Whey Protein Isolate and β-Lactoglobulin-Modified Alginate Hydrogel Scaffolds Enhance Cell Proliferation for Cultivated Meat Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alginate–RGD Synthesis
2.3. Scaffold Fabrication and Characterization
2.4. Equilibrium Swell Ratio and Weight Loss
2.5. Unconfined Compression Testing
2.6. Primary Bovine Muscle Cell (pBMC) Culture and Characterization
2.6.1. Cell Culture and Seeding on Scaffolds
2.6.2. Cell Imaging
2.6.3. Cell Metabolic Activity
2.7. Statistics
3. Results
3.1. Scaffold Fabrication and Characterization
3.2. Equilibrium Swell Ratio and Weight Loss
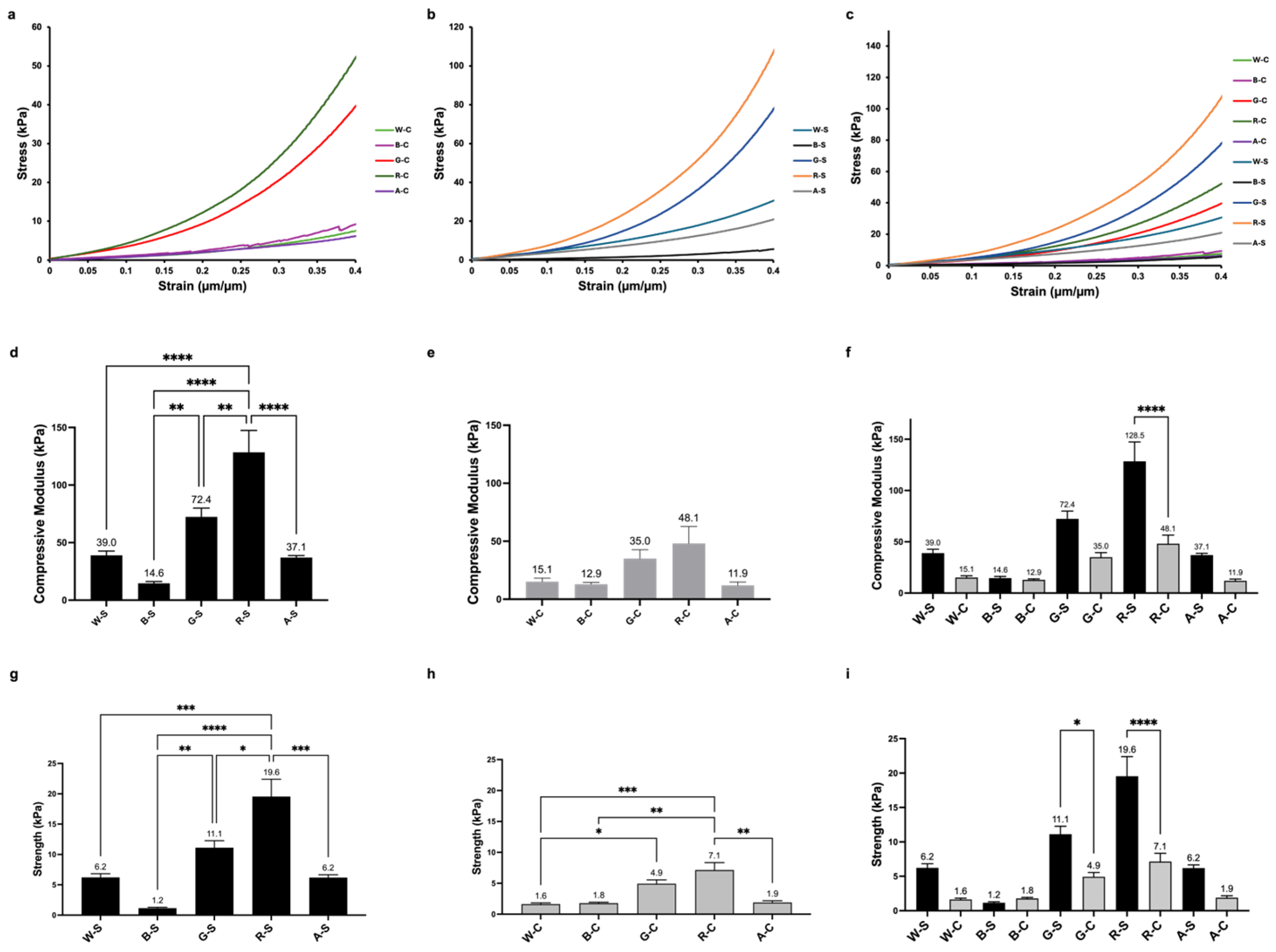
3.3. Mechanical Properties
3.4. pBMC Bioactivity and Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- OECD. OECD-FAO Agricultural Outlook 2021–2030. 2021. Available online: https://www.oecd.org/en/publications/2021/07/oecd-fao-agricultural-outlook-2021-2030_31d65f37.html (accessed on 26 February 2024).
