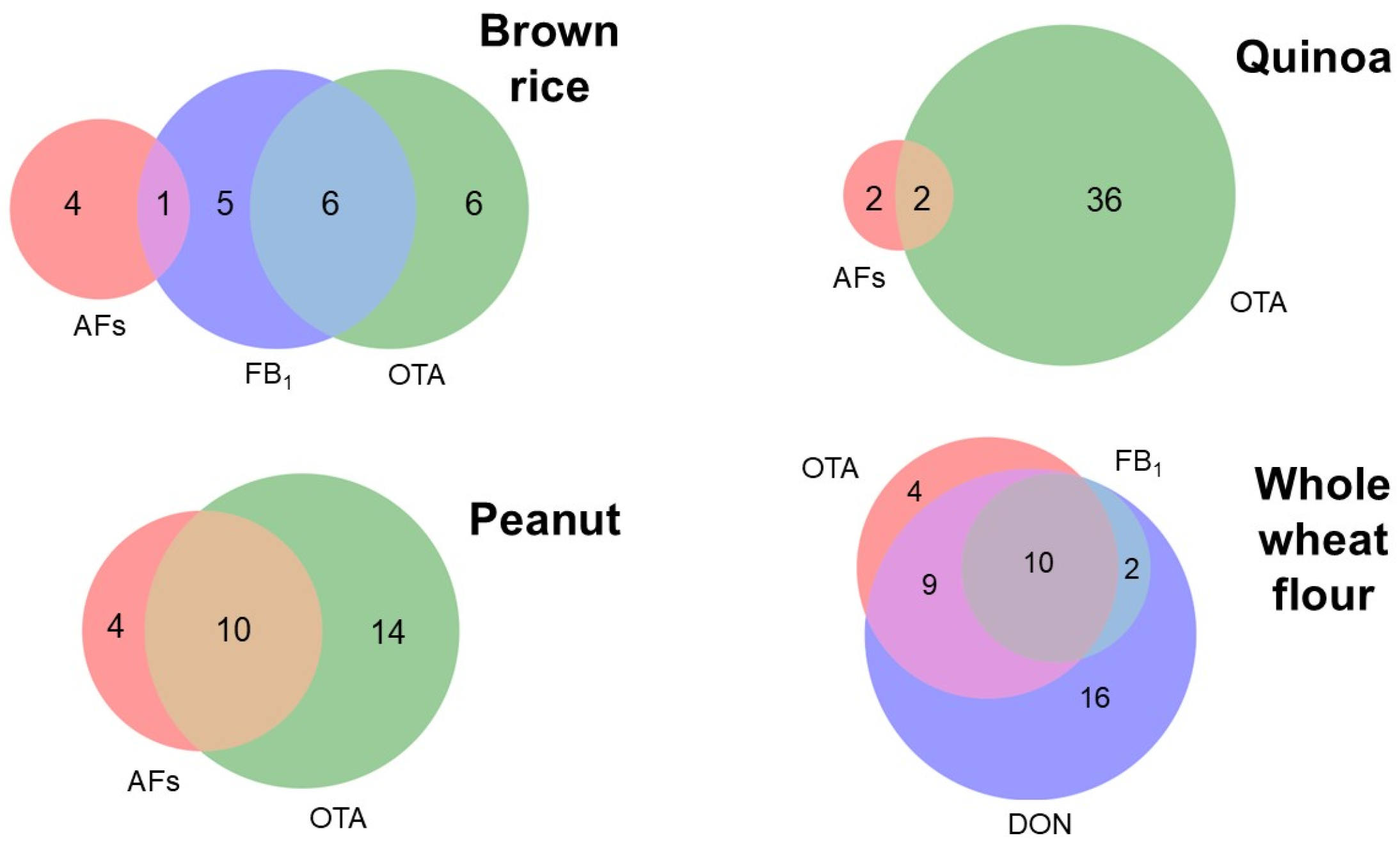
3.3. Mycotoxin Co-Occurrence in Minimally Processed Traditional Foods in Ecuador
3.4. Mycotoxin Occurrence in Quinoa and Lupin
3.5. Mycotoxin Occurrence in Peanut Samples
3.6. Mycotoxin Occurrence in Whole Grains: Brown Rice and Whole-Wheat Flour
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFs | Aflatoxins (AFB1, AFB2, AFG1, AFG2) |
| AFt | Total aflatoxins (AFB1 + AFB2 + AFG1 + AFG2) |
| OTA | Ochratoxin A |
| FB1 | Fumonisin B1 |
| DON | Deoxynivalenol |
| MPL | Maximum permitted level |
| RP-HPLC | Reversed-Phase High-Performance Liquid Chromatography |
| DAD | Diode Array Detector |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
References
- Iqbal, S.Z. Mycotoxins in Food, Recent Development in Food Analysis and Future Challenges; a Review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An Overview on the Major Mycotoxins in Food Products: Characteristics, Toxicity, and Analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in Cereal-Based Products during 24 Years (1983–2017): A Global Systematic Review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Nešić, K.; Habschied, K.; Mastanjević, K. Possibilities for the Biological Control of Mycotoxins in Food and Feed. Toxins 2021, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Sadik, J.A.; Fentahun, N.; Brouwer, I.D.; Tessema, M.; van der Fels-Klerx, H.J. Preharvest and Postharvest Management Practices Related to Mycotoxin Contamination in Maize in Ethiopia—A Review. World Mycotoxin J. 2023, 16, 211–226. [Google Scholar] [CrossRef]
- Alina Marc, R. Implications of Mycotoxins in Food Safety. In Mycotoxins and Food Safety—Recent Advances; IntechOpen: London, UK, 2022; ISBN 9781839629044. [Google Scholar]
- Santos Pereira, C.; Cunha, S.C.; Fernandes, J.O. Prevalent Mycotoxins in Animal Feed: Occurrence and Analytical Methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef]
- Stoev, S.D. Food Security, Underestimated Hazard of Joint Mycotoxin Exposure and Management of the Risk of Mycotoxin Contamination. Food Control 2024, 159, 110235. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Zhang, W.; Guang, C.; Xu, W.; Mu, W. Deoxynivalenol: Occurrence, Toxicity, and Degradation. Food Control 2024, 155, 110027. [Google Scholar] [CrossRef]
- Ramos-Diaz, J.M.; Sulyok, M.; Jacobsen, S.E.; Jouppila, K.; Nathanail, A.V. Comparative Study of Mycotoxin Occurrence in Andean and Cereal Grains Cultivated in South America and North Europe. Food Control 2021, 130, 108260. [Google Scholar] [CrossRef]
