Untargeted Metabolomics Reveals Distinct Anthocyanin Profiles in Napier Grass (Pennisetum purpureum Schumach.) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Reagents
2.3. Sample Preparation
2.4. Instruments and Conditions
2.5. Identification Strategy
2.6. Quantification Criteria
2.7. Differential Metabolites Selected
3. Results and Discussion
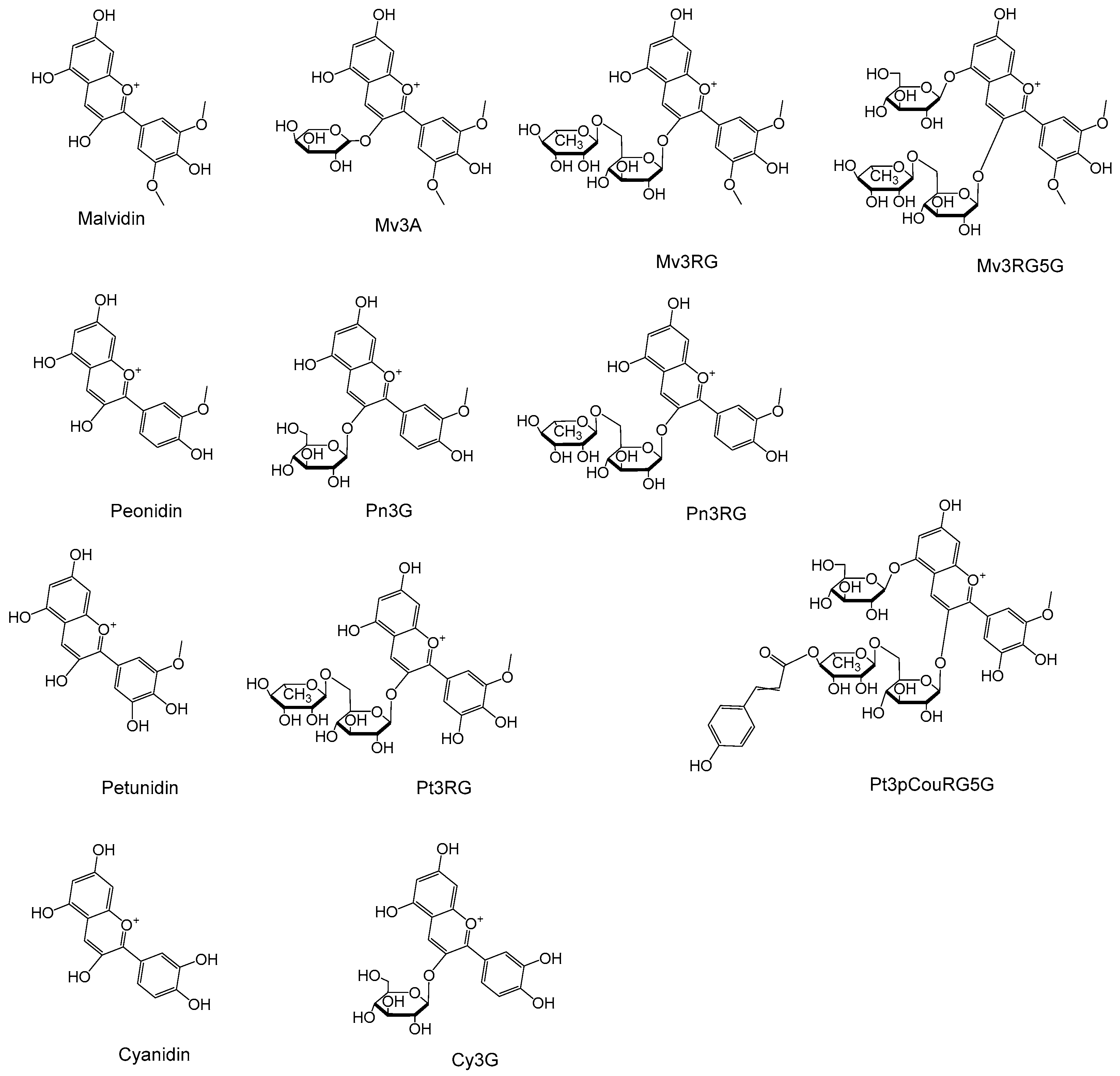
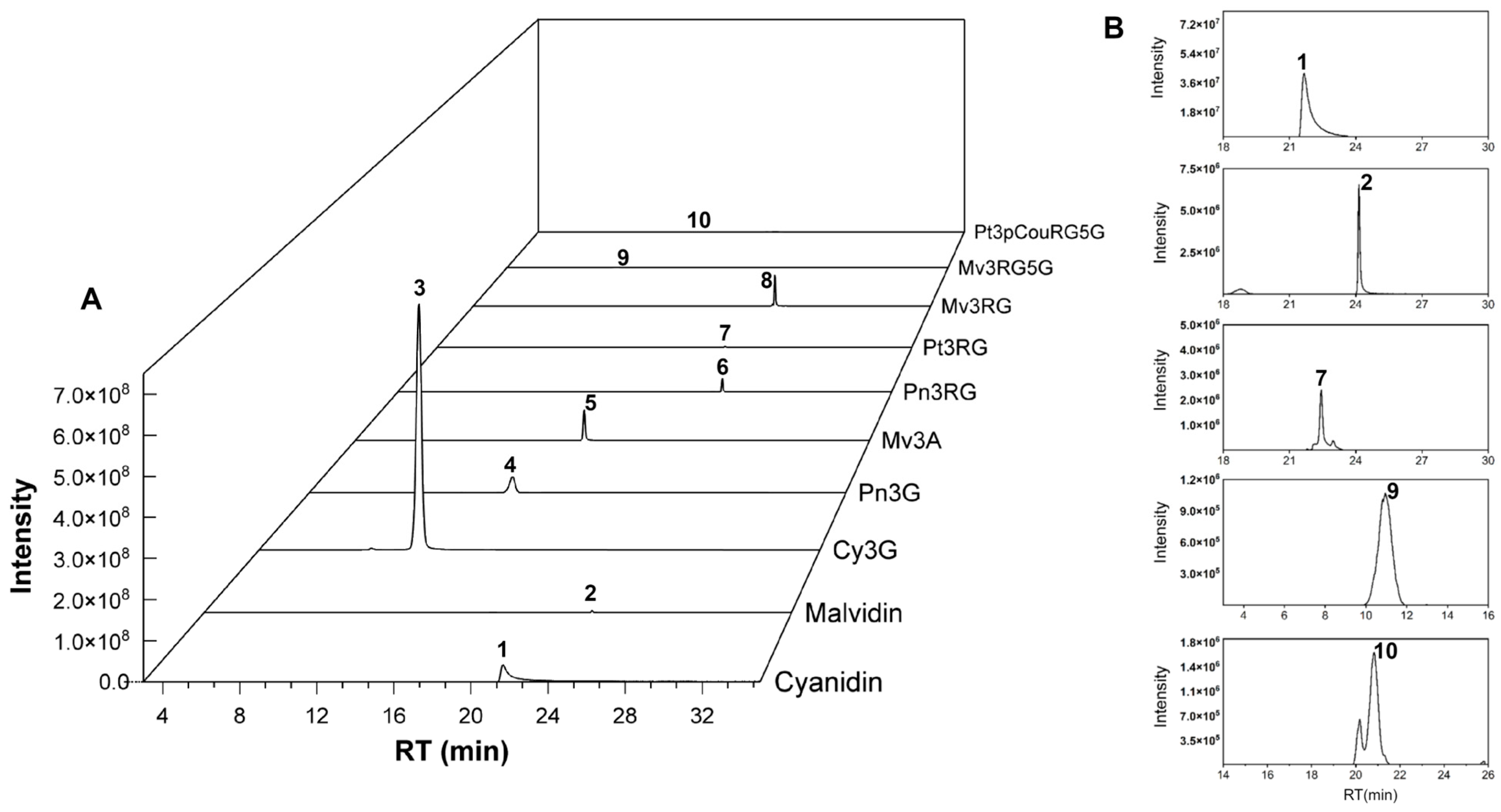
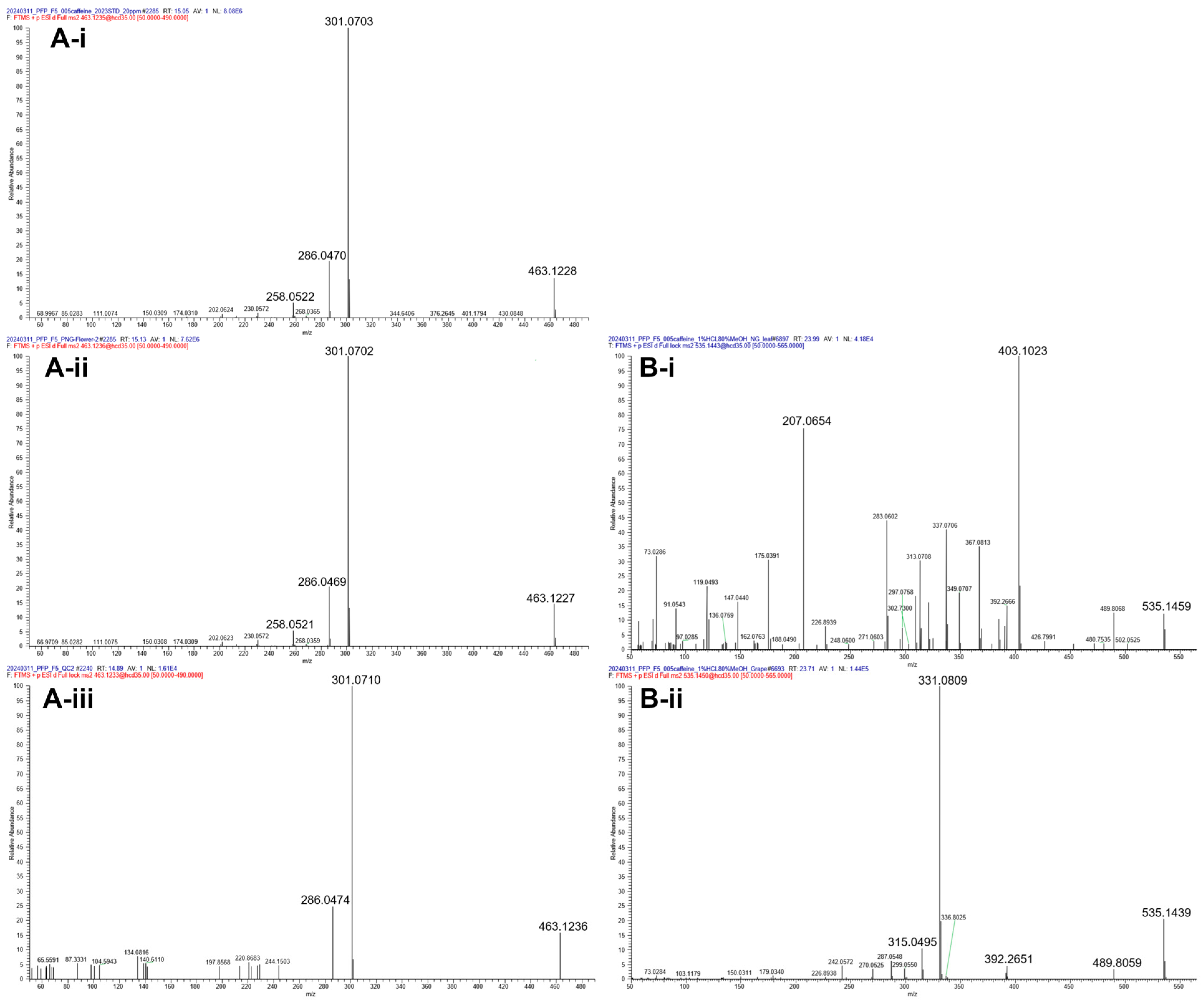
3.1. Qualitative Analysis of Anthocyanidins and Anthocyanins
| Peak NO | Tentative Identification | RT (min) | Molecular Formula | [M]+ Theoretical m/z | [M]+ Observed m/z (Mass Error) | Characteristic Fragment Ion | UV/Vis Absorption Bands (nm) | Level | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cyanidin | 21.67 | C15H11O6+ | 287.0550 | 287.0545 (1.7) | 269.0444 [M-H2O]2•+ 241.0495 [M-CO-H2O]2•+ 213.0546 [M-2CO-H2O]2•+ 137.0233 [M-C6H4-2CO-H2O]2•+ | 520 | 1 | [15,16] |
| 2 | Malvidin | 23.90 | C17H15O7+ | 331.0807 | 331.0813 (1.8) | 315.0496 [M-CH3-H]2•+ 270.0523 [M-CH3OH-COH]•+ 242.0571 [M-CH3OH-COH-CO]•+ | 520 | 1 | [15,17] |
| 3 | Cy3G | 12.15 | C21H21O11+ | 449.1078 | 449.1072 (1.3) | 287.0545 [M-Glc]+ | 235,520,280 | 1 | [15] |
| 4 | Pn3G | 16.32 | C22H23O11+ | 463.1235 | 463.1229 (1.3) | 301.0703 [M-Glc]+ | 234,520 | 1 | [15] |
| 5 | Mv3A | 17.22 | C22H23O11+ | 463.1235 | 463.1236 (0.2) | 331.0809 [M-Ara]+ | 234,520,350 | 1 | [15] |
| 6 | Pn3RG | 24.04 | C28H33O15+ | 609.1814 | 609.1814 (0.0) | 463.1231 [M-Rha]+ 301.0702 [M-Rha-Glc]+ | 235,520 | 2 | [17,18] |
