Novel Synbiotic Yogurt Formulation Supplemented with Fucoidan from Phaeophyceae Algae to Promote Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus GG
Abstract
1. Introduction
2. Materials and Methods
2.1. Lactobacillus spp. Strains and Culture
2.2. Fucoidan
2.3. In Vitro Growth Trials Under Fucoidan Exposure
2.4. Estimation of the Prebiotic Effect of Fucoidan
2.5. Mathematical Modelling
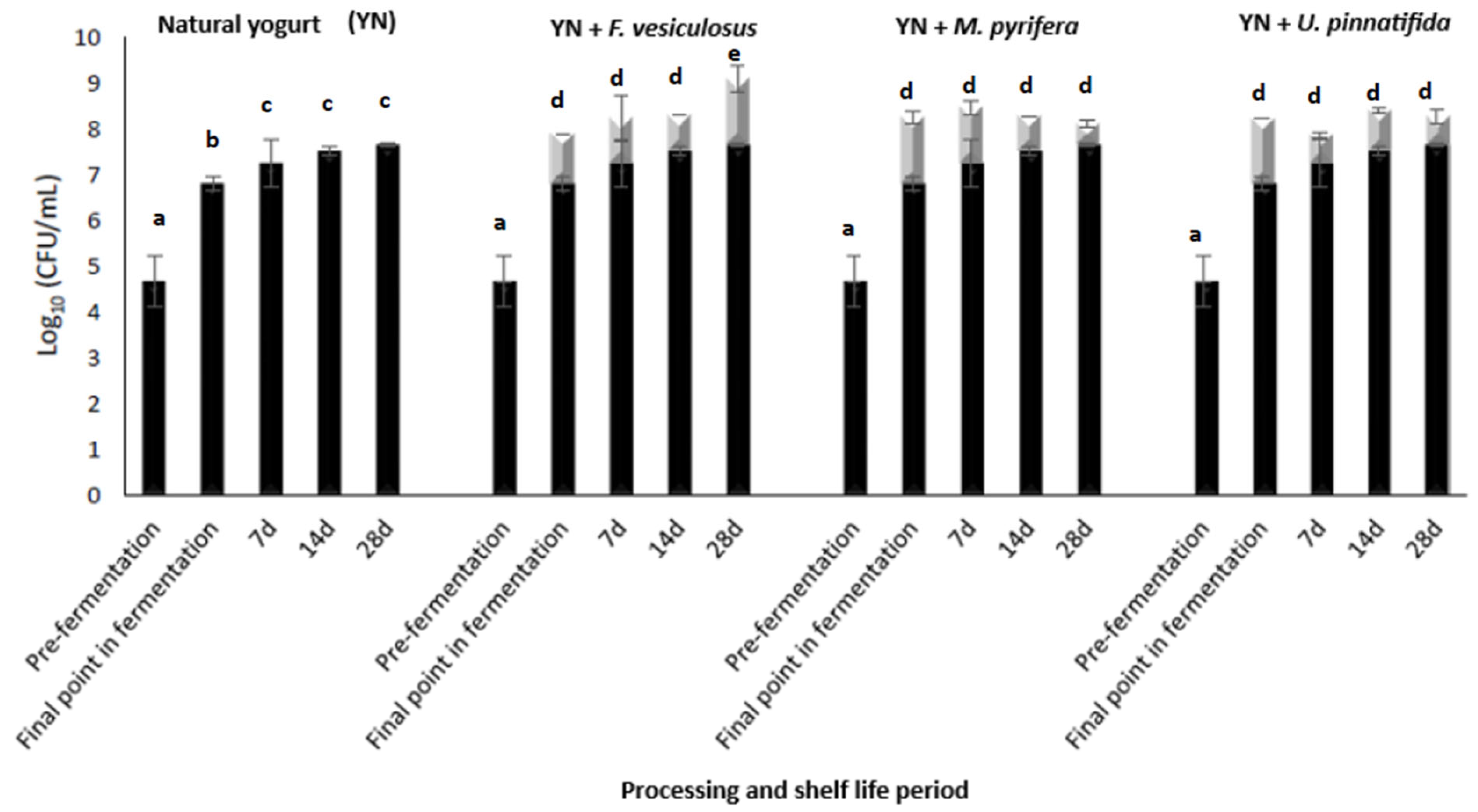
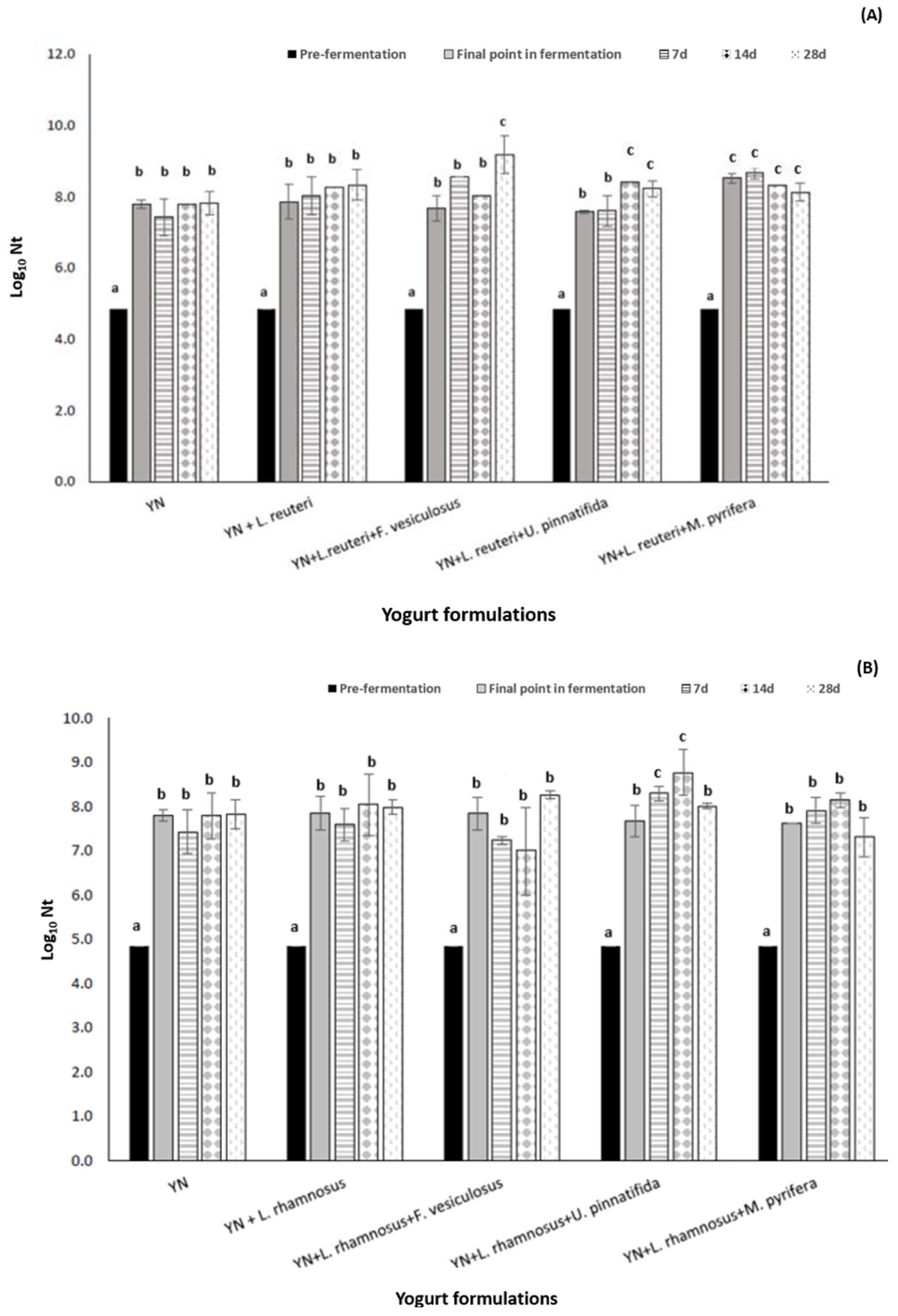
2.6. Fucoidan Yogurt Formulation and Microbiological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. The In Vitro Fucoidan Prebiotic Effect on L. reuteri and L. rhamnosus Growth
3.2. The Impact of Fucoidan Addition to L. retueri and L. rhamnosus Growth/Viability in a New Formulated Synbiotic Yogurt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Potential Utility of High-Pressure Processing to Address the Risk of Food Allergen Concerns. Comp. Rev. Food Sci. Food Saf. 2014, 13, 78–90. [Google Scholar] [CrossRef] [PubMed]
- IFFAM Integrated Approaches to Food Allergen and Allergy Risk Management. Grant Agreement EU ID: 312147. The University of Manchester. 2017. Available online: https://cordis.europa.eu/article/id/165890-food-allergy-management (accessed on 15 March 2025).