- Imran, M. Mycotoxins—A Global One Health Concern: A Review. Agrobiol. Rec. 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, P.; Du, H.; Ge, L.; Liu, S.; Huang, K.; Chen, X. The Combined Effect of Deoxynivalenol and Fumonisin B1 on Small Intestinal Inflammation Mediated by Pyroptosis in Vivo and in Vitro. Toxicol. Lett. 2023, 372, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Speijers, G.J.A.; Speijers, M.H.M. Combined Toxic Effects of Mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Creppy, E.E.; Chiarappa, P.; Baudrimont, I.; Borracci, P.; Moukha, S.; Carratù, M.R. Synergistic Effects of Fumonisin B1 and Ochratoxin A: Are in Vitro Cytotoxicity Data Predictive of in Vivo Acute Toxicity? Toxicology 2004, 201, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, Y.; Xie, Y.; Yan, C.; Du, H.; Li, Z. Ochratoxin A and Fumonisin B1 Exhibit Synergistic Cytotoxic Effects by Inducing Apoptosis on Rat Liver Cells. Toxicon 2020, 181, 19–27. [Google Scholar] [CrossRef]
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and Biotransformation in Animal Cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef]
- Radzka-Pogoda, A.; Radzki, R.P.; Bieńko, M.; Szponar, J.; Sokołowska, B.; Kulik, A.; Lewicka, M.; Borzęcki, A. Ochratoxin A and Aflatoxin B1 as Factors of Bone Damage and Neurodegeneration through the Influence on the Immunomodulation Processes of TNF-α and IL-6 Concentrations. Pol. Hyperb. Res. 2023, 80, 61–72. [Google Scholar] [CrossRef]
- Yu, J.; Pedroso, I.R. Mycotoxins in Cereal-Based Products and Their Impacts on the Health of Humans, Livestock Animals and Pets. Toxins 2023, 15, 480. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Toxicological Interactions between Mycotoxins from Ubiquitous Fungi: Impact on Hepatic and Intestinal Human Epithelial Cells. Chemosphere 2018, 202, 538–548. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited “FAO Estimate” of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize Food Chain and Mycotoxins: A Review on Occurrence Studies. Trends Food Sci. Technol. 2021, 115, 307–331. [Google Scholar] [CrossRef]
- Sarmast, E.; Fallah, A.A.; Jafari, T.; Mousavi Khaneghah, A. Occurrence and Fate of Mycotoxins in Cereals and Cereal-Based Products: A Narrative Review of Systematic Reviews and Meta-Analyses Studies. Curr. Opin. Food Sci. 2021, 39, 68–75. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A Review of Recent Innovative Strategies for Controlling Mycotoxins in Foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Vásquez-Ocmín, P.G.; Marti, G.; Gadea, A.; Cabanac, G.; Vásquez-Briones, J.A.; Casavilca-Zambrano, S.; Ponts, N.; Jargeat, P.; Haddad, M.; Bertani, S. Metabotyping of Andean Pseudocereals and Characterization of Emerging Mycotoxins. Food Chem. 2023, 407, 135134. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A Major Source of Sustainable Protein for Health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Nugent, A.P.; Tee, E.-S.; Thielecke, F. Whole-Grain Dietary Recommendations: The Need for a Unified Global Approach. Br. J. Nutr. 2016, 115, 2031–2038. [Google Scholar] [CrossRef]