| 7 | Pt3RG | 22.49 | C28H33O16+ | 625.1763 | 625.1764 (0.1) | 463.1240 [M-Rha-H2O]+ 317.0656 [M-Rha-Glc]+ 301.0707 [M-Rha-Glc-H2O]+ | 233 | 2 | [18] |
| 8 | Mv3RG | 24.10 | C29H35O16+ | 639.1920 | 639.1921 (0.2) | 493.1337 [M-Rha]+ 331.0810 [M-Rha-Glc]+ 315.0497 [M-Rha-Glc-H2O]+ | 235,520 | 2 | [18] |
| 9 | Mv3RG5G | 10.95 | C35H45O21+ | 801.2448 | 801.2445 (0.3) | 639.1932 [M-Glc]+ 493.1342 [M-Rha-Glc]+ 331.0811 [M-Rha-2Glc]+ 315.0500 [M-Rha-Glc-H2O]+ | 234 | 2 | [18] |
| 10 | Pt3pCouRG5G | 20.88 | C43H49O23+ | 933.2659 | 933.2656 (0.3) | 771.2119 [M-Glc]+ 479.1179 [M-C9H8O3-Rha-Glc]+ 317.0655 [M-C9H8O3-Rha-2Glc]+ | 234 | 2 | [18] |
| IS | Caffeine | 8.81 | C8H10N4O2 | 195.0877 * | 195.0877 (0.0) | 138.0662, 109.9588 | 1 |
3.2. Relative Changes in Anthocyanin Abundance Across Three Cultivars and Different Organs of TS5
3.3. Results from the Quantitative Analysis of Anthocyanins
3.3.1. Comparison of Anthocyanin Levels in TS2, TS5, and TS6
3.3.2. Organ-Specific Anthocyanin Distribution in TS5
3.4. Compound-Level Insights into Anthocyanin-Related Metabolite Accumulation in Napier Grass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cy3G | Cyanidin 3-O-glucoside |
| Mv3A | Malvidin 3-O-arabinoside |
| Pn3G | Peonidin 3-O-glucoside |
| Pn3RG | Peonidin 3-O-rutinoside |
| Pt3RG | Petunidin 3-O-rutinoside |
| Mv3RG | Malvidin 3-O-rutinoside |
| Mv3RG5G | Malvidin 3-O-rutinoside-5-O-glucoside |
| Pt3pCouRG5G | Petunidin 3-O-(p-coumaroyl)rutinoside-5-O-glucoside |
