Evaluation of Packaging Effects on the Phenolic Profile and Sensory Characteristics of Extra Virgin Olive Oil During Storage Using Liquid Chromatography Coupled with Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Olive Samples and Oil Production
2.3. Prediction of Oil Shelf-Life
- Method 1: Shelf-life (months) = Rancimat hours at 110 °C × 1.
- Method 2: Shelf-life (months) = [17.0% − Pyropheophytin a (PPPs)]/0.6%.
- Method 3: Shelf-life (months) = [1,2-Diacylglycerol Content (DAGs) − 35.0%]/Acidity factor (FFA factor).
2.4. Phenolic Compound Analysis
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Oil Stability and Shelf-Life Analysis
3.2. Phenolic Profile Analysis
3.2.1. Effect of Storage Time on Polyphenols
3.2.2. Effect of Packaging on (Poly) Phenolic Compounds
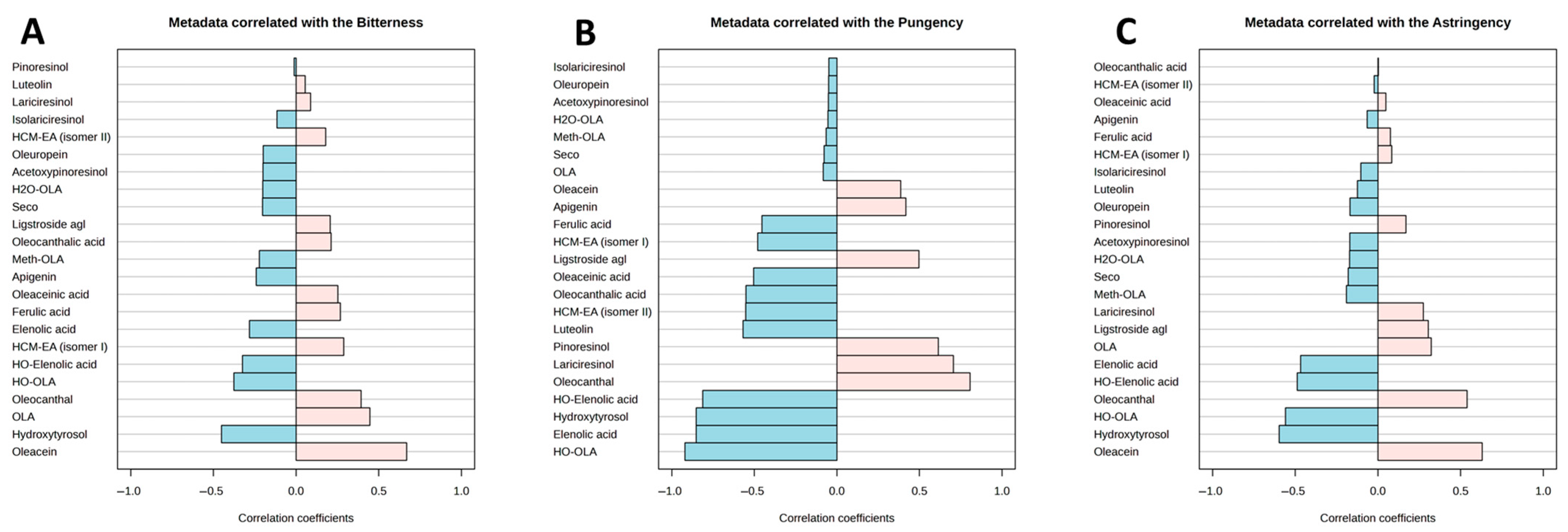
3.3. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olmo-Cunillera, A.; Pérez, M.; López-Yerena, A.; Abuhabib, M.M.; Ninot, A.; Romero-Aroca, A.; Vallverdú-Queralt, A.; Maria Lamuela-Raventós, R. Targeted Metabolic Profiling of the Revived Ancient ‘Corbella’ Olive Cultivar during Early Maturation. Food Chem. 2024, 430, 137024. [Google Scholar] [CrossRef] [PubMed]
- Olmo-Cunillera, A.; Pérez, M.; López-Yerena, A.; Abuhabib, M.M.; Ninot, A.; Romero-Aroca, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Oleacein and Oleocanthal: Key Metabolites in the Stability of Extra Virgin Olive Oil. Antioxidants 2023, 12, 1776. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Gallardo-Gomez, M.; Simal-Gandara, J.; Carpena, M.; Lorenzo, J.M.; Lourenço-Lopes, C.; Barba, F.J.; Prieto, M.A. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Rinaldi De Alvarenga, J.F.; Torrado, X.; Lamuela-Raventos, R.M. Home Cooking and Phenolics: Effect of Thermal Treatment and Addition of Extra Virgin Olive Oil on the Phenolic Profile of Tomato Sauces. J. Agric. Food Chem. 2014, 62, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castellón, J.; López-Yerena, A.; Olmo-Cunillera, A.; Jáuregui, O.; Pérez, M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Total Analysis of the Major Secoiridoids in Extra Virgin Olive Oil: Validation of an Uhplc-Esi-Ms/Ms Method. Antioxidants 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Caravaca, A.M.; Maggio, R.M.; Cerretani, L. Chemometric Applications to Assess Quality and Critical Parameters of Virgin and Extra-Virgin Olive Oil. A Review. Anal. Chim. Acta 2016, 913, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Drira, M.; Kelebek, H.; Guclu, G.; Jabeur, H.; Selli, S.; Bouaziz, M. Targeted Analysis for Detection the Adulteration in Extra Virgin Olive Oil’s Using LC-DAD/ESI–MS/MS and Combined with Chemometrics Tools. Eur. Food Res. Technol. 2020, 246, 1661–1677. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; Laveriano-Santos, E.P.; Abuhabib, M.M.; Pozzoli, C.; Pérez, M.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Proven Traceability Strategies Using Chemometrics for Organic Food Authenticity. Trends Food Sci. Technol. 