- Bryant, L.; Rangan, A.; Grafenauer, S. Lupins and Health Outcomes: A Systematic Literature Review. Nutrients 2022, 14, 327. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and Biological Value of Quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. Whole Grains and Pulses: A Comparison of the Nutritional and Health Benefits. J. Agric. Food Chem. 2014, 62, 7029–7049. [Google Scholar] [CrossRef]
- Settaluri, V.S.; Kandala, C.V.K.; Puppala, N.; Sundaram, J. Peanuts and Their Nutritional Aspects—A Review. Food Nutr. Sci. 2012, 3, 1644–1650. [Google Scholar] [CrossRef]
- MacGregor, S. Ecuadorian Cuisine: Discover 5 of the Country’s Most Fascinating Foods. Forbes Magazine, 1 June 2023. [Google Scholar]
- Deaconu, A.; Ekomer; Mercille, G.; Batal, M. Promoting Traditional Foods for Human and Environmental Health: Lessons from Agroecology and Indigenous Communities in Ecuador. BMC Nutr. 2021, 7, 1. [Google Scholar] [CrossRef]
- Assaf, S.; Park, J.; Chowdhry, N.; Ganapuram, M.; Mattathil, S.; Alakeel, R.; Kelly, O.J. Unraveling the Evolutionary Diet Mismatch and Its Contribution to the Deterioration of Body Composition. Metabolites 2024, 14, 379. [Google Scholar] [CrossRef]
- Ortiz, J.; Van Camp, J.; Mestdagh, F.; Donoso, S.; De Meulenaer, B. Mycotoxin Co-Occurrence in Rice, Oat Flakes and Wheat Noodles Used as Staple Foods in Ecuador. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- April-Lalonde, G.; Deaconu, A.; Cole, D.C.; Batal, M. Traditional Food Consumption in Andean Ecuador and Associated Consumer Characteristics, Shopping and Eating Habits. Ecol. Food Nutr. 2023, 62, 308–333. [Google Scholar] [CrossRef]
- Rezende Machado de Sousa, L.; Saint-Ville, A.; Samayoa-Figueroa, L.; Melgar-Quiñonez, H. Changes in Food Security in Latin America from 2014 to 2017. Food Secur. 2019, 11, 503–513. [Google Scholar] [CrossRef]
- Ortiz, J.; Astudillo, G.; Castro, M.; Castro, C.; Astudillo, S.; Donoso, S. 12th IFDC 2017 Special Issue—Seasonal Variations in Nutrient Composition of Plant-Based Foods Produced at the Southern Highlands of Ecuador. J. Food Compost. Anal. 2019, 83, 103284. [Google Scholar] [CrossRef]
- CIA. Ecuador. Available online: https://www.cia.gov/the-world-factbook/countries/ecuador/ (accessed on 2 September 2024).
