3.1. Metabolite Profile of Fermented ZSLs
Analysis of differential metabolites before and after fermentation (
Figure S1) showed a notable increase in flavonoids such as vitexin, catechin, kaempferol, and poncirin, which are key contributors to antioxidant activity. These results align with Xu et al. [
17], who reported that flavonoids and flavanones in sainfoin silage serve as critical differential metabolites. This increase likely stems from microbial enzyme activity during fermentation, which facilitates the release of active compounds from plant cell walls.
Oxidative stress negatively impacts animal productivity and meat quality [
18]. Polyphenol-rich plant supplements and fermented feeds provide natural protection against oxidative stress, thereby improving performance and meat quality [
19]. For example,
B. papyrifera silage enhances antioxidant status due to its bioactive components, including flavonoids, phenolic acids, and alkaloids [
20]. Similarly, our study found that fermented ZSLs are rich in bioactive compounds and protein, making them a viable and sustainable protein source for livestock, including Sanhuang chickens. Dietary inclusion of fermented ZSLs has the potential to improve growth performance and meat quality in Sanhuang chickens. This study highlights the value of utilizing
Z. schinifolium by-products, offering a sustainable strategy for enhancing animal nutrition and feed production.
3.2. Growth Performance and Carcass Characteristics
As shown in
Table 2, initial body weight (ABW1) did not differ significantly between the control and treatment groups (
p > 0.05). However, dietary supplementation with fermented ZSLs significantly enhanced feed intake, final body weight (ABW2), ADG, and ADFI, while reducing the feed-to-gain ratio (F: G) (
p < 0.05), thereby improving feed efficiency. The MDG group showed the highest ADG, followed by HDG, with feed efficiency significantly higher in MDG than in the control group (NC) (
p < 0.05). Carcass traits, DP, was higher in the treatment groups than in NC. Additionally, AFP was significantly lower in MDG compared to NC (
p < 0.05). These results suggest that supplementation with 6% fermented ZSLs improved growth performance and carcass traits, simultaneously reducing the abdominal fat percentage and avoiding excessive abdominal fat deposition in Sanhuang chickens, thereby improving feed utilization efficiency. Studies have demonstrated that excessive fermented feed generates high concentrations of lactic acid and acetic acid, which disrupt intestinal acid–base balance and depress digestive enzyme activity. Furthermore, the accumulation of certain anti-nutritional factors directly compromises the intestinal mucosal barrier, thereby inhibiting poultry growth.
Similar studies have reported that incorporating 5%, 10%, or 15% fermented feed based on cottonseed and rapeseed meal into broiler diets significantly enhances growth performance [
21]. The improved performance of Sanhuang chickens in this study may be attributed to the superior protein quality and biological value of ZSLs, which aligns closely with animal protein profiles. Fermentation further enhances nutrient digestibility, particularly amino acids [
22]. Our previous study demonstrated that fermentation increased ZSL digestibility by raising the cellulose degradation rate to 42.86% and boosting acid-soluble protein content by 5.14-fold, facilitating nutrient absorption. Fermentative microorganisms secrete a spectrum of digestive enzymes, including proteases and cellulases. Acid proteases exhibit elevated metabolic activity in acidic environments, facilitating protein degradation that increases acid-soluble protein (ASP) content, thereby enhancing feed utilization efficiency. Consistent with Ashayerizadeh et al. [
23], fermented feed may also enhance gut health similarly to probiotics. The improved dressing percentage and carcass traits in treatment groups likely result from increased intake of bioavailable nutrients, supporting superior growth performance. These results align with previous findings showing that fermented feed supplementation enhances broiler growth rates [
24].
3.3. Meat Quality Parameters
Table 3 indicates that intramuscular fat content was significantly higher in the MDG and HDG groups compared to NC (
p < 0.05). Muscle protein content and pH45min values showed no significant differences between the treatment and control groups (
p > 0.05). After 24 h, MDG and HDG displayed higher pH values and lower a* and b* values than the control group (
p < 0.05). The HDG group had the lowest cooking and drip loss rates, followed by MDG (
p < 0.05). Shear force was significantly greater in NC compared to all treatment groups (
p < 0.05).