| Cy5G | Cyanidin 5-O-glucoside |
| Cy3G5G | Cyanidin 3-O-glucoside-5-O-glucoside |
| Cy3RG | Cyanidin 3-O-rutinoside |
| Pentahydroxychalcone4′OG | 2′,3,4,4′,6′-Pentahydroxychalcone 4′-O-β-D-glucoside |
| Aureusidin6OG | Aureusidin 6-O-glucoside |
| Hesperetin7OG | Hesperetin 7-O-glucoside |
| UHPLC-HRMS | Ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry |
| NCE | Normalized collision energy |
| PVDF | Polyvinylidene difluoride |
| QC | Quality control |
| OPLS-DA | Orthogonal partial-least-squares discriminant analysis |
| DIA | Data-independent acquisition |
| XICs | Extracted ion chromatograms |
| TS2 | Taishiu No. 2 |
| TS5 | Taishiu No. 5 |
| TS6 | Taishiu No. 6 |
| L | Leaves |
| F | Flowers |
| St | Ground tissue of stem |
| Sk | Epidermis of stem |
| CHS | Chalcone synthase |
| CHI | Chalcone isomerase |
| UDP-Rha | UDP-rhamnose |
| F3H | Flavanone 3-hydroxylase |
References
- Thaisungnoen, K.; Umar, M.; Singh, M.; Anal, A. Ultrasonic-Assisted Extraction of Bioactive Extract from Napier Grass (Pennisetum purpureum), Evaluation of Its Bioactivity, Antimutagenicity and Cytotoxicity. Nat. Life Sci. Commun. 2024, 23, e2024014. [Google Scholar] [CrossRef]
- Jack, I.; Clark, P.; Ndukwe, G. Evaluation of Phytochemical, Antimicrobial and Antioxidant Capacities of Pennisetum purpureum (Schumach) Extracts. Chem. Sci. Int. J. 2020, 29, 1–14. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ojo, A.B.; Barnabas, M.; Iyobhebhe, M.; Elebiyo, T.C.; Evbuomwan, I.O.; Michael, T.; Ajiboye, B.O.; Oyinloye, B.E.; Oloyede, O.I. Phytochemical properties and pharmacological activities of the genus Pennisetum: A review. Sci. Afr. 2022, 16, e01132. [Google Scholar] [CrossRef]