- World Bank Group. Ecuador Climatology. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 1 July 2025).
- Jornet, D.; Busto, O.; Guasch, J. Solid-Phase Extraction Applied to the Determination of Ochratoxin A in Wines by Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. A 2000, 882, 29–35. [Google Scholar] [CrossRef]
- European Union. EU Commission Regulation 2023/915; European Union: Brussels, Belgium, 2023; pp. 103–157. [Google Scholar]
- Taverniers, I.; De Loose, M.; Van Bockstaele, E. Trends in Quality in the Analytical Laboratory. II. Analytical Method Validation and Quality Assurance. Trends Analyt. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and Their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Sanchis, V.; Abadias, M.; Oncins, L.; Sala, N.; Viñas, I.; Canela, R. Fumonisins B1 and B2 and Toxigenic Fusarium Strains in Feeds from the Spanish Market. Int. J. Food Microbiol. 1995, 27, 37–44. [Google Scholar] [CrossRef]
- Farhadi, A.; Nowrozi, H.; Kachuei, R. Metabolism, Toxicity, Detoxification, Occurrence, Intake and Legislations of Fumonisins—A Review. J. Pharm. Res. Int. 2019, 29, 1–35. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of Mycotoxins on Humans and Animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, F.; Bazile, D.; Biaggi, C.; Callo-Concha, D.; Jacquet, J.; Jemal, O.M.; King, O.I.; Mbosso, C.; Padulosi, S.; Speelman, E.N.; et al. When Neglected Species Gain Global Interest: Lessons Learned from Quinoa’s Boom and Bust for Teff and Minor Millet. Glob. Food Sec. 2022, 32, 100613. [Google Scholar] [CrossRef]
- Gómez Pando, L.; Aguilar Castellanos, E. Guía de Cultivo de la Quinua; Food & Agriculture Organization of the United Nations (FAO): Rome, Italy, 2016; ISBN 9789253090693. [Google Scholar]
- Ducos, C.; Pinson-Gadais, L.; Chereau, S.; Richard-Forget, F.; Vásquez-Ocmín, P.; Cerapio, J.P.; Casavilca-Zambrano, S.; Ruiz, E.; Pineau, P.; Bertani, S.; et al. Natural Occurrence of Mycotoxin-Producing Fusaria in Market-Bought Peruvian Cereals: A Food Safety Threat for Andean Populations. Toxins 2021, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, V.C.; Peralta, I.E. El Cultivo de Chocho Lupinus mutabilis Sweet: Fitonutrición, Enfermedades y Plagas, En El Ecuador; INIAP, Estación Experimental Santa Catalina, Programa Nacional de Leguminosas: Quito, Ecuador, 2001. [Google Scholar]
- van de Noort, M. Lupin: An Important Protein and Nutrient Source. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–183. ISBN 9780128027783. [Google Scholar]
- Schryvers, S.; Arinzechukwu, C.; Miserez, B.; Eeckhout, M.; Jacxsens, L. The Fate of Quinolizidine Alkaloids during the Processing of Lupins (Lupinus spp.) for Human Consumption. Food Chem. 2023, 429, 136847. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Magan, N.; Geisen, R. Stress Induction of Mycotoxin Biosynthesis Genes by Abiotic Factors. FEMS Microbiol. Lett. 2008, 284, 142–149. [Google Scholar] [CrossRef]
- Wang, L.; Hua, X.; Shi, J.; Jing, N.; Ji, T.; Lv, B.; Liu, L.; Chen, Y. Ochratoxin A: Occurrence and Recent Advances in Detoxification. Toxicon 2022, 210, 11–18. [Google Scholar] [CrossRef]