- Tuomisto, H.L.; Teixeira de Mattos, M.J. Environmental Impacts of Cultured Meat Production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Lei, Q.; Yan, Q.; Li, X.; Zhou, J.; Du, G.; Chen, J. Trends and ideas in technology, regulation and public acceptance of cultured meat. Future Foods 2021, 3, 100032. [Google Scholar] [CrossRef]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, 2102908. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Kurisawa, M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 2021, 124, 108–129. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Lim, J.-H.; Lee, E.-J.; Chun, H.-J.; Ali, S.; Ahmad, S.S.; Shaikh, S.; Choi, I. Extracellular Matrix and the Production of Cultured Meat. Foods 2021, 10, 3116. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhao, Y.; Jin, X.; Zhu, X.; Fang, Y. Material Perspective on the Structural Design of Artificial Meat. Adv. Sustain. Syst. 2021, 5, 2100017. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Zhang, S.; Li, G.; Midgley, A.C.; Fang, Y.; Zhao, M.; Nishinari, K.; Yao, X. The important role of cellular mechanical microenvironment in engineering structured cultivated meat: Recent advances. Curr. Res. Food Sci. 2024, 9, 100865. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Abmayr, S.M.; Pavlath, G.K. Myoblast fusion: Lessons from flies and mice. Development 2012, 139, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Charron, P.N.; Fenn, S.L.; Poniz, A.; Floreani, R. Mechanical properties and failure analysis of visible light crosslinked alginate-based tissue sealants. J. Mech. Behav. Biomed. Mater. 2016, 59, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Tahir, I.; Floreani, R. Dual-Crosslinked Alginate-Based Hydrogels with Tunable Mechanical Properties for Cultured Meat. Foods 2022, 11, 2829. [Google Scholar] [CrossRef] [PubMed]
- Huettner, N.; Dargaville, T.R.; Forget, A. Discovering Cell-Adhesion Peptides in Tissue Engineering: Beyond RGD. Trends Biotechnol. 2018, 36, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Yun, S.H.; Lee, S.Y.; Lee, J.; Mariano, E., Jr.; Joo, S.T.; Choi, I.; Choi, J.S.; Kim, G.D.; Lee, J.; et al. Analysis of commercial fetal bovine serum (FBS) and its substitutes in the development of cultured meat. Food Res. Int. 2023, 174, 113617. [Google Scholar] [CrossRef] [PubMed]
- Fenn, S.L.; Floreani, R. Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2016, 104, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, G.; Chen, H. The applications of alginate in functional food products. J. Nutr. Food Sci. 2020, 3, 100013. [Google Scholar]
- Lapin, M.R.; Gonzalez, J.M.; Johnson, S.E. Substrate elasticity affects bovine satellite cell activation kinetics in vitro. J. Anim. Sci. 2013, 91, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Dai, S.; Huang, J.; Hu, X.; Ge, C.; Zhang, X.; Yang, K.; Shao, P.; Sun, P.; Xiang, N. Soy Protein Amyloid Fibril Scaffold for Cultivated Meat Application. ACS Appl. Mater. Interfaces 2023, 15, 15108–15119. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Plazzotta, S.; Powell, J.; de Vries, A.; Rousseau, D.; Calligaris, S. Structural characterisation and sorption capability of whey protein aerogels obtained by freeze-drying or supercritical drying. Food Hydrocolloid 2022, 122, 107117. [Google Scholar] [CrossRef]
- Keri Marshall, N. Therapeutic applications of whey protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar]