2024, 147, 104430. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; Olmo-Cunillera, A.; Casadei, E.; Valli, E.; Domínguez-López, I.; Miliarakis, E.; Pérez, M.; Ninot, A.; Romero-Aroca, A.; Bendini, A.; et al. A Targeted Foodomic Approach to Assess Differences in Extra Virgin Olive Oils: Effects of Storage, Agronomic and Technological Factors. Food Chem. 2024, 435, 137539. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.I.; Falqué, E. Effect of Storage Time and Container Type on the Quality of Extra-Virgin Olive Oil. Food Control 2007, 18, 521–529. [Google Scholar] [CrossRef]
- Lanza, B.; Di Serio, M.G.; Giansante, L.; Di Loreto, G.; Di Giacinto, L. Effect of Shelf Conditions on the Phenolic Fraction and Oxidation Indices of Monovarietal Extra Virgin Olive Oil from Cv. ‘Taggiasca’. Acta Aliment. 2015, 44, 585–592. [Google Scholar] [CrossRef]
- Lechhab, T.; Lechhab, W.; Cacciola, F.; Salmoun, F. Sets of Internal and External Factors Influencing Olive Oil (Olea Europaea L.) composition: A review. Eur. Food Res. Technol. 2022, 248, 1069–1088. [Google Scholar] [CrossRef]
- Sgherri, C.; Pinzino, C.; Quartacci, M.F. Reactive Oxygen Species and Photosynthetic Functioning: Past and Present. In Reactive Oxygen Species in Plants; Singh, V.P., Singh, S., Tripathi, D.K., Prasad, S.M., Chauhan, D.K., Eds.; John Wiley & Sons Ltd.: Pisa, Italy, 2018; pp. 137–155. [Google Scholar]
- Kontominas, M.G. Olive Oil Packaging: Recent Developments. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing; Kiritsakis, A., Shahidi, F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 279–294. [Google Scholar]
- Pristouri, G.; Badeka, A.; Kontominas, M.G. Effect of Packaging Material Headspace, Oxygen and Light Transmission, Temperature and Storage Time on Quality Characteristics of Extra Virgin Olive Oil. Food Control 2010, 21, 412–418. [Google Scholar] [CrossRef]
- Lolis, A.; Badeka, A.V.; Kontominas, M.G. Effect of Bag-in-Box Packaging Material on Quality Characteristics of Extra Virgin Olive Oil Stored under Household and Abuse Temperature Conditions. Food Packag. Shelf Life 2019, 21, 100368. [Google Scholar] [CrossRef]
- de Leonardis, A.; Macciola, V.; Spadanuda, P.; Cuomo, F.; Diacylglycerols, D.A.G. Effects of Bag-in-box Packaging on Long-Term Shelf Life of Extra Virgin Olive Oil. Eur. Food Res. Technol. 2021, 247, 839–850. [Google Scholar] [CrossRef]
- Guillaume, C.; Ravetti, L. Shelf-Life Prediction of Extra Virgin Olive Oils Using an Empirical Model Based on Standard Quality Tests. J. Chem. 2016, 2016, 6393962. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Mylona, A.; Chiou, A.; Ioannou, M.S.; Andrikopoulos, N.K. Retention and Distribution of Natural Antioxidants (α-Tocopherol, Polyphenols and Terpenic Acids) after Shallow Frying of Vegetables in Virgin Olive Oil. LWT-Food Sci. Technol. 2007, 40, 1008–1017. [Google Scholar] [CrossRef]
- The Commission of the European Communities Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Off. J. Eur. Communities 1991, 248, 1–83.
