Advancing Kiwifruit Maturity Assessment: A Comparative Study of Non-Destructive Spectral Techniques and Predictive Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Non-Destructive Analysis
2.2.1. Hyperspectral Methodology
2.2.2. Colour Parameters Acquired by the Computer Vision System
2.3. Destructive Analysis
2.3.1. Firmness, Titratable Acidity, pH, Soluble Solids Content, and Dry Matter
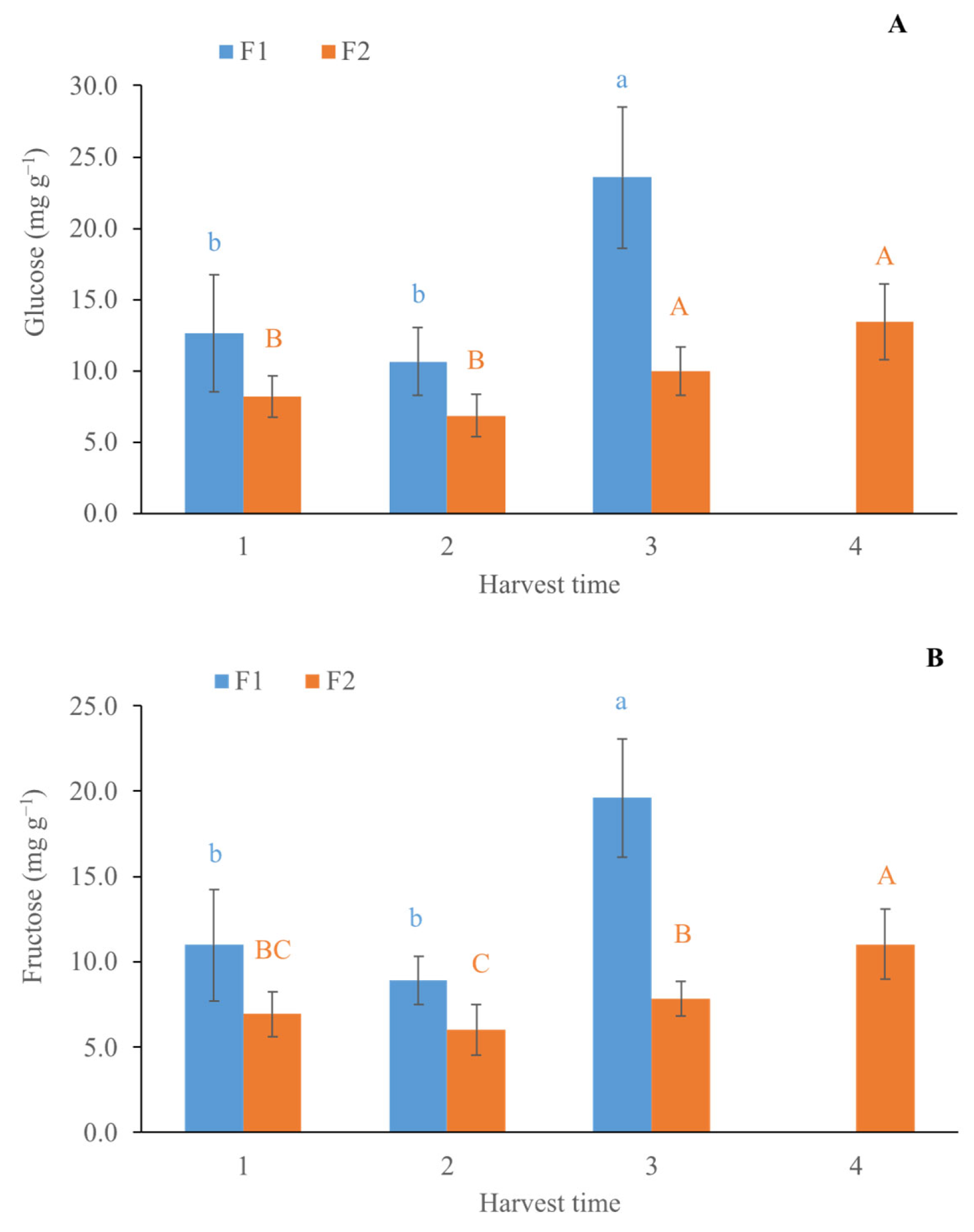
2.3.2. Determination of Glucose and Fructose
2.4. Statistical Analysis
3. Results and Discussion
3.1. Results on Colour Parameters Acquired by the Computer Vision System
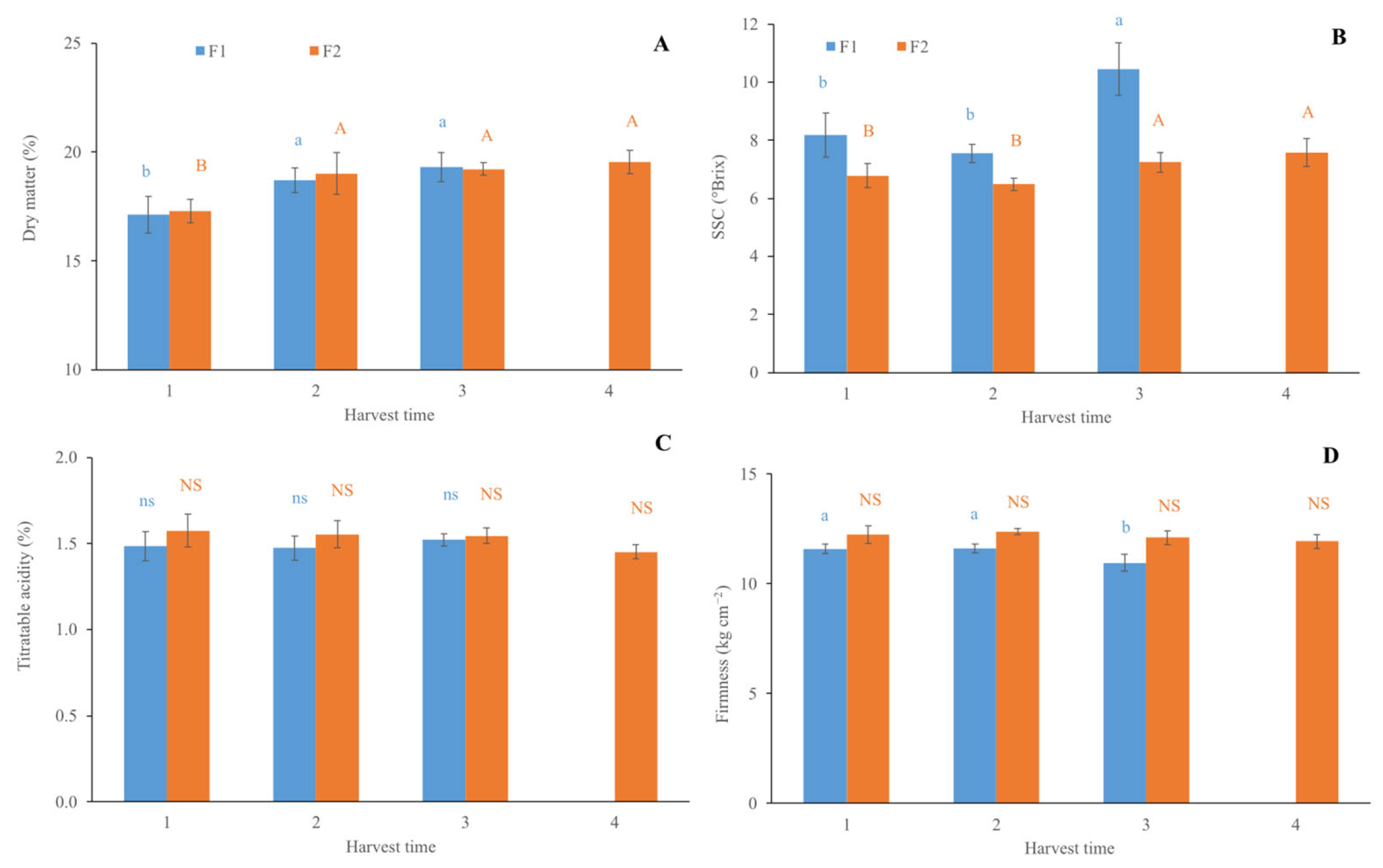