- Ho, P.Y.; Koh, Y.C.; Lu, T.J.; Liao, P.L.; Pan, M.H. Purple Napiergrass (Pennisetum purpureum Schumach) Hot Water Extracts Ameliorate High-Fat Diet-Induced Obesity and Metabolic Disorders in Mice. J. Agric. Food Chem. 2023, 71, 20701–20712. [Google Scholar] [CrossRef] [PubMed]
- Okaraonye, C.; Ikewuchi, J. Nutritional and antinutritional components of Pennisetum purpureum (Schumach). Pak. J. Nutr. 2009, 8, 32–34. [Google Scholar] [CrossRef][Green Version]
- Tsai, P.-J.; Wu, S.-C.; Cheng, Y.-K. Role of polyphenols in antioxidant capacity of napiergrass from different growing seasons. Food Chem. 2008, 106, 27–32. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the biological activity of anthocyanins and polyphenols in blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Duncan, G.J.; Neacsu, M.; Russell, W.R. Rapid method for quantification of anthocyanidins and anthocyanins in human biological samples. Food Chem. 2019, 290, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Onjai-uea, N.; Paengkoum, S.; Taethaisong, N.; Sorasak, T.; Sinpru, B.; Surakhunthod, J.; Meethip, W.; Purba, R.; Paengkoum, P. Effect of Cultivar, Plant Spacing and Harvesting Age on Yield, Characteristics, Chemical Composition, and Anthocyanin Composition of Purple Napier Grass. Animals 