- COI/T.20/Doc. No 15/Rev. 10; Sensory Analysis of Olive Oil. Method for the Organoleptic Assessment of Virgin Olive Oil. International Olive Council: Madrid, Spain, 2018.
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 662:2016; Animal and Vegetable Fats and Oils—Determination of Moisture and Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 659:2009; Oilseeds—Determination of Oil Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2009.
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, Antioxidant and Anti-Inflammatory Phenolic Activities in Extra Virgin Olive Oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, R.; Losito, I.; Castellaneta, A.; de Ceglie, C.; Calvano, C.D.; Cataldi, T.R.I. Insight into the Storage-Related Oxidative/Hydrolytic Degradation of Olive Oil Secoiridoids by Liquid Chromatography and High-Resolution Fourier Transform Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 12310–12325. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Zanoni, B.; Breschi, C.; Angeloni, G.; Masella, P.; Calamai, L.; Parenti, A. Understanding Olive Oil Stability Using Filtration and High Hydrostatic Pressure. Molecules 2020, 25, 420. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Willis, C.; Napolitano, M.P.; Keefe, A.J.O.; Kimball, B.A.; Slade, L.; Beauchamp, G.K. Oleocanthal and Oleacein from Privet Leaves: An Alternative Source for High-Value Extra Virgin Olive Oil Bioactives. Int. J. Mol. Sci. 2024, 25, 12020. [Google Scholar] [CrossRef] [PubMed]
- Salsano, J.E.; Digiacomo, M.; Cuffaro, D.; Bertini, S.; Macchia, M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods 2022, 11, 1354. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Markulin, L.; Corbin, C.; Renouard, S.; Drouet, S.; Gutierrez, L.; Mateljak, I.; Auguin, D.; Hano, C.; Fuss, E.; Lainé, E. Pinoresinol-Lariciresinol Reductases, Key to the Lignan Synthesis in Plants. Planta 2019, 249, 1695–1714. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Gharbi, I.; Dabbou, S.; Brahmi, F.; Nakbi, A. Impact of Packaging Material and Storage Time on Olive Oil Quality. Afr. J. Biotechnol. 2011, 10, 16937–16947. [Google Scholar] [CrossRef]