3.2. Quality Parameters from Destructive Analysis
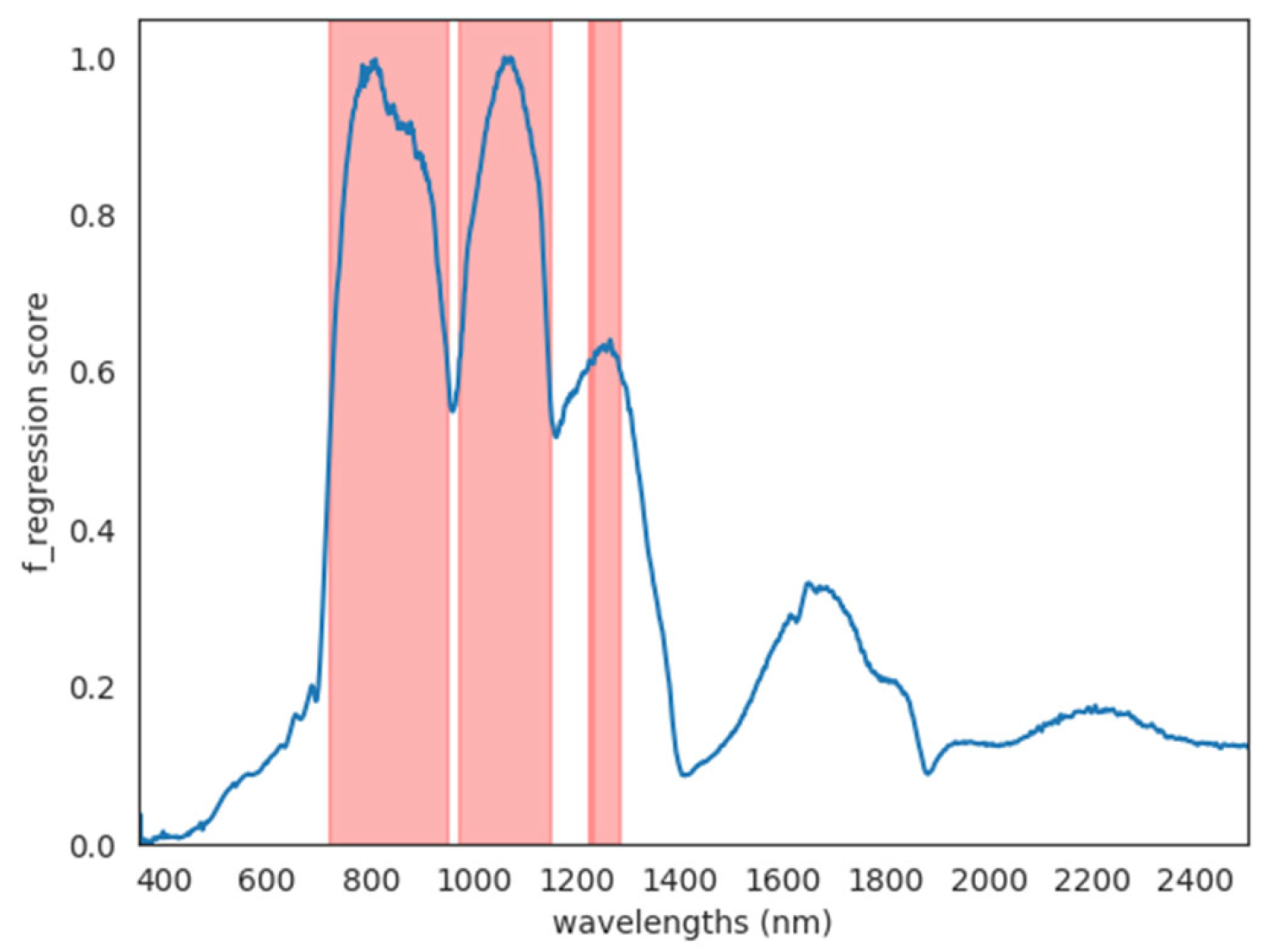
3.3. Prediction of Soluble Solids Content and Sugars by Hyperspectral Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; FAOSTAT. Production Statistics, Crops. 2024. Available online: https://www.fao.org/faostat/en/#home (accessed on 10 January 2025).