- Bastiaan-Net, S.; Pina-Pérez, M.C.P.; Dekkers, B.J.W.; Westphal, A.H.; America, A.H.P.; Ariëns, R.M.C.; de Jong, N.W.; Wichers, H.J.; Mes, J.J. Identification and in silico bioinformatics analysis of PR10 proteins in cashew nut. Protein Sci. 2020, 29, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Lynch, S.V. Microbiota in Allergy and Asthma and the Emerging Relationship with the Gut Microbiome. Cell Host Microbe 2015, 17, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Goedert, J.J.; Pu, A.; Yu, G.; Shi, J. Allergy associations with the adult fecal microbiota: Analysis of the American Gut Project. E Bio Med. 2015, 27, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Peroni, D.G.; Nuzzi, G.; Trambusti, I.; Di Cicco, M.E.; Comberiati, P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front. Immunol. 2020, 11, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Song, Y.; Wu, W.; Yu, W.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Chan, Y.L.; Tsai, M.H.; Wang, C.J.; Chiang, M.H.; Chiu, C.C. Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Org. J. 2019, 12, 100021. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.Y.; et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 2019, 178, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, Z.Q.; Yang, P.C. Treatment of Allergic Rhinitis with Probiotics: An Alternative Approach. N. Am. J. Med. Sci. 2013, 8, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Mira, A.; Artacho, A.; Abrahamsson, T.R.; Jenmalm, M.C.; Collado, M.C. Allergy development is associated with consumption of breastmilk with a reduced microbial richness in the first month of life. Pediatr. Allergy Immunol. 2019, 31, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Z.; Liu, X.; Hu, W.; Lu, W.; Lee, J.K.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes. PLoS ONE 2020, 21, e0231865. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.M.; Rao, G. Health benefits of yogurt among infants and toddlers aged 4 to 24 months: A systematic review. Nutr. Rev. 2019, 77, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Eslamia, M.; Baharb, A.; Keikhac, M.; Karbalaeid, M.; Kobyliake, N.M.; Yousefif, B. Probiotics function and modulation of the immune system in allergic diseases. Allergol. Immunopathol. 2020, 48, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Skoletzko, S. Probiotics and Prebiotics for Prevention of Food Allergy. Indications and Recommendations by Societies and Institutions. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 9–10. [Google Scholar]
- Jerzynska, J.; Stelmach, W.; Balcerak, J.; Woicka-Kolejwa, K.; Rychlik, B.; Blauz, A.; Wachulec, M.; Stelmach, P.; Majak, P.; Stelmach, J. Effect of Lactobacillus rhamnosus GG and vitamin D supplementation on the immunologic effectiveness of grass-specific sublingual immunotherapy in children with allergy. Allergy Asthma Proc. 2016, 37, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and Preventive Effects in Allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Iji, P.A.; Kadam, M.M. Chapter 21—Prebiotic properties of algae and algae-supplemented products. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2013; pp. 658–670. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Brück, W.M.; Brück, T.; Beyrer, M. Chapter 4—Microalgae as healthy ingredients for functional foods. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Academic Press: Cambridge, MA, USA, 2020; pp. 103–137. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ali Ayadi, M.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Matos, J.; Afonso, C.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M. Yogurt Enriched with Isochrysis galbana: An Innovative Functional Food. Foods 2021, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Ofosu Mensah, E.; Nabayire Kanwugu, O.; Kumar Panda, P.; Adadi, P. Marine fucoidans: Structural, extraction, biological activities and their applications in the food industry. Foods Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nie, S.; Fu, X.; Nan, S.; Ren, X.; Li, R. Exploring the prebiotic potential of hydrolyzed fucoidan fermented in vitro with human fecal inocula: Impact on microbiota and metabolome. Int. J. Biol. Macromol. 2024, 267, 131202. [Google Scholar] [CrossRef] [PubMed]