- European Commission. Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards marketing standards for olive oil, and repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union 2022, 284, 1–22. [Google Scholar]
- Predieri, S.; Medoro, C.; Magli, M.; Gatti, E.; Rotondi, A. Virgin Olive Oil Sensory Properties: Comparing Trained Panel Evaluation and Consumer Preferences. Food Res. Int. 2013, 54, 2091–2094. [Google Scholar] [CrossRef]
- Mateos, R.; Cert, A.; Carmen Pérez-Camino, M.; García, J.M. Evaluation of Virgin Olive Oil Bitterness by Quantification of Secoiridoid Derivatives. J. Am. Oil Chem. Soc. 2004, 81, 71–75. [Google Scholar] [CrossRef]
- Peyrot Des Gachons, C.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B.; Beauchamp, G.K.; Breslin, P.A.S. Unusual Pungency from Extra-Virgin Olive Oil Is Attributable to Restricted Spatial Expression of the Receptor of Oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Caporaso, N.; Sacchi, R. Flavor Chemistry of Virgin Olive Oil: An Overview. Appl. Sci. 2021, 11, 1639. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Barba-Palomeque, F.; Calderón-Santiago, M.; Penco-Valenzuela, J.M.; Priego-Capote, F. Phenolic Metabolism Explains Bitterness and Pungency of Extra Virgin Olive Oils. Foods 2025, 14, 1620. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Mondola, F.; Paduano, A.; Sacchi, R. Biophenolic Compounds Influence the In-Mouth Perceived Intensity of Virgin Olive Oil Flavours and off-Flavours. Molecules 2020, 25, 1969. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; O’Keefe, A.J.; Slade, L.; Beauchamp, G.K. Protein Suppresses Both Bitterness and Oleocanthal-Elicited Pungency of Extra Virgin Olive Oil. Sci. Rep. 2021, 11, 11851. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Moio, L. Salivary Protein-Tannin Interaction: The Binding behind Astringency. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: London, UK, 2021. [Google Scholar]