- Tilahun, S.; Choi, H.R.; Park, D.S.; Lee, Y.M.; Choi, J.H.; Baek, M.W.; Hyok, K.; Park, S.M.; Jeong, C.S. Ripening Quality of Kiwifruit Cultivars Is Affected by Harvest Time. Sci. Hortic. 2020, 261, 108936. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient Compositions and Antioxidant Capacity of Kiwifruit (Actinidia) and Their Relationship with Flesh Color and Commercial Value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.; Pidakala, P.; Martin, P.; McAtee, P.A.; Boldingh, H.L.; Hall, A.; Schaffer, R.J. Postharvest Performance of the Yellow-Fleshed ‘Hort16A’ Kiwifruit in Relation to Fruit Maturation. Postharvest Biol. Technol. 2014, 92, 98–106. [Google Scholar] [CrossRef]
- Cozzolino, R.; De Giulio, B.; Petriccione, M.; Martignetti, A.; Malorni, L.; Zampella, L.; Laurino, C.; Pellicano, M.P. Comparative Analysis of Volatile Metabolites, Quality and Sensory Attributes of Actinidia Chinensis Fruit. Food Chem. 2020, 316, 126340. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.; Pidakala, P.; Martin, P.; Billing, D.; Boldingh, H. Fruit Maturation and the Soluble Solids Harvest Index for ‘Hayward’ Kiwifruit. Sci. Hortic. 2016, 213, 193–198. [Google Scholar] [CrossRef]
- Asiche, W.O.; Witere Mitalo, O.; Kasahara, Y.; Tosa, Y.; Gituma Mworia, E.; Ushijima, K.; Nakano, R.; Kubo, Y. Effect of Storage Temperature on Fruit Ripening in Three Kiwifruit Cultivars. Hortic. J. 2017, 86, 403–410. [Google Scholar] [CrossRef]
- Famiani, F.; Baldicchi, A.; Farinelli, D.; Cruz-Castillo, J.G.; Marocchi, F.; Mastroleo, M.; Moscatello, S.; Proietti, S.; Battistelli, A. Yield Affects Qualitative Kiwifruit Characteristics and Dry Matter Content May Be an Indicator of Both Quality and Storability. Sci. Hortic. 2012, 146, 124–130. [Google Scholar] [CrossRef]
- Lim, Y.J.; Eom, S.H. Kiwifruit Cultivar ‘Halla Gold’ Functional Component Changes during Preharvest Fruit Maturation and Postharvest Storage. Sci. Hortic. 2018, 234, 134–139. [Google Scholar] [CrossRef]
- Commission Regulation (EC). Commission Regulation (EC) No. 1221/2008. Official J. Eur. Union 2008, L336, 1–80. [Google Scholar]
- Afonso, A.M.; Antunes, M.D.; Cruz, S.; Cavaco, A.M.; Guerra, R. Non-Destructive Follow-up of ‘Jintao’ Kiwifruit Ripening through VIS-NIR Spectroscopy—Individual vs. Average Calibration Model’s Predictions. Postharvest Biol. Technol. 2022, 188, 111895. [Google Scholar] [CrossRef]
- Afonso, A.M.; Guerra, R.; Cruz, S.; Antunes, M.D. Sensory Evaluation and Spectra Evolution of Two Kiwifruit Cultivars during Cold Storage. Horticulturae 2023, 9, 772. [Google Scholar] [CrossRef]
- Benelli, A.; Cevoli, C.; Fabbri, A.; Ragni, L. Ripeness Evaluation of Kiwifruit by Hyperspectral Imaging. Biosyst. Eng. 2022, 223, 42–52. [Google Scholar] [CrossRef]