Meat quality is a multifaceted evaluation of characteristics that shape consumer preferences [
25]. Higher intramuscular fat content enhances muscle juiciness, flavor, and tenderness while it lowers shear force [
26]. This study corroborates these findings, as the treatment groups showed increased intramuscular fat and decreased shear force. Similarly, Xu et al. [
27] demonstrated that supplementing pork with 800 mg/kg of apple polyphenols reduced L* and b* values. In this study, ZSL supplementation significantly decreased the a* and b* values in chicken meat. This reduction may result from the oxidation of myoglobin, which transforms bright red oxymyoglobin into brown metmyoglobin, affecting redness [
28]. Additionally, polyphenols can bind to proteins, reducing their chromogenic potential [
29]. Quinones generated from polyphenol oxidation can form covalent bonds with myosin sulfhydryl groups (-SH), thereby masking the chromophoric heme iron site of myoglobin. Additionally, polyphenols may bind iron atoms to accelerate metmyoglobin accumulation, adversely impacting meat redness. ZSLs contain functional plant secondary metabolites, such as flavonoids, which inhibit free radical formation. The bioactive compounds in ZSLs likely contributed to the observed changes in meat color. Natural antioxidants, like those in ZSLs, stabilize cell membranes and enhance the water-holding capacity of meat [
30]. Thus, incorporating ZSLs may improve water retention and tenderness in chicken.
3.4. Fatty Acid Composition
No significant differences were observed in C18:1n9t and C20:3n6 levels among the groups (
p > 0.05;
Table 4). However, C14:1 and C20:2 levels were significantly higher in all treatment groups compared to the control group (
p < 0.05). Fermented ZSL supplementation significantly increased C18:0 and C22:6n3 levels in the treatment groups relative to NC and NF (
p < 0.05), while C16:0, C16:1, C18:2n6c, and C20:4n6 levels were significantly lower than in the control (
p < 0.05). Total saturated fatty acids (SFAs) were highest in NC (
p < 0.05). In contrast, MDG had significantly higher levels of C18:0, C20:2, and polyunsaturated fatty acids (PUFAs) compared to the other treatment groups (
p < 0.05). NC exhibited the highest total n-6 PUFA content and the lowest n-3 PUFA content (
p < 0.05), whereas MDG had the highest total n-3 PUFA levels (
p < 0.05).
Previous studies have demonstrated that plant-based diets rich in natural antioxidants can increase n-3 PUFA concentrations in meat [
31]. Marcinčák et al. [
32] reported that supplementing poultry feed with 10% fermented corn meal significantly elevated the proportions of oleic acid, α-linolenic acid, and γ-linolenic acid in chicken breast fat while concurrently improving the n-3/n-6 PUFA ratio. Kwiecień et al. [
33] revealed that thigh muscle exhibited the highest n-3 PUFA content when supplemented with low-dose alfalfa (
Medicago sativa), which demonstrated antioxidant potential capable of modulating fatty acid profiles. In this study, supplementation with 6% fermented ZSLs enhanced C18:3n3 and C22:6n3 levels in Sanhuang chicken breast meat, suggesting potential health benefits for consumers. These improvements in PUFA content likely result from the synergistic modulation of lipid synthesis and oxidation by the probiotics and bioactive compounds in fermented ZSLs. However, HDG showed lower total n-3 PUFA and overall PUFA levels compared to MDG. This reduction may be due to the higher phenolic content in HDG, which could exert pro-oxidant activity and promote lipid peroxidation [
34]. The pro-oxidant activity of phenolic compounds primarily occurs in the presence of transition metal ions or at elevated concentrations. Its core mechanism involves radical chain reactions and metal ion-mediated redox cycling, demonstrating that certain natural antioxidants also exhibit pro-oxidant effects [
33].