| 0 Months | 6 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| Chemical Class | Compound * | Initial Oil | Bag-in-Box | Stainless Steel with N2 | Bag-in-Box | Stainless Steel with N2 |
| Secoiridoids | Oleuropein | 0.558 ± 0.005 | ULQ | 0.568 ± 0.004 | 0.560 ± 0.020 | ULQ |
| Oleuropein aglycone | 51.000 ± 3.000 | 59.000 ± 4.000 | 64.000 ± 3.000 | 56.000 ± 2.000 | 67.000 ± 4.000 | |
| Ligstroside aglycone | 43.301 ± 1.700 | 57.000 ± 4.000 | 60.000 ± 3.000 | 45.000 ± 3.000 | 52.000 ± 2.000 | |
| Oleacein | 46.000 ± 2.000 | 44.000 ± 3.000 | 48.000 ± 2.000 | 41.500 ± 1.700 | 53.000 ± 3.000 | |
| Oleocanthal | 23.100 ± 1.300 | 24.300 ± 1.500 | 26.030 ± 1.120 | 17.900 ± 1.500 | 24.700 ± 1.400 | |
| Oleacenic acid | 0.569 ± 0.006 | 0.580 ± 0.015 | 0.592 ± 0.003 | 0.664 ± 0.016 | 0.686 ± 0.007 | |
| Oleocanthalic acid | 0.758 ± 0.019 | 0.950 ± 0.080 | 0.983 ± 0.019 | 1.770 ± 0.040 | 1.810 ± 0.060 | |
| Hydroxy oleuropein aglycone | 0.974 ± 0.017 | 1.030 ± 0.030 | 1.120 ± 0.040 | 1.520 ± 0.020 | 1.152 ± 0.016 | |
| Hydro oleuropein aglycone | 0.558 ± 0.005 | ULQ | 0.568 ± 0.004 | 0.560 ± 0.020 | 0.558 ± 0.002 | |
| Methyl oleuropin aglycone | 0.560 ± 0.005 | ULQ | 0.569 ± 0.005 | 0.560 ± 0.020 | 0.559 ± 0.002 | |
| Hydroxyelolonolic acid | 0.621 ± 0.016 | 0.613 ± 0.013 | 0.650 ± 0.030 | 0.710 ± 0.030 | 0.663 ± 0.009 | |
| Secoiridoid derivatives | Elenolic acid | 3.500 ± 0.800 | 4.500 ± 1.200 | 5.200 ± 0.900 | 10.500 ± 0.300 | 6.200 ± 0.900 |
| HCM-EA (isomer I) | 0.581 ± 0.006 | 0.600 ± 0.020 | ULQ | 0.748 ± 0.015 | 0.790 ± 0.007 | |
| HCM-EA (Isomer II) | 0.577 ± 0.005 | 0.595 ± 0.017 | 0.606 ± 0.002 | 0.660 ± 0.020 | ULQ | |
| Phenolic alcohols | Hydroxytyrosol | ULQ | ULQ | ULQ | 0.120 ± 0.050 | ULQ |
| Flavonoids | Apigenin | 1.280 ± 0.03 | ULQ | 1.290 ± 0.020 | 1.190 ± 0.030 | 1.210 ± 0.040 |