- Berardinelli, A.; Benelli, A.; Tartagni, M.; Ragni, L. Kiwifruit Flesh Firmness Determination by a NIR Sensitive Device and Image Multivariate Data Analyses. Sens. Actuators Phys. 2019, 296, 265–271. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Paliotta, M.; Amoriello, T.; Carbone, K. FT-NIR Spectroscopy and Multivariate Classification Strategies for the Postharvest Quality of Green-Fleshed Kiwifruit Varieties. Sci. Hortic. 2019, 257, 108622. [Google Scholar] [CrossRef]
- Zhu, H.; Chu, B.; Fan, Y.; Tao, X.; Yin, W.; He, Y. Hyperspectral Imaging for Predicting the Internal Quality of Kiwifruits Based on Variable Selection Algorithms and Chemometric Models. Sci. Rep. 2017, 7, 7845. [Google Scholar] [CrossRef] [PubMed]
- McGlone, V.A.; Jordan, R.B.; Seelye, R.; Martinsen, P.J. Comparing Density and NIR Methods for Measurement of Kiwifruit Dry Matter and Soluble Solids Content. Postharvest Biol. Technol. 2002, 26, 191–198. [Google Scholar] [CrossRef]
- Nie, S.; Al Riza, D.F.; Ogawa, Y.; Suzuki, T.; Kuramoto, M.; Miyata, N.; Kondo, N. Potential of a Double Lighting Imaging System for Characterization of “Hayward” Kiwifruit Harvest Indices. Postharvest Biol. Technol. 2020, 162, 111113. [Google Scholar] [CrossRef]
- Ma, T.; Xia, Y.; Inagaki, T.; Tsuchikawa, S. Non-Destructive and Fast Method of Mapping the Distribution of the Soluble Solids Content and pH in Kiwifruit Using Object Rotation near-Infrared Hyperspectral Imaging Approach. Postharvest Biol. Technol. 2021, 174, 111440. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, J.; Inagaki, T.; Su, Y.; Tsuchikawa, S. Rapid and Nondestructive Prediction of Firmness, Soluble Solids Content, and pH in Kiwifruit Using Vis–NIR Spatially Resolved Spectroscopy. Postharvest Biol. Technol. 2022, 186, 111841. [Google Scholar] [CrossRef]
- Mishra, P.; Verschoor, J.; Vries, M.N.; Polder, G.; Boer, M.P. Portable Near-Infrared Spectral Imaging Combining Deep Learning and Chemometrics for Dry Matter and Soluble Solids Prediction in Intact Kiwifruit. Infrared Phys. Technol. 2023, 131, 104677. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E.; Eddins, S.L. Digital Image Processing Using MATLAB; 5. impr.; Pearson Education [u.a.]: New Delhi, India, 2009; ISBN 978-81-7758-898-9. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B.; Sergio, L.; Baruzzi, F.; Gatto, M.A.; Carito, A.; Linsalata, V.; Cascarano, N.A.; Di Venere, D. Postharvest Performance of Fresh-Cut ‘Big Top’ Nectarine as Affected by Dipping in Chemical Preservatives and Packaging in Modified Atmosphere. Int. J. Food Sci. Technol. 2014, 49, 1184–1195. [Google Scholar] [CrossRef]
- Montefusco, A.; Durante, M.; Migoni, D.; De Caroli, M.; Ilahy, R.; Pék, Z.; Helyes, L.; Fanizzi, F.P.; Mita, G.; Piro, G.; et al. Analysis of the Phytochemical Composition of Pomegranate Fruit Juices, Peels and Kernels: A Comparative Study on Four Cultivars Grown in Southern Italy. Plants 2021, 10, 2521. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Resear 2011, 12, 2825–2830. [Google Scholar]
- Rogers, T.J.; Mclean, J.; Cross, E.J.; Worden, K. Rabczuk, T., Bathe, K.-J., Eds.; Gaussian Processes. In Machine Learning in Modeling and Simulation; Computational Methods in Engineering & the Sciences; Springer International Publishing: Cham, Switzerland, 2023; pp. 121–147. ISBN 978-3-031-36643-7. [Google Scholar]