- Kilara, A.; Vaghela, M. Whey proteins. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 93–126. [Google Scholar]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Xavier Malcata, F. Bovine whey proteins—Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, Z.; Liu, N.; Ashaolu, T.J. Whey Protein Nutrition in Sports: Action Mechanisms and Gaps in Research. Curr. Nutr. Rep. 2025, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Khan, S.; Kunstmann, S.; Aachmann, F.L.; Ipsen, R.; Westh, P.; Emanuelsson, C.; Svensson, B. Unaided efficient transglutaminase cross-linking of whey proteins strongly impacts the formation and structure of protein alginate particles. Food Chem-Mol. Sci. 2022, 5, 100137. [Google Scholar] [CrossRef] [PubMed]
- Aboumahmoud, R.; Savello, P. Crosslinking of Whey Protein by Transglutaminase. J. Dairy Sci. 1990, 73, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Castillo, E.; Felix, M.; Bengoechea, C.; Guerrero, A. Proteins from Agri-Food Industrial Biowastes or Co-Products and Their Applications as Green Materials. Foods 2021, 10, 981. [Google Scholar] [CrossRef] [PubMed]
- Comfort, S.; Howell, N.K. Gelation properties of soya and whey protein isolate mixtures. Food Hydrocolloid 2002, 16, 661–672. [Google Scholar] [CrossRef]
- Liu, X.; Qin, X.; Wang, Y.; Zhong, J. Physicochemical properties and formation mechanism of whey protein isolate-sodium alginate complexes: Experimental and computational study. Food Hydrocolloid 2022, 131, 107786. [Google Scholar] [CrossRef]
- Madsen, M.; Ronne, M.E.; Li, R.F.; Greco, I.; Ipsen, R.; Svensson, B. Simulated gastrointestinal digestion of protein alginate complexes: Effects of whey protein cross-linking and the composition and degradation of alginate. Food Funct. 2022, 13, 8375–8387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of whey protein isolate (WPI)-dextran conjugates in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Subirade, M. Alginate-whey protein granular microspheres as oral delivery vehicles for bioactive compounds. Biomaterials 2006, 27, 4646–4654. [Google Scholar] [CrossRef] [PubMed]
- Deat-Laine, E.; Hoffart, V.; Garrait, G.; Beyssac, E. Whey protein and alginate hydrogel microparticles for insulin intestinal absorption: Evaluation of permeability enhancement properties on Caco-2 cells. Int. J. Pharm. 2013, 453, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Deat-Laine, E.; Hoffart, V.; Garrait, G.; Jarrige, J.F.; Cardot, J.M.; Subirade, M.; Beyssac, E. Efficacy of Mucoadhesive Hydrogel Microparticles of Whey Protein and Alginate for Oral Insulin Delivery. Pharm. Res.-Dordr. 2013, 30, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Wichchukit, S.; Oztop, M.H.; McCarthy, M.J.; McCarthy, K.L. Whey protein/alginate beads as carriers of a bioactive component. Food Hydrocolloid 2013, 33, 66–73. [Google Scholar] [CrossRef]
- Madsen, M.; Mohammad-Beigi, H.; Westh, P.; Aachmann, F.L.; Svensson, B. Tuning alginate β-lactoglobulin complex coacervation by modulating pH and temperature. Soft Matter 2023, 19, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Westh, P.; Khan, S.; Ipsen, R.; Almdal, K.; Aachmann, F.L.; Svensson, B. Impact of Alginate Mannuronic-Guluronic Acid Contents and pH on Protein Binding Capacity and Complex Size. Biomacromolecules 2021, 22, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Martocq, L.; Douglas, T.E.L. Amine-Rich Coatings to Potentially Promote Cell Adhesion, Proliferation and Differentiation, and Reduce Microbial Colonization: Strategies for Generation and Characterization. Coatings 2021, 11, 983. [Google Scholar] [CrossRef]