- Thang, C.L.; Baurhoo, B.; Boye, J.J.; Simpson, B.K.; Zhao, X. Effects of Lactobacillus rhamnosus GG supplementation on cow’s milk allergy in a mouse model. Allergy Asthma Clin. Immunol. 2011, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Black, P.; Stanley, T.V.; Mitchell, E.; Barthow, C.; Fitzharris, P.; Purdie, G.; Crane, J. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy 2012, 42, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Rubel, I.; Pérez, E.E.; Genovese, D.B.; Manrique, G.D. In vitro prebiotic activity of inulin-rich carbohydrates extracted from Jerusalem artichoke (Helianthus tuberosus L.) tubers at different storage times by Lactobacillus paracasei. Food Res. Int. 2014, 62, 59–65. [Google Scholar] [CrossRef]
- Belda-Galbis, C.M.; Jimenez-Carreton, A.; Pina-Perez, M.C.; Martínez, A.; Rodrigo, D. Antimicrobial activity of açaí against Listeria innocua. Food Control 2015, 53, 212–216. [Google Scholar] [CrossRef]
- Hadebe, N. Isolation and Characterization of Prebiotic Oligosaccharides from Algal Extracts and Their Effect on Gut Microflora. Doctoral Dissertation, University of Technology, Durban, South Africa, 2018; pp. 1–117. Available online: https://pdfs.semanticscholar.org/014e/eac7b4b1d8389921e88db9f4ae73acb6d817.pdf (accessed on 15 April 2025).
- Iraporda, C.; Rubel, I.A.; Manrique, G.D.; Abraham, A.G. Influence of inulin rich carbohydrates from Jerusalem artichoke (Helianthus tuberosus L.) tubers on probiotic properties of Lactobacillus strains. LWT-Food Sci. Technol. 2019, 101, 738–746. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Chapter 7—Prebiotic–Probiotic Relationship: The Genetic Fundamentals of Polysaccharides Conversion by Bifidobacterium and Lactobacillus Genera. In Food Bioconversion. Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2017; pp. 237–278. [Google Scholar] [CrossRef]
- Pérez-López, E.; Cela, D.; Costabile, A.; Mateos-Aparicio, I.; Rupérez, A. In vitro fermentability and prebiotic potential of soyabean Okara by human faecal microbiota. Br. J. Nutr. 2016, 116, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Obasola Adebola, O.; Corcoran, O.; Morgan, W.A. Synbiotics: The impact of potential prebiotics inulin, lactulose and lactobionic acid on the survival and growth of lactobacilli probiotics. J. Funct. Foods 2014, 10, 75–84. [Google Scholar] [CrossRef]
- Shibata, H.; Iimuro, M.; Uchiya, N.; Kawamori, T.; Nagaoka, M.; Ueyama, S.; Hashimoto, S.; Yokokura, T.; Sugimura, T.; Wakabayashi, K. Preventive Effects of Cladosiphon Fucoidan Against Helicobacter pylori Infection in Mongolian gerbils. Helicobacter 2003, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Golmakani, M.T.; Hadi Eskandari, M.; Hashem Hosseini, S.M. Potential prebiotic and antibacterial activities of fucoidan from Laminaria japonica. Int. J. Biol. Macromol. 2024, 268, 131776. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Gorba, C.; Pina, R.; Tortajada-Girbés, M.; Jiménez-Belenguer, A.; Siguemoto, E.; Ferrús, M.A.; Rodrigo, D.; Pina-Pérez, M.C. Caenorhabditis elegans as an in vivo model to assess fucoidan bioactivity preventing Helicobacter pylori infection. Food Funct. 2020, 10, 4525–4534. [Google Scholar] [CrossRef] [PubMed]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 9, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Hieu, B.T.N.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Sakaguchi, K.; Sakane, I. Oral Administration of Fucoidan Can Exert Anti-Allergic Activity after Allergen Sensitization by Enhancement of Galectin-9 Secretion in Blood. Biomolecules 2020, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from seaweed Fucus vesiculosus inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Pelto, L.; Isolauri, E.; Lilius, E.M.; Nuutila, J.; Salminen, S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin. Exp. Allergy 1998, 28, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Hyeog, S. Probiotics as a Potential Immunomodulating Pharmabiotics in Allergic Diseases: Current Status and Future Prospects. Allergy Asthma Immunol. Res. 2018, 10, 575–590. [Google Scholar] [CrossRef] [PubMed]
- FDA. Fucoidan from Undaria Pinnatifida; GRAS Notice No. GRN 000661. 1-888-INFO-FDA (1-888-463-6332). 2017. Available online: https://www.fda.gov/media/104562/download (accessed on 15 April 2025).