2022, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, J.; Lai, Y.; Yin, G.; Chen, P.; Pennerman, K.K.; Yan, H.; Wu, B.; Zhang, H.; Yi, X.; et al. Integrative analysis of metabolome and transcriptome reveals anthocyanins biosynthesis regulation in grass species Pennisetum purpureum. Ind. Crops Prod. 2019, 138, 111470. [Google Scholar] [CrossRef]
- Bittremieux, W.; Wang, M.; Dorrestein, P.C. The critical role that spectral libraries play in capturing the metabolomics community knowledge. Metabolomics 2022, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Chen, J.; Huang, W.; Huang, G.; Deng, M.; Hong, S.; Ai, P.; Gao, C.; Zhou, H. OmicShare tools: A zero-code interactive online platform for biological data analysis and visualization. Imeta 2024, 3, e228. [Google Scholar] [CrossRef] [PubMed]
- García-Beneytez, E.; Cabello, F.; Revilla, E. Analysis of Grape and Wine Anthocyanins by HPLC-MS. J. Agric. Food Chem. 2003, 51, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Šmídová, B.; Šatínský, D.; Dostálová, K.; Solich, P. The pentafluorophenyl stationary phase shows a unique separation efficiency for performing fast chromatography determination of highbush blueberry anthocyanins. Talanta 2017, 166, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Straßmann, S.; Passon, M.; Schieber, A. Chemical Hemisynthesis of Sulfated Cyanidin-3-O-Glucoside and Cyanidin Metabolites. Molecules 2021, 26, 2146. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Schug, K.A. Structural characterization of cyanidin-3,5-diglucoside and pelargonidin-3,5-diglucoside anthocyanins: Multi-dimensional fragmentation pathways using high performance liquid chromatography-electrospray ionization-ion trap-time of flight mass spectrometry. Int. J. Mass Spectrom. 2011, 308, 71–80. [Google Scholar] [CrossRef]
- Xu, W.; Luo, G.; Yu, F.; Jia, Q.; Zheng, Y.; Bi, X.; Lei, J. Characterization of anthocyanins in the hybrid progenies derived from Iris dichotoma and I. domestica by HPLC-DAD-ESI/MS analysis. Phytochemistry 2018, 150, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Whittall, J.B.; Voelckel, C.; Kliebenstein, D.J.; Hodges, S.A. Convergence, constraint and the role of gene expression during adaptive radiation: Floral anthocyanins in Aquilegia. Mol. Ecol. 2006, 15, 4645–4657. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Rubinskiene, M.; Jasutiene, I.; Venskutonis, P.R.; Viskelis, P. HPLC determination of the composition and stability of blackcurrant anthocyanins. J. Chromatogr. Sci. 2005, 43, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Color and stability of anthocyanins in strawberry nectars containing various co-pigment sources and sweeteners. Food Chem. 2020, 310, 125856. [Google Scholar] [CrossRef] [PubMed]
- Sendri, N.; Bhandari, P. Anthocyanins: A comprehensive review on biosynthesis, structural diversity, and industrial applications. Phytochem. Rev. 2024, 23, 1913–1974. [Google Scholar] [CrossRef]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Chen, W.-K.; Yu, K.-J.; Ji, X.-N.; Duan, C.