- Dvora, M.; Warwick, P.; Henry, J.E. WPI hydrogels as a potential substrate for tissue scaffolds: Mechanical properties. Mech. Soft Mater. 2022, 4, 6. [Google Scholar] [CrossRef]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamula, E.; Reilly, G.C.; Douglas, T.E.L. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
- Dvora, M.; Warwick, P.; Henry, J.E. Proliferation kinetics and mineralization properties of MC3T3-E1 cells on whey protein isolate scaffolds for bone tissue regeneration. Nano Sel. 2023, 4, 333–345. [Google Scholar] [CrossRef]
- Dziadek, M.; Douglas, T.E.L.; Dziadek, K.; Zagrajczuk, B.; Serafim, A.; Stancu, I.C.; Cholewa-Kowalska, K. Novel whey protein isolate-based highly porous scaffolds modified with therapeutic ion-releasing bioactive glasses. Mater. Lett. 2020, 261, 127115. [Google Scholar] [CrossRef]
- Rabe, R.; Hempel, U.; Martocq, L.; Keppler, J.K.; Aveyard, J.; Douglas, T.E.L. Dairy-Inspired Coatings for Bone Implants from Whey Protein Isolate-Derived Self-Assembled Fibrils. Int. J. Mol. Sci. 2020, 21, 5544. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Palka, K.; Truszkiewicz, W.; Douglas, T.E.L.; Nurzynska, A.; Ginalska, G. Could Curdlan/Whey Protein Isolate/Hydroxyapatite Biomaterials Be Considered as Promising Bone Scaffolds?-Fabrication, Characterization, and Evaluation of Cytocompatibility towards Osteoblast Cells In Vitro. Cells 2022, 11, 3251. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Tarczynska, M.; Truszkiewicz, W.; Gaweda, K.; Douglas, T.E.L.; Ginalska, G. Freeze-Dried Curdlan/Whey Protein Isolate-Based Biomaterial as Promising Scaffold for Matrix-Associated Autologous Chondrocyte Transplantation-A Pilot In-Vitro Study. Cells 2022, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Norris, K.; Kocot, M.; Tryba, A.M.; Chai, F.; Talari, A.; Ashton, L.; Parakhonskiy, B.V.; Samal, S.K.; Blanchemain, N.; Pamuła, E.; et al. Marine-Inspired Enzymatic Mineralization of Dairy-Derived Whey Protein Isolate (WPI) Hydrogels for Bone Tissue Regeneration. Mar. Drugs 2020, 18, 294. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zhao, J.; Zhu, Q.; Yi, W.; Hau, E.; Ren, D. Design of edible whey protein isolate hydrogels with cell adhesion via a two-step crosslinking method for cultured meat scaffolds. Food Hydrocolloid 2025, 168, 111562. [Google Scholar] [CrossRef]
- Charron, P.N.; Tahir, I.; Foley, C.; White, G.; Floreani, R.A. Whey Protein Isolate Composites as Potential Scaffolds for Cultivated Meat. ACS Appl. Bio Mater. 2024, 7, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Charron, P.N.; Garcia, L.M.; Tahir, I.; Floreani, R. Bio-inspired green light crosslinked alginate-heparin hydrogels support HUVEC tube formation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104932. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Rao, K.S.; Spees, J.L.; Floreani, R. Osteogenic differentiation of human mesenchymal stem cells through alginate-graft-poly(ethylene glycol) microsphere-mediated intracellular growth factor delivery. J. Control Release 2014, 192, 57–66. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Standard Test Methods for Determination of Gel Content and Swell Ratio of Crosslinked Ethylene Plastics; ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Farman, G.P.; Miller, M.S.; Reedy, M.C.; Soto-Adames, F.N.; Vigoreaux, J.O.; Maughan, D.W.; Irving, T.C. Phosphorylation and the N-terminal extension of the regulatory light chain help orient and align the myosin heads in Drosophila flight muscle. J. Struct. Biol. 2009, 168, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Maughan, D.; Henkin, J.; Vigoreaux, J. Stoichiometry of glycolytic enzymes in a diffusible component of the rabbit skeletal muscle proteome. Biophys. J. 2005, 88, 398A. [Google Scholar]
- Miller, M.; Brown, E.; Braddock, J.; Maughan, D.; Vigoreaux, J. Age related changes in Drosophila flight muscle mechanics and structure. Biophys. J. 2005, 88, 19A. [Google Scholar]
- Vigoreaux, J.O. Genetics of the Drosophila flight muscle myofibril: A window into the biology of complex systems. Bioessays 2001, 23, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Swelling of Homogeneous Alginate Gels with Multi-Stimuli Sensitivity. Int. J. Mol. Sci. 2023, 24, 5064. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Fan, X.; Tian, C.; Luo, J.; Zhang, Y.; Deng, L.; Qin, T.; Lv, Q. Decellularization of porcine skeletal muscle extracellular matrix for the formulation of a matrix hydrogel: A preliminary study. J. Cell. Mol. Med. 2016, 20, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, V.; Singh, S.K.; Gupta, J.; Kumar, M.; Sarma, D.K.; Verma, V. Recent advances in bioengineered scaffold for in vitro meat production. Cell Tissue Res. 2023, 391, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Malos, I.G.; Ghizdareanu, A.-I.; Vidu, L.; Matei, C.B.; Pasarin, D. The Role of Whey in Functional Microorganism Growth and Metabolite Generation: A Biotechnological Perspective. Foods 2025, 14, 1488. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, T.S.; Giromini, C.; Rebucci, R.; Lanzoni, D.; Petrosillo, E.; Baldi, A.; Cheli, F. Milk whey as a sustainable alternative growth supplement to fetal bovine serum in muscle cell culture. J. Dairy. Sci. 2025, 108, 4749–4760. [Google Scholar] [CrossRef] [PubMed]





| Group Name | Grouping | Composition |
|---|---|---|
| W-C, W-S | Experimental | WPI + Alginate |
| B-C, B-S | Experimental | β-LG + Alginate |
| G-C, G-S | Positive Control | Gelatin + Alginate |
| R-C, R-S | Positive Control | RGD–Alginate |
| A-C, A-S | Negative Control | Alginate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, I.; Foley, C.; Floreani, R. Whey Protein Isolate and β-Lactoglobulin-Modified Alginate Hydrogel Scaffolds Enhance Cell Proliferation for Cultivated Meat Applications. Foods 2025, 14, 2534. https://doi.org/10.3390/foods14142534
Tahir I, Foley C, Floreani R. Whey Protein Isolate and β-Lactoglobulin-Modified Alginate Hydrogel Scaffolds Enhance Cell Proliferation for Cultivated Meat Applications. Foods. 2025; 14(14):2534. https://doi.org/10.3390/foods14142534
Chicago/Turabian StyleTahir, Irfan, Christopher Foley, and Rachael Floreani. 2025. "Whey Protein Isolate and β-Lactoglobulin-Modified Alginate Hydrogel Scaffolds Enhance Cell Proliferation for Cultivated Meat Applications" Foods 14, no. 14: 2534. https://doi.org/10.3390/foods14142534
APA StyleTahir, I., Foley, C., & Floreani, R. (2025). Whey Protein Isolate and β-Lactoglobulin-Modified Alginate Hydrogel Scaffolds Enhance Cell Proliferation for Cultivated Meat Applications. Foods, 14(14), 2534. https://doi.org/10.3390/foods14142534