3.5. Free Amino Acid Profile
Table 5 shows that the concentrations of five essential amino acids (EAA)—isoleucine, leucine, methionine, phenylalanine, and valine—were significantly higher in the group supplemented with fermented ZSLs compared to the control group (
p < 0.05). Similarly, seven non-essential amino acids (NEAA), including alanine, arginine, aspartic acid, cysteine, glycine, proline, and serine, also increased significantly in the same group (
p < 0.05). Notably, lysine was absent across all groups, while histidine was detected only in MDG. Overall, the inclusion of fermented ZSLs in the diet significantly enhanced total EAA, NEAA, and Umami amino acids (UAA) levels in Sanhuang chicken meat (
p < 0.05). Additionally, UAA and sweet amino acids (SAAs) concentrations increased proportionally with the amount of fermented ZSLs in the diet (
p < 0.05).
Amino acids, serving as the material foundation of protein metabolism, are not only of vital importance for biological functions but also key precursors in the formation of flavor compounds in meat. Feed composition is known to enhance chicken flavor, with appropriate supplementation further optimizing sensory characteristics [
35]. Mohammed et al. [
36] highlighted that elevated levels of total free amino acids enhance meat’s nutritional value. In this study, all treatment groups showed significant increases in total FAA, UAA, and SAA levels. Among the amino acids, glutamic acid was most prevalent, followed by threonine and alanine. Glutamic acid, a key precursor for glutathione synthesis, is crucial for antioxidant defense and is a primary umami compound, enhancing aroma and taste [
37]. Umami amino acids also help reduce dietary salt and fat intake while increasing food consumption among the elderly [
38]. In addition, the increased levels of SAA, such as alanine and glycine, may further enhance the sensory characteristics of chicken meat through synergistic interactions [
35]. The improvement in amino acid composition by fermented ZSLs can be attributed to their abundant probiotics and bioactive compounds, which promote protein hydrolysis and inhibit amino acid oxidation, thereby increasing the release and accumulation of free amino acids. However, the increase in some amino acids was lower in HDG compared to MDG, suggesting that excessive addition may reduce amino acid synthesis efficiency due to increased metabolic load or substrate competition. Fermented ZSLs significantly enhanced the nutritional quality and flavor characteristics of chicken by regulating amino acid metabolism, with the 6% addition yielding the best results.
3.6. Sensory Assessment
The sensory evaluation results of boiled chicken are illustrated in
Figure 1. Compared with the control group, the dietary supplementation of fermented ZSLs improved the flavor, taste, and texture attributes of chicken. In terms of flavor, treatment groups showed significantly lower smoky flavor scores compared to the control group, while nutty flavors were more pronounced in the MDG and NF groups (
Figure 1A). It is worth noting that the intensity of fat and cardboard flavors in the treatment groups was significantly higher than in the control group. This may be attributed to the accumulation of lipid oxidation products, such as aldehydes and ketones, particularly due to the oxidative degradation of PUFAs during processing or storage. This result indicates that while fermented Sichuan pepper leaves enhance the fatty acid composition (such as increasing n-3 PUFA), it is also essential to consider oxidative stability and shelf-life requirements.
In the sensory evaluation of taste, the MDG and HDG groups demonstrated higher scores for umami and saltiness (
Figure 1B), which may be closely linked to the increased content of free amino acids. This study revealed that fermented ZSLs significantly elevated the levels of umami amino acids, such as glutamic acid and threonine (
Table 5). Glutamic acid, the primary activator of umami receptors, can directly enhance the umami flavor of meat when its concentration rises. Furthermore, the improvement in saltiness is likely attributed to the synergistic flavor-enhancing effects of sodium ions and amino acids [
30].
Texture analysis showed notable variation across attributes such as juiciness, elasticity, chewiness, and hardness (
Figure 1C), with the MDG group scoring highest for juiciness and elasticity and lowest for hardness. This is thought to be attributed to the modification of the muscle fiber membrane structure and the enhancement of water-holding capacity by PUFAs. Although direct molecular evidence of PUFA–myofibrillar protein interactions is currently lacking, integrated analysis of lipidomic profiles, texture parameters, and sensory data suggests that PUFA enrichment likely enhances meat tenderness through improved myocyte membrane hydration capacity and the inhibition of collagen cross-linking in connective tissue. Generalized Procrustes analysis (GPA) based on sensory data (
Figure 1D) further revealed a clear distinction in the sensory characteristics of chicken breast meat among the different treatment groups. In conclusion, dietary supplementation with ZSLs influenced the sensory qualities of Sanhuang chicken, improving flavor and overall acceptance. However, the specific reasons remain unclear, and further exploration through instrumental analysis is required.