| Luteolin | ULQ | 2.604 ± 0.114 | 2.690 ± 0.170 | 3.060 ± 0.060 | 3.000 ± 0.200 | |
| Phenolic acids | Ferulic acid | ULQ | 0.075 ± 0.009 | ULQ | 0.0920 ± 0.004 | ULQ |
| Lignans | Pinoresinol | 4.180 ± 0.090 | 4.200 ± 0.200 | 4.164 ± 0.105 | 3.760 ± 0.020 | 3.900 ± 0.080 |
| Acetoxypinoresinol | 0.605 ± 0.005 | 0.604 ± 0.013 | 0.616 ± 0.005 | 0.600 ± 0.030 | 0.605 ± 0.002 | |
| Lariciresinol | 15.400 ± 1.400 | 13.900 ± 0.600 | 14.200 ± 0.700 | 9.400 ± 1.400 | 12.300 ± 1.300 | |
| Isolariciresinol | 3.700 ± 0.600 | 3.700 ± 0.400 | 4.500 ± 0.600 | 3.500 ± 0.400 | 4.200 ± 0.300 | |
| Secoisolariciresinol | ULQ | 0.156 ± 0.003 | ULQ | 0.155 ± 0.006 | ULQ | |
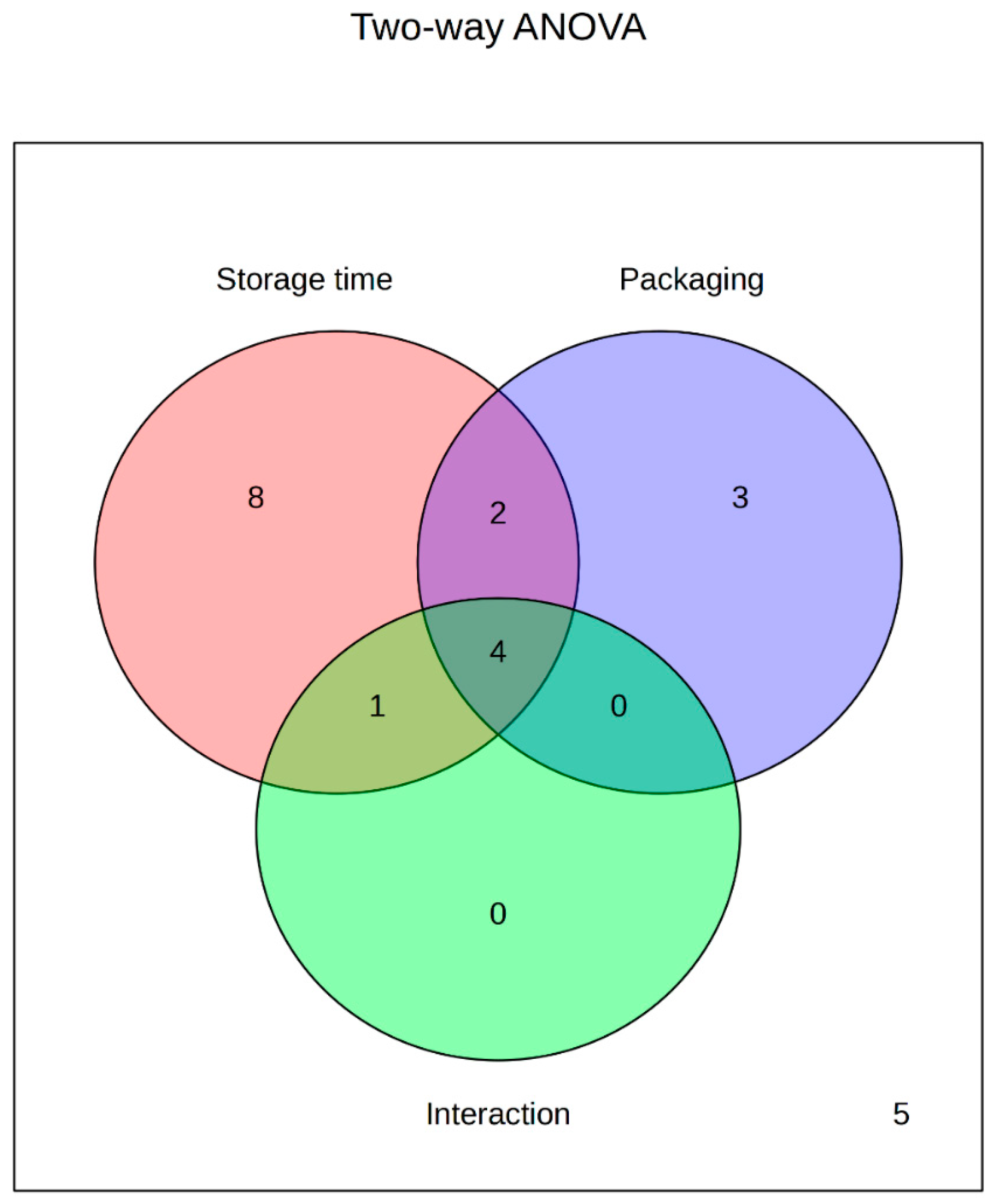
| Chemical Class | Compound | Storage Time | Packaging | Interaction: Storage Time–Packaging |
|---|---|---|---|---|
| Secoiridoids | Oleuropein | |||
| Oleuropein aglycone | ||||
| Ligstroside aglycone | * | * | ||
| Oleacein | * | |||
| Oleocanthal | * | * | * | |
| Oleacenic acid | * | * | ||
| Oleocanthalic acid | * | |||
| Hydroxy oleuropein aglycone | * | * | * | |
| Hydro oleuropein aglycone | ||||
| Methyl oleuropin aglycone | ||||
| Hydroxyelolonolic acid | * | * | ||
| Secoiridoid derivatives | Elenolic acid | * | * | * |
| HCM-EA (isomer I) | * | * | ||
| HCM-EA (Isomer II) | * | |||
| Phenolic alcohols | Hydroxytyrosol | * | * | * |
| Flavonoids | Apigenin | * | ||
| Luteolin | * | |||
| Phenolic acids | Ferulic acid | * | ||
| Lignans | Pinoresinol | * | ||
| Acetoxypinoresinol | ||||
| Lariciresinol | * | |||
| Isolariciresinol | * | * | ||
| Secoisolariciresinol |
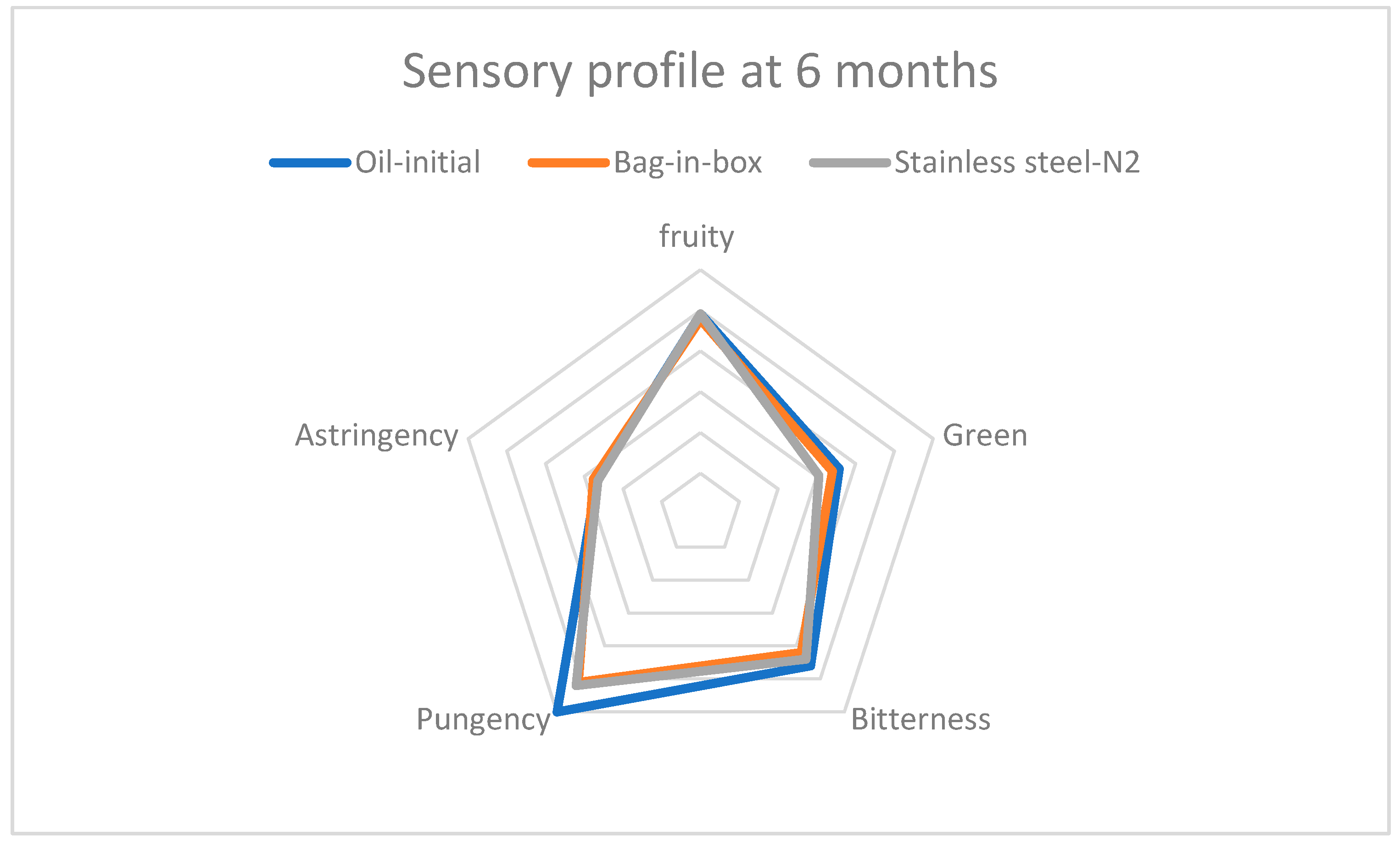
| 0 Months | 6 Months | 12 Months | |||
|---|---|---|---|---|---|
| Attribute * | Initial Oil | Bag-in-Box | Stainless Steel with N2 | Bag-in-Box | Stainless Steel with N2 |
| Fruity | 4.92 ± 0.51 | 4.78 ± 0.13 | 4.92 ± 0.30 | 5.00 ± 0.13 | 4.40 ± 0.44 |
| Green | 3.58 ± 0.45 | 3.40 ± 0.23 | 3.05 ± 0.48 | 3.55 ± 0.26 | 2.87 ± 0.15 |