- Prabhurajeshwar, C.; Chandrakanth, K. Evaluation of antimicrobial properties and their substances against pathogenic bacteria in-vitro by probiotic Lactobacilli strains isolated from commercial yoghurt. Clin. Nutr. Exp. 2019, 23, 97–115. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, S.; Soltani, H.; Reid, G. Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as a functional food. Innov. Food Sci. Emerg. Technol. 2009, 10, 293–296. [Google Scholar] [CrossRef]
- Farhana Fazilah, N.; Ariff, A.B.; Ezuan Khayat, M.; Rios-Solis, L.; Halim, M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J. Funct. Foods 2018, 48, 387–399. [Google Scholar] [CrossRef]
- Kamel, D.G.; Hammam, A.R.A.; Alsaleem, K.A.; Osman, D.M. Addition of inulin to probiotic yogurt: Viability of probiotic bacteria (Bifidobacterium bifidum) and sensory characteristics. Food Sci. Nutr. 2021, 9, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Canbulat, Z.; Ozcan, T. Effects of Short-Chain and Long-Chain Inulin on the Quality of Probiotic Yogurt Containing Lactobacillus rhamnosus. J. Food Proc. Preserv. 2014, 39, 1251–1260. [Google Scholar] [CrossRef]
- Schouten, B.; van Esch, B.C.A.M.; Hofman, G.A.; van Doorn, S.A.C.M.; Knol, J.; Nauta, A.J.; Garssen, J.; Willemsen, L.E.M.; Knippels, L.M.J. Cow Milk Allergy Symptoms Are Reduced in Mice Fed Dietary Synbiotics during Oral Sensitization with Whey. J. Nutr. 2009, 139, 1398–1403. [Google Scholar] [CrossRef] [PubMed]