- Xu, W.; Wei, L.; Cheng, W.; Yi, X.; Lin, Y. Non-Destructive Assessment of Soluble Solids Content in Kiwifruit Using Hyperspectral Imaging Coupled with Feature Engineering. Front. Plant Sci. 2024, 15, 1292365. [Google Scholar] [CrossRef] [PubMed]
- Heidarian Dehkordi, R.; Candiani, G.; Nutini, F.; Carotenuto, F.; Gioli, B.; Cesaraccio, C.; Boschetti, M. Towards an Improved High-Throughput Phenotyping Approach: Utilizing MLRA and Dimensionality Reduction Techniques for Transferring Hyperspectral Proximal-Based Model to Airborne Images. Remote Sens. 2024, 16, 492. [Google Scholar] [CrossRef]
- Mishra, P.; Rutledge, D.N.; Roger, J.-M.; Wali, K.; Khan, H.A. Chemometric Pre-Processing Can Negatively Affect the Performance of near-Infrared Spectroscopy Models for Fruit Quality Prediction. Talanta 2021, 229, 122303. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, F.; Dong, J. Nondestructive Measurement of Soluble Solids Content of Kiwifruits Using Near-Infrared Hyperspectral Imaging. Food Anal. Methods 2016, 9, 38–47. [Google Scholar] [CrossRef]
- Hu, W.; Sun, D.-W.; Blasco, J. Rapid Monitoring 1-MCP-Induced Modulation of Sugars Accumulation in Ripening ‘Hayward’ Kiwifruit by Vis/NIR Hyperspectral Imaging. Postharvest Biol. Technol. 2017, 125, 168–180. [Google Scholar] [CrossRef]
- Pullanagari, R.R.; Li, M. Uncertainty Assessment for Firmness and Total Soluble Solids of Sweet Cherries Using Hyperspectral Imaging and Multivariate Statistics. J. Food Eng. 2021, 289, 110177. [Google Scholar] [CrossRef]
- Rasmussen, C.E. Gaussian Processes in Machine Learning. In Proceedings of the Summer School on Machine Learning, Canberra, Australia, 2–14 February 2003; Springer: Berlin/Heidelberg, Germany, 2003; pp. 63–71. [Google Scholar]
| Model | Hyperparameter Name | Hyperparameter Description | Range Min. | Range Max |
|---|---|---|---|---|
| PLS | NCOMPS | Number of components retained by PLS | 1 | 40 |
| SVR | EPS | Defines the distance from the predicted to the actual value (i.e., “tube” width) for which no penalty is applied in the training loss function | 1 × 10−40 | 1 × 103 |
| SVR | C | Regularisation strength | 1 × 10−3 | 1 × 105 |
| GPR | LSCALE | Length scale of RBF kernel | 1 × 10−4 | 1 × 104 |
| GPR | ALPHA | Noise level | 1 × 10−20 | 2 |
| Colour Parameters | Farm 1 | Farm 2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HT1 | HT2 | HT3 | p-Value | HT1 | HT2 | HT3 | HT4 | p-Value | ||||||||
| L* | 17.98 | 17.38 | 17.40 | ns | 17.44 | 16.60 | 16.39 | 17.29 | ns | |||||||
| a* | 2.87 | 2.83 | 3.09 | ns | 3.60 | 3.42 | 3.44 | 3.16 | ns | |||||||
| b* | 13.67 | a | 13.10 | ab | 12.79 | b | * | 13.38 | a | 12.45 | b | 12.28 | b | 12.21 | b | ** |
| Chroma | 13.99 | a | 13.42 | ab | 13.16 | b | * | 13.87 | a | 12.92 | b | 12.76 | b | 12.68 | b | ** |
| hue angle | 78.13 | 77.77 | 76.34 | ns | 74.95 | 74.57 | 74.29 | 74.84 | ns | |||||||