- el Khoury, A.; Atoui, A. Ochratoxin A: General Overview and Actual Molecular Status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Schollenberger, M.; Müller, H.-M.; Rüfle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural Occurrence of 16 Fusarium Toxins in Grains and Feedstuffs of Plant Origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar] [CrossRef]
- Carvajal-Larenas, F.E.; Linnemann, A.R.; Nout, M.J.R.; Koziol, M.; van Boekel, M.A.J.S. Lupinus mutabilis: Composition, Uses, Toxicology, and Debittering. Crit. Rev. Food Sci. Nutr. 2016, 56, 1454–1487. [Google Scholar] [CrossRef]
- Acuña-Gutiérrez, C.; Jiménez, V.M.; Müller, J. Occurrence of Mycotoxins in Pulses. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4002–4017. [Google Scholar] [CrossRef]
- Commey, L.; Tengey, T.K.; Cobos, C.J.; Dampanaboina, L.; Dhillon, K.K.; Pandey, M.K.; Sudini, H.K.; Falalou, H.; Varshney, R.K.; Burow, M.D.; et al. Peanut Seed Coat Acts as a Physical and Biochemical Barrier against Aspergillus flavus Infection. J. Fungi 2021, 7, 1000. [Google Scholar] [CrossRef] [PubMed]
- Amézqueta, S.; Schorr-Galindo, S.; Murillo-Arbizu, M.; González-Peñas, E.; López de Cerain, A.; Guiraud, J.P. OTA-Producing Fungi in Foodstuffs: A Review. Food Control 2012, 26, 259–268. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Conditions of Formation of Ochratoxin A in Drying, Transport and in Different Commodities. Food Addit. Contam. 2005, 22 (Suppl. 1), 10–16. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, C.; Astoreca, A.; Ponsone, L.; Fernández-Juri, M.G.; Chiacchiera, S.; Dalcero, A. Ochratoxin A and the Occurrence of Ochratoxin A-producing Black Aspergilli in Stored Peanut Seeds from Córdoba, Argentina. J. Sci. Food Agric. 2006, 86, 2369–2373. [Google Scholar] [CrossRef]
- Sangare-Tigori, B.; Moukha, S.; Kouadio, H.J.; Betbeder, A.-M.; Dano, D.S.; Creppy, E.E. Co-Occurrence of Aflatoxin B1, Fumonisin B1, Ochratoxin A and Zearalenone in Cereals and Peanuts from Côte d’Ivoire. Food Addit. Contam. 2006, 23, 1000–1007. [Google Scholar] [CrossRef]
- Aristil, J.; Venturini, G.; Maddalena, G.; Toffolatti, S.L.; Spada, A. Fungal Contamination and Aflatoxin Content of Maize, Moringa and Peanut Foods from Rural Subsistence Farms in South Haiti. J. Stored Prod. Res. 2020, 85, 101550. [Google Scholar] [CrossRef]
- Ding, X.; Wu, L.; Li, P.; Zhang, Z.; Zhou, H.; Bai, Y.; Chen, X.; Jiang, J. Risk Assessment on Dietary Exposure to Aflatoxin B1 in Post-Harvest Peanuts in the Yangtze River Ecological Region. Toxins 2015, 7, 4157–4174. [Google Scholar] [CrossRef]
- Hoeltz, M.; Einloft, T.C.; Oldoni, V.P.; Dottori, H.A.; Noll, I.B. The Occurrence of Aflatoxin B1 Contamination in Peanuts and Peanut Products Marketed in Southern Brazil. Braz. Arch. Biol. Technol. 2012, 55, 313–317. [Google Scholar] [CrossRef]
- Nakai, V.K.; de Oliveira Rocha, L.; Gonçalez, E.; Fonseca, H.; Ortega, E.M.M.; Corrêa, B. Distribution of Fungi and Aflatoxins in a Stored Peanut Variety. Food Chem. 2008, 106, 285–290. [Google Scholar] [CrossRef]