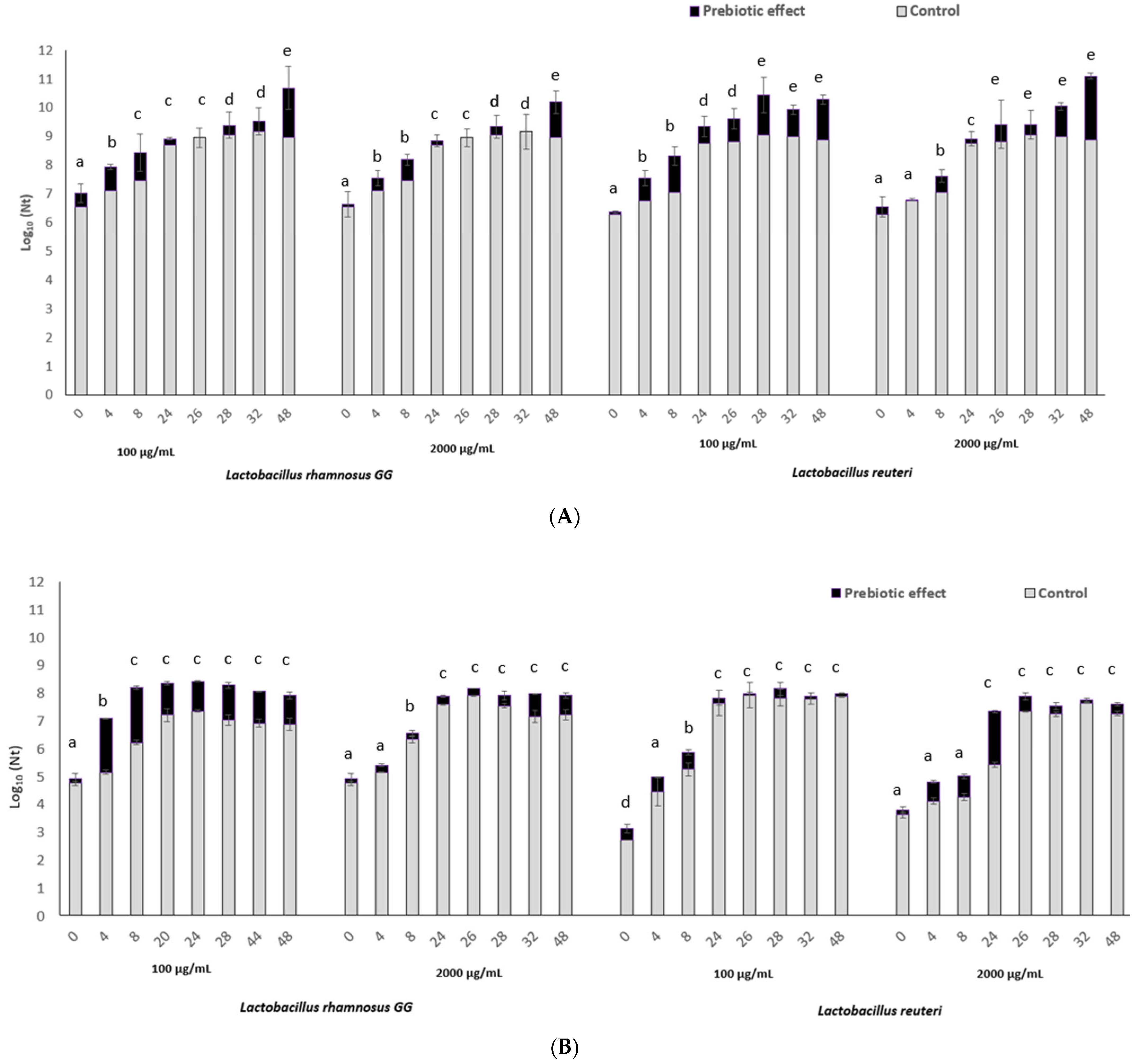
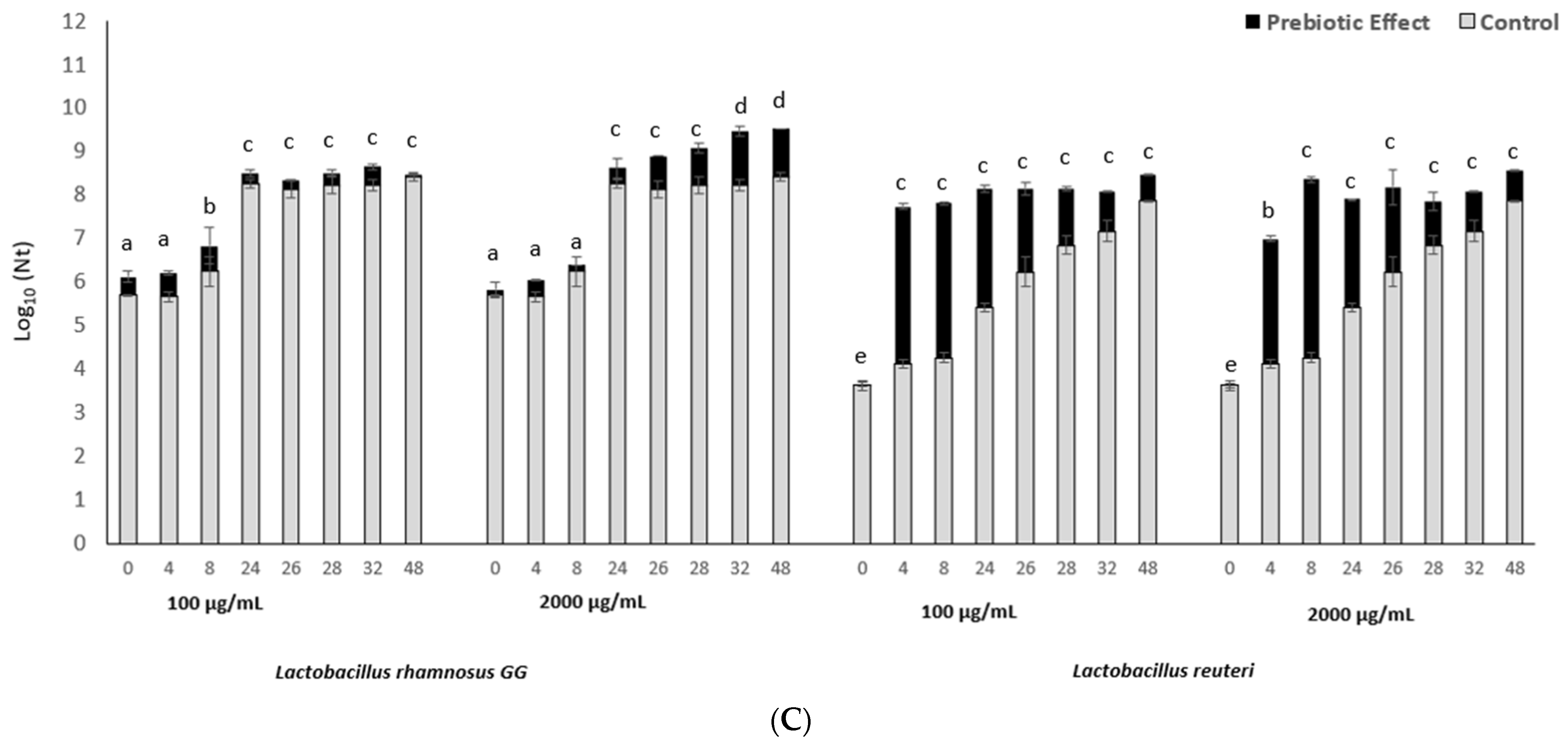
| Microbial Strain | Fucoidan Concentration (µg/mL) | A | C | B | M | R2-Adjusted | RMSE | ʎ | µmax | Af |
|---|---|---|---|---|---|---|---|---|---|---|
| Fucus vesiculosus | ||||||||||
| L. reuteri | 0 (Control) | 6.31 ± 0.19 | 2.72 ± 0.27 | 0.15 ± 0.04 | 9.04 ± 1.31 | 0.98 | 0.08 | 2.23 ± 1.23 a | 0.15 ± 0.03 a | 1.03 |
| 100 | 6.97 ± 0.87 | 7.28 ± 1.25 | 0.18 ± 0.01 | 7.23 ± 0.43 | 0.96 | 0.19 | 0.43 ± 0.67 b | 0.48 ± 0.02 b | 1.19 | |
| 2000 | 4.20 ± 0.12 | 15.98 ± 0.67 | 0.21 ± 0.01 | 4.12 ± 1.22 | 0.93 | 0.18 | 0.85 ± 0.11 b | 1.23 ± 0.04 c | 1.18 | |
| L. rhamnosus GG | 0 (Control) | 6.37 ± 0.09 | 2.76 ± 0.08 | 0.13 ± 0.01 | 6.91 ± 1.15 | 0.98 | 0.09 | 0.68 ± 0.23 a | 0.13 ± 0.07 a | 1.03 |
| 100 | 5.03 ± 0.45 | 12.72 ± 0.24 | 0.14 ± 0.04 | 5.11 ± 1.43 | 0.91 | 0.23 | 0.99 ± 0.14 a | 1.55 ± 0.11 b | 1.09 | |
| 2000 | 3.11 ± 0.41 | 13.68 ± 0.24 | 0.25 ± 0.04 | 6.23 ± 2.22 | 0.92 | 0.19 | −0.24 ± 0.06 b | 1.34 ± 0.22 c | 1.11 | |
| Undaria pinnatifida | ||||||||||
| L. reuteri | 0 (Control) | 0.03 ± 0.01 | 8.01 ± 0.32 | 0.13 ± 0.01 | 0.5 ± 0.05 | 0.99 | 0.11 | −24.1 ± 1.12 a | 0.37 ± 0.03 a | 1.22 |