| Prediction of Soluble Solids Content (°Brix) | |||||||
| model | stage | min | max | mean | std | R2 avg | MSE avg |
| PLS | test | 6.22 | 11.39 | 7.75 | 1.32 | 0.60 ± 0.16 | 0.75 ± 0.26 |
| PLS | train | 6.03 | 11.58 | 7.75 | 1.31 | 0.77 ± 0.06 | 0.39 ± 0.08 |
| SVR | test | 6.22 | 11.39 | 7.75 | 1.32 | 0.55 ± 0.13 | 0.97 ± 0.27 |
| SVR | train | 6.03 | 11.58 | 7.75 | 1.31 | 0.75 ± 0.08 | 0.49 ± 0.15 |
| GPR | test | 6.22 | 11.39 | 7.75 | 1.32 | 0.6 ± 0.12 | 0.89 ± 0.16 |
| GPR | train | 6.03 | 11.58 | 7.75 | 1.31 | 0.99 ± 0.00 | 0.0 ± 0.0 |
| Prediction of Glucose in fresh kiwifruit(mg g−1) | |||||||
| model | stage | min | max | mean | std | R2 avg | MSE avg |
| PLS | test | 5.89 | 29.56 | 12.23 | 5.88 | 0.58 ± 0.16 | 17.38 ± 5.34 |
| PLS | train | 4.46 | 30.78 | 12.17 | 5.69 | 0.77 ± 0.08 | 7.31 ± 1.97 |
| SVR | test | 5.89 | 29.56 | 12.23 | 5.88 | 0.54 ± 0.13 | 19.48 ± 4.09 |
| SVR | train | 4.46 | 30.78 | 12.17 | 5.69 | 0.78 ± 0.09 | 7.66 ± 2.35 |
| GPR | test | 5.89 | 29.56 | 12.23 | 5.88 | 0.55 ± 0.12 | 18.22 ± 3.72 |
| GPR | train | 4.46 | 30.78 | 12.17 | 5.69 | 0.99 ± 0.00 | 0.37 ± 0.06 |
| Prediction of Fructose in fresh kiwifruit (mg g−1) | |||||||
| model | stage | min | max | mean | std | R2 avg | MSE avg |
| PLS | test | 4.70 | 24.24 | 10.29 | 4.87 | 0.58 ± 0.16 | 11.59 ± 3.22 |
| PLS | train | 3.72 | 24.37 | 10.13 | 4.55 | 0.76 ± 0.08 | 4.92 ± 1.03 |
| SVR | test | 4.70 | 24.24 | 10.29 | 4.87 | 0.48 ± 0.13 | 14.68 ± 2.80 |
| SVR | train | 3.72 | 24.37 | 10.13 | 4.55 | 0.76 ± 0.09 | 5.55 ± 1.94 |
| GPR | test | 4.70 | 24.24 | 10.29 | 4.87 | 0.59 ± 0.12 | 11.29 ± 1.58 |
| GPR | train | 3.72 | 24.37 | 10.13 | 4.55 | 0.99 ± 0.00 | 0.27 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, M.; Pace, B.; Corvino, A.; Serio, F.; Carotenuto, F.; Cavaliere, A.; Genangeli, A.; Cefola, M.; Gioli, B. Advancing Kiwifruit Maturity Assessment: A Comparative Study of Non-Destructive Spectral Techniques and Predictive Models. Foods 2025, 14, 2581. https://doi.org/10.3390/foods14152581
Palumbo M, Pace B, Corvino A, Serio F, Carotenuto F, Cavaliere A, Genangeli A, Cefola M, Gioli B. Advancing Kiwifruit Maturity Assessment: A Comparative Study of Non-Destructive Spectral Techniques and Predictive Models. Foods. 2025; 14(15):2581. https://doi.org/10.3390/foods14152581
Chicago/Turabian StylePalumbo, Michela, Bernardo Pace, Antonia Corvino, Francesco Serio, Federico Carotenuto, Alice Cavaliere, Andrea Genangeli, Maria Cefola, and Beniamino Gioli. 2025. "Advancing Kiwifruit Maturity Assessment: A Comparative Study of Non-Destructive Spectral Techniques and Predictive Models" Foods 14, no. 15: 2581. https://doi.org/10.3390/foods14152581
APA StylePalumbo, M., Pace, B., Corvino, A., Serio, F., Carotenuto, F., Cavaliere, A., Genangeli, A., Cefola, M., & Gioli, B. (2025). Advancing Kiwifruit Maturity Assessment: A Comparative Study of Non-Destructive Spectral Techniques and Predictive Models. Foods, 14(15), 2581. https://doi.org/10.3390/foods14152581