- Pinto, L.; Santos, A.; Vargas, E.; Madureira, F.; Faria, A.; Augusti, R. Validation of an Analytical Method Based on QuEChERS and LC-MS/MS to Quantify Nine Mycotoxins in Plant-Based Milk. World Mycotoxin J. 2021, 14, 339–346. [Google Scholar] [CrossRef]
- Dhanshetty, M.; Elliott, C.T.; Banerjee, K. Decontamination of Aflatoxin B1 in Peanuts Using Various Cooking Methods. J. Food Sci. Technol. 2021, 58, 2547–2554. [Google Scholar] [CrossRef] [PubMed]
- Mannani, N.; El Boujamaai, M.; Sifou, A.; Bennani, M.; El Adlouni, C.; Abdennebi, E.H.; Zinedine, A. Aflatoxins and Ochratoxin A in Dried Fruits from Morocco: Monitoring, Regulatory Aspects, and Exposure Assessment. Regul. Toxicol. Pharmacol. 2023, 145, 105503. [Google Scholar] [CrossRef]
- Trucksess, M.W.; Abbas, H.K.; Weaver, C.M.; Shier, W.T. Distribution of Aflatoxins in Shelling and Milling Fractions of Naturally Contaminated Rice. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1076–1082. [Google Scholar] [CrossRef]
- Abou Ghaida, A. Ochratoxin A in Rice Marketed in United Arab Emirates. Master’s Thesis, Lebanese American University, Beirut, Lebanon, 2022. [Google Scholar]
- Kolawole, O.; Meneely, J.; Petchkongkaew, A.; Elliott, C. A Review of Mycotoxin Biosynthetic Pathways: Associated Genes and Their Expressions under the Influence of Climatic Factors. Fungal Biol. Rev. 2021, 37, 8–26. [Google Scholar] [CrossRef]
- Chhaya, R.S.; O’Brien, J.; Cummins, E. Feed to Fork Risk Assessment of Mycotoxins under Climate Change Influences—Recent Developments. Trends Food Sci. Technol. 2022, 126, 126–141. [Google Scholar] [CrossRef]
- Sales, A.C.; Yoshizawa, T. Updated Profile of Aflatoxin and Aspergillus Section Flavi Contamination in Rice and Its Byproducts from the Philippines. Food Addit. Contam. 2005, 22, 429–436. [Google Scholar] [CrossRef]
- Fredlund, E.; Thim, A.-M.; Gidlund, A.; Brostedt, S.; Nyberg, M.; Olsen, M. Moulds and Mycotoxins in Rice from the Swedish Retail Market. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 527–533. [Google Scholar] [CrossRef]
- El Telégrafo. Ecuatorianos Comen 53.2 Kg de Arroz al Año. El Telégrafo, 2 June 2014. [Google Scholar]
- Ecuador’s Wheat Awakening. Available online: https://www.cimmyt.org/news/ecuadors-wheat-awakening/ (accessed on 2 September 2024).
- Chen, C.; Turna, N.S.; Wu, F. Risk Assessment of Dietary Deoxynivalenol Exposure in Wheat Products Worldwide: Are New Codex DON Guidelines Adequately Protective? Trends Food Sci. Technol. 2019, 89, 11–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, F.; Fang, Y.; Li, P.; Zhao, Y.; Shen, F.; Zou, Y.; Hu, Q. Comparison of Concentration and Health Risks of 9 Fusarium Mycotoxins in Commercial Whole Wheat Flour and Refined Wheat Flour by Multi-IAC-HPLC. Food Chem. 2019, 275, 763–769. [Google Scholar] [CrossRef]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical Factors Responsible for Fungi Growth in Stored Food Grains and Non-Chemical Approaches for Their Control. Ind. Crops Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Ahou, L.L.; Aholia, J.-B.A.; Atse, N.R.A.; Kouakou, B. Evaluation of Practices Favoring the Development of Mycotoxigenic Molds in Rice Sold in the Retail Markets of Abidjan, Cte DIvoire. J. Toxicol. Environ. Health Sci. 2021, 13, 45–51. [Google Scholar] [CrossRef]