| 100 | 2.17 ± 0.22 | 5.76 ± 0.87 | 0.24 ± 0.02 | 4.71 ± 0.36 | 0.99 | 0.07 | −7.12 ± 1.23 a | 0.50 ± 0.01 b | 1.12 | |
| 2000 | 0.59 ± 0.06 | 7.18 ± 0.25 | 0.09 ± 0.04 | 1.71 ± 0.82 | 0.97 | 0.21 | −38.1 ± 2.58 a | 0.53 ± 0.01 b | 1.03 | |
| L. rhamnosus GG | 0 (Control) | 6.35 ± 0.56 | 8.22 ± 0.36 | 0.13 ± 0.01 | 6.81 ± 0.11 | 0.98 | 0.09 | −0.50 ± 0.05 a | 0.20 ± 0.03 a | 1.04 |
| 100 | 4.91 ± 0.08 | 8.56 ± 0.25 | 1.04 ± 0.37 | 3.15 ± 0.17 | 0.98 | 0.10 | −1.75 ± 0.36 b | 0.65 ± 0.01 b | 1.15 | |
| 2000 | 4.90 ± 0.32 | 9.23 ± 0.11 | 0.51 ± 0.08 | 7.75 ± 0.22 | 0.99 | 0.09 | −3.16 ± 0.85 b | 1.26 ± 0.01 b | 1.23 | |
| Macrocystis pyrifera | ||||||||||
| L. reuteri | 0 (Control) | 3.99 ± 0.71 | 6.81 ± 0.51 | 0.25 ± 0.03 | 23.7 ± 1.23 | 0.98 | 0.16 | 13.10 ± 1.74 a | 0.75 ± 0.03 a | 1.08 |
| 100 | 3.22 ± 0.12 | 8.36 ± 1.32 | 0.66 ± 0.12 | 3.63 ± 0.87 | 0.98 | 0.09 | −0.51 ± 1.21 b | 2.55 ± 0.01 b | 1.03 | |
| 2000 | 3.53 ± 0.08 | 8.61 ± 0.15 | 3.37 ± 1.22 | 3.33 ± 0.62 | 0.98 | 0.15 | 2.78 ± 1.23 b | 5.71 ± 0.07 c | 1.11 | |
| L. rhamnosus GG | 0 (Control) | 4.75 ± 0.66 | 6.31 ± 0.12 | 0.35 ± 0.03 | 5.67 ± 0.73 | 0.97 | 0.011 | −2.39 ± 0.26 a | 0.29 ± 0.03 a | 1.25 |
| 100 | 5.22 ± 0.24 | 8.69 ± 1.21 | 0.36 ± 0.05 | 9.11 ± 1.21 | 0.99 | 0.06 | 2.80 ± 0.15 b | 0.43 ± 0.01 b | 1.04 | |