-Q.; Reeves, M.J.; Wang, J. Molecular and biochemical characterization of the UDP-glucose: Anthocyanin 5-O-glucosyltransferase from Vitis amurensis. Phytochemistry 2015, 117, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Liu, S.; Fan, L.; Yang, J.; Li, H.; Xia, Y.; Li, Y. Nutrient stoichiometric and resorption characteristics of the petals of four common urban greening Rosaceae tree species. Front. Plant Sci. 2023, 14, 1201759. [Google Scholar] [CrossRef] [PubMed]
- Kitao, M.; Yazaki, K.; Tobita, H.; Agathokleous, E.; Kishimoto, J.; Takabayashi, A.; Tanaka, R. Anthocyanins act as a sugar-buffer and an alternative electron sink in response to starch depletion during leaf senescence: A case study on a typical anthocyanic tree species, Acer japonicum. J. Exp. Bot. 2024, 75, 3521–3541. [Google Scholar] [CrossRef] [PubMed]
- Saffer, A.M.; Irish, V.F. Flavonol rhamnosylation indirectly modifies the cell wall defects of RHAMNOSE BIOSYNTHESIS1 mutants by altering rhamnose flux. Plant J. 2018, 94, 649–660. [Google Scholar] [CrossRef] [PubMed]

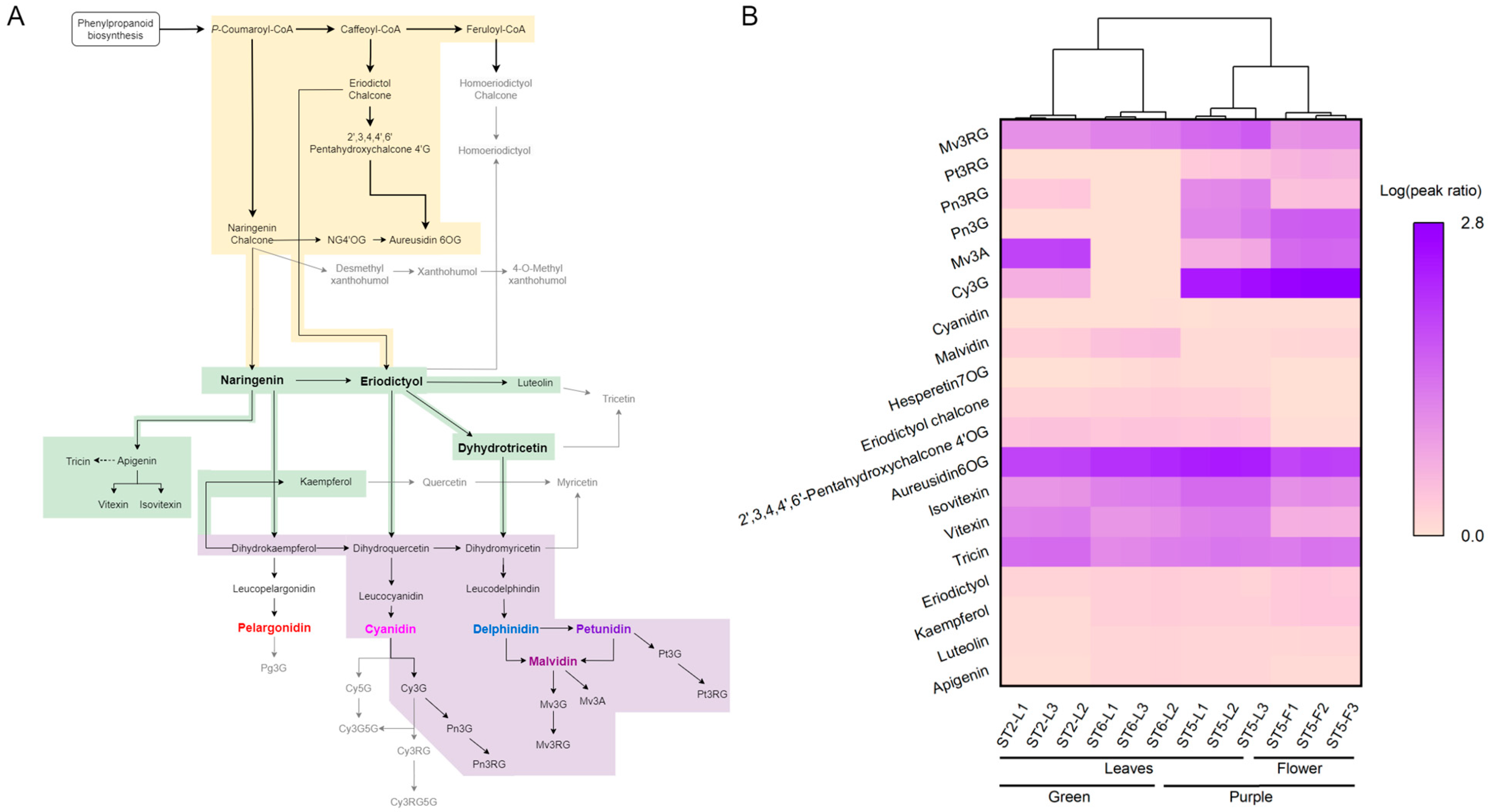
| Compounds | Samples (μg/g) | |||||
|---|---|---|---|---|---|---|
| Green | Purple | |||||
| TS2-L | TS6-L | TS5-L | TS5-F | TS5-St | TS5-Sk | |
| Malvidin | Trace | Trace | Trace | Trace | N.D. | Trace |
| Cyanidin * | Trace | Trace | Trace | Trace | N.D. | Trace |
| Cy3G | 31.99 ± 2.80 | N.D. | 2470.67 ± 504.49 | 5019.13 ± 451.39 | 24.50 ± 1.21 | 508.39 ± 21.64 |
| Mv3A | 703.85 ± 38.01 | N.D. | Trace | 224.73 ± 20.72 | Trace | N.D. |