3.7. E-Tongue and E-Nose
To systematically investigate the impact of fermented ZSLs on the flavor profile of chicken breast meat, we employed e-tongue and e-nose technologies for quantitative analysis. These methods are recognized for their precision in characterizing flavor attributes [
39]. The e-tongue measured parameters including sourness, sweetness, bitterness, aftertaste (bitterness), astringency, aftertaste (astringency), umami, saltiness, and richness. Using tasteless thresholds (sourness: −13; umami: −6; others: 0) as reference points. Meaningful responses were detected for bitterness, umami, richness, and sweetness, while sourness, aftertaste (bitterness), aftertaste (astringency), and saltiness remained consistently below the threshold across all groups (
Figure 2A). The lack of responses may reflect the low concentration of flavor compounds in diluted liquid samples. The MDG group demonstrated significantly higher umami levels compared to the control group (
p < 0.05). Richness was notably greater in the LDG and MDG groups (
p < 0.05), and sweetness was significantly elevated in chickens fed with fermented ZSLs (
p < 0.05). This result is closely linked to the increased content of FAA, particularly the elevated concentrations of glutamic acid (the primary precursor of umami), as well as alanine and glycine (sweet amino acids) [
40]. This further supports the enhanced perception of umami and saltiness observed in the sensory evaluation (
Figure 1B). The umami flavor formation theoretically relies on Glu-IMP synergism; however, instrumental constraints precluded quantification of IMP in this study. Principal component analysis (PCA) (
Figure 2B) showed clear segregation between the control and treatment groups, indicating that the electronic tongue can effectively differentiate the flavor characteristics of the treatment group from those of the control group.
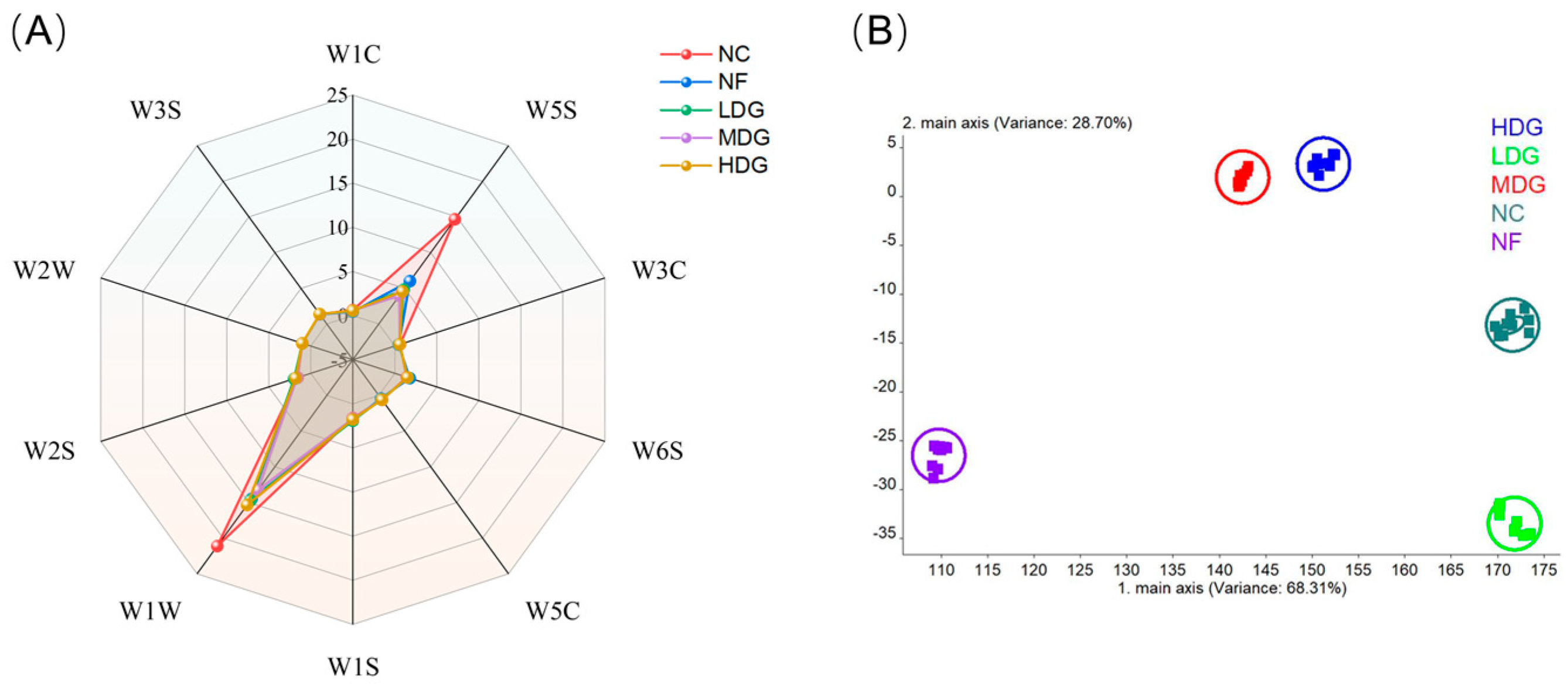
E-nose analysis further revealed the differences in volatile aromas (
Figure 3). The result demonstrated that the chicken samples exhibited relatively high sensitivity to the W5S and W1W sensors, indicating greater concentrations of nitrogen oxides and organic sulfides in the chicken. Among all the treatment groups, the response values of W5S and W1W were significantly lower than those of the control group (
Figure 3A), with the MDG group presenting the most significant decrease. The reduction in the response values of nitrogen oxides and organic sulfides indicates a decrease in unpleasant odors, contributing to a more balanced and milder flavor profile [