| Bitterness | 4.60 ± 0.33 | 4.20 ± 0.23 | 4.40 ± 0.20 | 4.72 ± 0.12 | 4.32 ± 0.29 |
| Pungency | 6.00 ± 0.43 | 5.10 ± 0.13 | 5.20 ± 0.18 | 5.65 ± 0.10 | 4.70 ± 0.20 |
| Astringency | 2.68 ± 0.66 | 2.77 ± 0.13 | 2.65 ± 0.28 | 3.35 ± 0.44 | 2.63 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuhabib, M.M.; Campins-Machado, F.M.; Lozano-Castellón, J.; Ninot, A.; Romero-Aroca, A.; Lamuela-Raventós, R.M.; Pérez, M.; Vallverdú-Queralt, A. Evaluation of Packaging Effects on the Phenolic Profile and Sensory Characteristics of Extra Virgin Olive Oil During Storage Using Liquid Chromatography Coupled with Mass Spectrometry. Foods 2025, 14, 2532. https://doi.org/10.3390/foods14142532
Abuhabib MM, Campins-Machado FM, Lozano-Castellón J, Ninot A, Romero-Aroca A, Lamuela-Raventós RM, Pérez M, Vallverdú-Queralt A. Evaluation of Packaging Effects on the Phenolic Profile and Sensory Characteristics of Extra Virgin Olive Oil During Storage Using Liquid Chromatography Coupled with Mass Spectrometry. Foods. 2025; 14(14):2532. https://doi.org/10.3390/foods14142532
Chicago/Turabian StyleAbuhabib, Mohamed M., Francesc M. Campins-Machado, Julián Lozano-Castellón, Antònia Ninot, Agustí Romero-Aroca, Rosa M. Lamuela-Raventós, Maria Pérez, and Anna Vallverdú-Queralt. 2025. "Evaluation of Packaging Effects on the Phenolic Profile and Sensory Characteristics of Extra Virgin Olive Oil During Storage Using Liquid Chromatography Coupled with Mass Spectrometry" Foods 14, no. 14: 2532. https://doi.org/10.3390/foods14142532
APA StyleAbuhabib, M. M., Campins-Machado, F. M., Lozano-Castellón, J., Ninot, A., Romero-Aroca, A., Lamuela-Raventós, R. M., Pérez, M., & Vallverdú-Queralt, A. (2025). Evaluation of Packaging Effects on the Phenolic Profile and Sensory Characteristics of Extra Virgin Olive Oil During Storage Using Liquid Chromatography Coupled with Mass Spectrometry. Foods, 14(14), 2532. https://doi.org/10.3390/foods14142532