| Pn3G | N.D. | N.D. | 119.89 ± 26.84 | 314.97 ± 21.85 | 1.84 ± 0.09 | 27.88 ± 1.32 |
| Pn3RG * | Trace | N.D. | 74.56 ± 24.58 | Trace | Trace | 12.10 ± 2.22 |
| Pt3RG * | Trace | N.D. | Trace | Trace | Trace | Trace |
| Mv3RG * | 43.56 ± 3.12 | 94.99 ± 15.09 | 256.70 ± 68.50 | 46.60 ± 6.74 | Trace | 47.86 ± 6.87 |
| Mv3RG5G * | N.D. | N.D. | N.D. | Trace | N.D. | N.D. |
| Pt3pCouRG5G * | N.D. | Trace | N.D. | N.D. | N.D. | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-Y.; Lin, P.-Y.; Hong, C.-Y.; Chou, K.C.-C.; Lu, T.-J. Untargeted Metabolomics Reveals Distinct Anthocyanin Profiles in Napier Grass (Pennisetum purpureum Schumach.) Cultivars. Foods 2025, 14, 2582. https://doi.org/10.3390/foods14152582
Wang Z-Y, Lin P-Y, Hong C-Y, Chou KC-C, Lu T-J. Untargeted Metabolomics Reveals Distinct Anthocyanin Profiles in Napier Grass (Pennisetum purpureum Schumach.) Cultivars. Foods. 2025; 14(15):2582. https://doi.org/10.3390/foods14152582
Chicago/Turabian StyleWang, Zhi-Yue, Pei-Yin Lin, Chwan-Yang Hong, Kevin Chi-Chung Chou, and Ting-Jang Lu. 2025. "Untargeted Metabolomics Reveals Distinct Anthocyanin Profiles in Napier Grass (Pennisetum purpureum Schumach.) Cultivars" Foods 14, no. 15: 2582. https://doi.org/10.3390/foods14152582
APA StyleWang, Z.-Y., Lin, P.-Y., Hong, C.-Y., Chou, K. C.-C., & Lu, T.-J. (2025). Untargeted Metabolomics Reveals Distinct Anthocyanin Profiles in Napier Grass (Pennisetum purpureum Schumach.) Cultivars. Foods, 14(15), 2582. https://doi.org/10.3390/foods14152582