| 2000 | 5.61 ± 0.13 | 8.23 ± 0.65 | 0.23 ± 0.12 | 9.67 ± 1.32 | 0.99 | 0.06 | 3.36 ± 0.33 b | 0.52 ± 0.01 b | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricós-Muñoz, N.; Maicas, S.; Tortajada-Girbés, M.; Pina-Pérez, M.C. Novel Synbiotic Yogurt Formulation Supplemented with Fucoidan from Phaeophyceae Algae to Promote Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus GG. Foods 2025, 14, 2589. https://doi.org/10.3390/foods14152589
Ricós-Muñoz N, Maicas S, Tortajada-Girbés M, Pina-Pérez MC. Novel Synbiotic Yogurt Formulation Supplemented with Fucoidan from Phaeophyceae Algae to Promote Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus GG. Foods. 2025; 14(15):2589. https://doi.org/10.3390/foods14152589
Chicago/Turabian StyleRicós-Muñoz, Neus, Sergi Maicas, Miguel Tortajada-Girbés, and Maria Consuelo Pina-Pérez. 2025. "Novel Synbiotic Yogurt Formulation Supplemented with Fucoidan from Phaeophyceae Algae to Promote Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus GG" Foods 14, no. 15: 2589. https://doi.org/10.3390/foods14152589
APA StyleRicós-Muñoz, N., Maicas, S., Tortajada-Girbés, M., & Pina-Pérez, M. C. (2025). Novel Synbiotic Yogurt Formulation Supplemented with Fucoidan from Phaeophyceae Algae to Promote Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus GG. Foods, 14(15), 2589. https://doi.org/10.3390/foods14152589