- Seal, C.J.; Courtin, C.M.; Venema, K.; de Vries, J. Health Benefits of Whole Grain: Effects on Dietary Carbohydrate Quality, the Gut Microbiome, and Consequences of Processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef]
- Thielecke, F.; Nugent, A.P. Contaminants in Grain-A Major Risk for Whole Grain Safety? Nutrients 2018, 10, 1213. [Google Scholar] [CrossRef]

| Mycotoxin | LOD | LOQ |
|---|---|---|
| FB1 | 3.15 | 6.30 |
| OTA | 0.07 | 0.14 |
| DON | 9.47 | 18.94 |
| AFB1 | 0.06 | 0.13 |
| AFB2 | 0.20 | 0.40 |
| AFG1 | 0.10 | 0.20 |
| AFG2 | 0.16 | 0.32 |
| Mycotoxin | MPLs (2023/915/EC) |
|---|---|
| FB1 | 1000 (FB1 + FB2) a |
| OTA | 5 (peanuts); 3 (others) b |
| DON | 600 c |
| AFB1 | 2 (quinoa) d; 5 (rice e); 8 (peanuts and lupins f) |
| AFB2 | - |
| AFG1 | - |
| AFG2 | - |
| AFt | 4 (quinoa) d; 10 (rice e); 15 (peanuts and lupins f) |
| Quinoa (n = 46) | Lupin (n = 36) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Per City | Overall | Per City | |||||||||
| Mycotoxin | CR (%) | % > MPL | City | Positive/Total | Mean ± SD | Range | CR (%) | % > MPL | City | Positive/Total | Mean ± SD | Range |
| FB1 | 8.9 | 0 | C | 1/15 | 21.3 a | - | n.d. | - | C | 0/15 | - | - |
| Q | 3/16 | 8.2 ± 1.7 | 6.3–9.6 | - | - | Q | 0/15 | - | - | |||
| G | 0/15 | - | - | - | - | G | 0/6 | - | - | |||
| OTA | 82.6 | 21.1 | C | 15/15 | 2.3 ± 3.0 | 0.78–10.7 | 48.6 | 5.7 | C | 15/15 | 1.1 ± 0.14 | 0.89–1.4 |
| Q | 11/16 | 3.5 ± 2.6 | 0.98–16.7 | - | - | Q | 2/15 | 15.9 ± 9.0 | 9.6–22.3 | |||
| G | 12/15 | 4.5 ± 4.8 | 1.3–9.3 | - | - | G | 0/6 | - | - | |||
| AFB1 | 6.5 | 0 | C | 0/15 | - | - | n.d. | - | C | 0/15 | - | - |
| Q | 2/16 | 0.03 ± 0.002 | 0.03–0.03 | - | - | Q | 0/15 | - | - | |||
| G | 1/15 | 0.05 a | - | - | - | G | 0/6 | - | - | |||
| AFB2 | n.d. | - | C | 0/15 | - | - | n.d. | - | C | 0/15 | - | - |
| Q | 0/16 | - | - | - | - | Q | 0/15 | - | - | |||
| G | 0/15 | - | - | - | - | G | 0/6 | - | - | |||
| AFG1 | 8.7 | - | C | 0/15 | - | - | n.d. | - | C | 0/15 | - | - |
| Q | 2/16 | 0.14 ± 0.05 | 0.11–0.18 | - | - | Q | 0/15 | - | - | |||
| G | 2/15 | 0.07 ± 0.02 | 0.06–0.08 | - | - | G | 0/6 | - | - | |||
| AFG2 | n.d. | - | C | 0/15 | - | - | n.d. | - | C | 0/15 | - | - |
| Q | 0/16 | - | - | - | - | Q | 0/15 | - | - | |||
| G | 0/15 | - | - | - | - | G | 0/6 | - | - | |||
| AFt | - | 0 | - | - | - | - | - | - | - | - | ||
| Peanuts (n = 45) | ||||||
|---|---|---|---|---|---|---|
| Overall | Per City | |||||
| Mycotoxin | CR (%) | % > MPL | City | Positive/Total | Mean ± SD | Range |
| OTA | 53.3 | 2.2 | C | 15/15 | 1.2 ± 0.23 | 0.9–1.7 |
| Q | 7/15 | 4.2 ± 5.6 | 1.2–16.8 | |||
| G | 2/15 | 2.9 ± 2.4 | 1.2–4.6 | |||
| AFB1 | 26.7 | 0 | C | 3/15 | 0.23 ± 0.22 | 0.06–0.47 |
| Q | 5/15 | 0.19 ± 0.28 | 0.05–0.69 | |||
| G | 4/15 | 0.05 ± 0.02 | 0.03–0.07 | |||
| AFB2 | n.d. | - | C | 0/15 | - | - |