41]. In other words, adding fermented ZSLs to the diet helps reduce unpleasant odors in chicken, with the MDG group showing the most significant effect. This result may be linked to the antioxidant activity of polyphenols in fermented ZSLs, which enhance the flavor of chicken by inhibiting lipid oxidation and the degradation of sulfur-containing amino acids (such as cysteine), while also reducing the formation of undesirable volatile compounds. Linear discriminant analysis (LDA) further confirmed the effective discrimination of the e-nose for each treatment group (
Figure 3B), indicating that the addition of fermented ZSLs to the diet influences the formation of chicken breast meat flavor, as verified through both sensory and instrumental analysis. However, the specific volatile compounds responsible for these differences remain unclear, warranting further investigation to identify and compare key aroma compounds.
3.8. Volatile Compound Profiling
The volatile compounds in chicken were analyzed, with the results summarized in
Table 6. GC-MS identified 63 volatile organic compounds across the samples, with 41, 48, 50, 53, and 52 compounds detected in the five chicken breast groups, respectively. The predominant volatile compounds included 1-octen-3-ol (3.49–5.22 µg/g), linalool (7.41–18.42 µg/g), hexanal (16.22–22.56 µg/g), benzaldehyde (4.28–10.91 µg/g), nonanal (9.32–13.85 µg/g), pentadecanal (2.33–8.09 µg/g), and hexadecanal (4.49–17.19 µg/g). Analysis of various compounds revealed that aldehydes were the predominant volatile compounds (53.15–77.78 µg/g), followed by alcohols (12.21–25.52 µg/g), with acids showing the lowest concentrations (
Figure 4A). Fermented ZSL supplementation significantly increased the total aldehyde and alcohol content compared to the control group, with the highest total volatile compound content observed in MDG, followed by HDG. The heatmap (
Figure 4B) indicates that the treatment groups, particularly MDG and HDG, displayed elevated concentrations and a greater diversity of volatile compounds compared to the control group. These results suggest that fermented ZSL supplementation enhances the abundance and variety of volatile compounds in chicken breast meat. This may be associated with the regulation of lipid and amino acid metabolism by probiotics, as well as enzymatic reactions during fermentation, which promote the oxidation and transformation of volatile precursor substances, such as unsaturated fatty acids and free amino acids [
42].
Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) further highlighted the differences in the volatile substance composition between the control and treatment groups.