| Q | 0/15 | - | - | |||
| G | 0/15 | - | - | |||
| AFG1 | 28.9 | - b | C | 3/15 | 0.77 ± 1 | 0.13–1.9 |
| Q | 5/15 | 0.57 ± 0.98 | 0.06–2.3 | |||
| G | 5/15 | 4.1 ± 13.5 | 0.07–44.7 | |||
| AFG2 | 2.2 | - b | C | 1/15 | 0.14 a | |
| Q | 0/15 | - | - | |||
| G | 0/15 | - | - | |||
| AFt | - c | 7.7 | - | - | - | |
| Brown Rice (n = 47) | Whole-Wheat Flour (n = 45) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Per City | Overall | Per City | |||||||||
| Mycotoxin | CR (%) | % > MPL | City | Positive/Total | Mean ± SD | Range | CR (%) | % > MPL | City | Positive/Total | Mean ± SD | Range |
| FB1 | 25.5 | 0 | C | 8/15 | 106 ± 16.3 | 74.8–122.8 | 26.7 | 50.0 | C | 9/15 | 812.3 ± 407.5 | 384.5–1430.9 |
| Q | 4/16 | 14.1 ± 2.9 | 10.8–13.0 | - | - | Q | 1/15 | 11.04 a | - | |||
| G | 0/16 | - | - | - | - | G | 2/15 | 5.9 ± 1.3 | 4.4–6.1 | |||
| OTA | 25.5 | 0 | C | 11/15 | 0.86 ± 0.08 | 0.79–1.06 | 76.7 | 0 | C | 15/15 | 0.96 ± 0.19 | 0.77–1.6 |
| Q | 0/16 | - | - | - | - | Q | 8/15 | 1.4 ± 0.39 | 0.9–2.1 | |||
| G | 1/16 | 1.12 a | - | - | - | G | n.a. | |||||
| DON | n.a. | - | - | - | - | 82.2 | 37.8 | C | 14/15 | 1878 ± 1181 | 166.2–4445.0 | |
| - | - | - | - | - | - | Q | 10/15 | 881.7 ± 741.1 | 139.0–2057.3 | |||
| - | - | - | - | - | - | G | 13/15 | 242 ± 48 | 115.0–286.8 | |||
| AFB1 | 6.4 | 0 | C | 0/15 | - | - | - | - | - | - | - | - |
| Q | 3/16 | 0.07 ± 0.05 | 0.03–0.11 | - | - | - | - | - | - | |||
| G | 0/16 | - | - | - | - | - | - | - | - | |||
| AFB2 | n.d. | -b | C | 0/15 | - | - | - | - | - | - | - | - |
| Q | 0/16 | - | - | - | - | - | - | - | - | |||
| G | 0/16 | - | - | - | - | - | - | - | - | |||
| AFG1 | 8.5 | - b | C | 0/15 | - | - | - | - | - | - | - | - |
| Q | 3/16 | 0.89 ± 1.02 | 0.17–1.6 | - | - | - | - | - | - | |||
| G | 1/16 | 0.07 a | - | - | - | - | - | - | ||||
| AFG2 | 2.1 | - b | C | 0/15 | - | - | - | - | - | - | - | - |
| Q | 0/16 | - | - | - | - | - | - | - | - | |||
| G | 1/16 | 0.07 a | - | - | - | - | - | - | - | |||
| AFt | - c | 0 | - | - | - | - | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Ulloa, J.; Saquicela, J.; Castro, M.; Cueva-Chamba, A.; Cevallos-Cevallos, J.M.; León, J. Mycotoxin Assessment in Minimally Processed Traditional Ecuadorian Foods. Foods 2025, 14, 2621. https://doi.org/10.3390/foods14152621
Ortiz-Ulloa J, Saquicela J, Castro M, Cueva-Chamba A, Cevallos-Cevallos JM, León J. Mycotoxin Assessment in Minimally Processed Traditional Ecuadorian Foods. Foods. 2025; 14(15):2621. https://doi.org/10.3390/foods14152621
Chicago/Turabian StyleOrtiz-Ulloa, Johana, Jorge Saquicela, Michelle Castro, Alexander Cueva-Chamba, Juan Manuel Cevallos-Cevallos, and Jessica León. 2025. "Mycotoxin Assessment in Minimally Processed Traditional Ecuadorian Foods" Foods 14, no. 15: 2621. https://doi.org/10.3390/foods14152621
APA StyleOrtiz-Ulloa, J., Saquicela, J., Castro, M., Cueva-Chamba, A., Cevallos-Cevallos, J. M., & León, J. (2025). Mycotoxin Assessment in Minimally Processed Traditional Ecuadorian Foods. Foods, 14(15), 2621. https://doi.org/10.3390/foods14152621