Figure 4C shows the PCA score plot of volatile compound content measured by GC-MS, with the first two principal components accounting for 63.8% of the total variance. The treatment groups with fermented ZSLs are positioned on the left side of the PCA score plot, with MDG and HDG partially overlapping, indicating similar aroma profiles. In contrast, NC and NF are located farther from the other groups, reflecting that their volatile compound composition differs from the other samples. These results are consistent with findings from sensory and e-nose analyses. PCA effectively highlights classification trends and groups samples based on their volatile profiles. PLS-DA, a supervised discriminant analysis, was applied to correlate GC-MS-identified volatile compounds with sample categories [
43]. As shown in
Figure 4D, the first and second components account for 41.9% and 31.6% of the variance, respectively. The PLS-DA plot reveals clear separations among all five groups, with greater distances indicating larger differences in composition [
40]. These findings confirm that GC-MS effectively discriminates between chicken samples based on their volatile compound profiles.
The supervised PLS-DA model was applied to identify aroma compounds with significant contributions to chicken sample classification and to pinpoint key differential aroma substances influencing chicken flavor. This approach evaluates the variable importance in projection (VIP) values, which measure the relevance and explanatory power of each volatile compound in distinguishing aroma profiles [
44]. Compounds with VIP values above 1.0 are deemed critical for classification. As illustrated in
Figure 4E, linalool, hexanal, benzaldehyde, pentadecanal, and hexadecanal all displayed VIP values exceeding 1.0, establishing them as key biomarkers for chicken flavor differentiation.
Table 6 outlines the concentrations of these compounds across the sample groups. Among the five biomarkers, four (excluding hexanal) demonstrated significant differences between groups (
p < 0.05). Therefore, linalool, benzaldehyde, pentadecanal, and hexadecanal were identified as the key differential compounds responsible for distinguishing the flavor characteristics of chicken. Fermented ZSL supplementation notably increased linalool levels, imparting floral and woody aromas. Benzaldehyde, recognized for its nutty scent, was significantly elevated in the NF, LDG, and MDG groups compared to NC and HDG, with MDG exhibiting the highest levels. This may be the primary reason for the intensified nut-like flavor observed in the sensory assessment of the MDG group. Furthermore, ZSL supplementation reduced pentadecanal, a compound associated with pungent and bitter flavor, which may explain the diminished bitterness in fermented groups [
45]. In contrast, hexadecanal, known for its mild floral and waxy aroma, was more abundant in the treatment groups, contributing to a smoother, more refined chicken flavor profile.
PLSR analysis was used to explore the relationship between aroma compounds identified by GC-MS (X variables,
n = 63) and six poultry aroma attributes evaluated through sensory analysis (Y variables,
n = 6). As shown in
Figure 4F, the weak sourness intensity may result from the limited presence of compounds near the acidic aroma zone. The NC sample was located closer to smoky and sulfurous aromas, while the treatment group was positioned farther away. This difference might be due to the higher content of aldehyde substances such as (E,E)-2,4-decadienal and nonanal in the treatment group, which produced stronger aldehyde-derived aromas and masked the smoky and sulfurous flavors. Ref. [
46] The synergistic effect of benzaldehyde and hexadecanal enhances the nut-like aroma, resulting in a more mellow aroma of the chicken [
47]. It is worth noting that compounds such as 1-octen-3-ol, with its broth-like scent, and (E,E)-2,4-decadienal, recognized for its rich chicken and chicken fat aromas, add a distinct chicken-like fragrance. Meanwhile, 1-octanol, nonanal, and (E)-2-octenal contribute oil or fried aroma, collectively enhancing the fat flavor profile of MDG and HDG. The cardboard-like aroma, associated with slightly oxidized fats and oils, evokes the impression of wet cardboard. The proximity of MDG and HDG to this aroma suggests a higher content of unsaturated fatty acids in these samples. During cooking, the oxidation and breakdown of unsaturated fatty acids generate aldehydes with oily characteristics, contributing to the unique flavor of cooked chicken [
16]. Aldehyde compounds, characterized by high concentrations and diverse olfactory properties, play a key role in creating the overall chicken flavor.