Genetic, Diversity, and Muscle Quality Among Red and Green Color Morphs of Asian Swimming Crab (Charybdis japonica): Implications for Accurate Species Recognition and Sustainable Management
Abstract
1. Introduction
2. Materials and Methods
2.1. C. japonica Collection
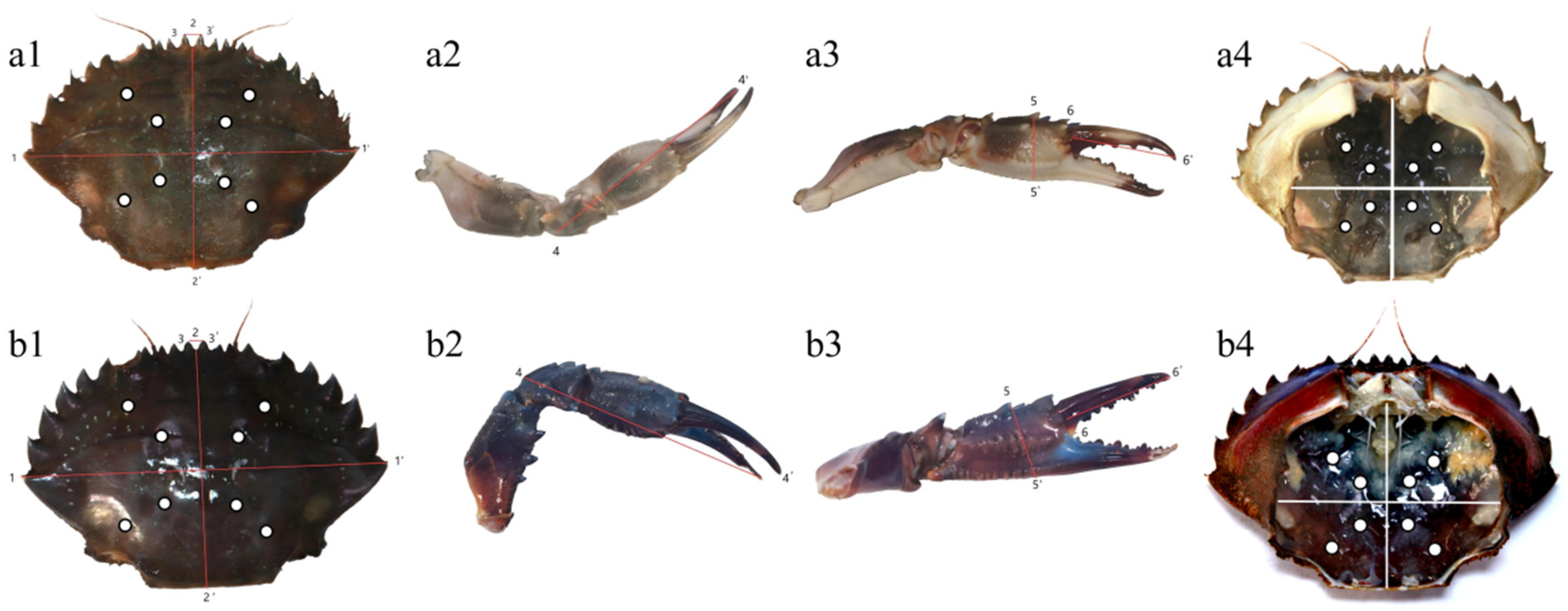
2.2. Determination of Morphological Parameters
2.3. DNA Extraction and Sequencing
2.4. Determination of Body Color and Histological Examination
2.5. Analysis of Muscle Texture and Amino Acid Content
2.6. Statistical Analysis
3. Results
3.1. Body Color and Histological Observations
3.2. Morphological Characteristics of C. japonica
3.3. Mitochondrial COI and ITS-1 Gene Sequence Differences Analysis of C. japonica
3.4. Differences in Muscle Quality Between Two Color Morphs of C. japonica
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ertl, N.G.; Elizur, A.; Brooks, P.; Kuballa, A.V.; Anderson, T.A.; Knibb, W.R. Molecular Characterisation of Colour Formation in the Prawn Fenneropenaeus merguiensis. PLoS ONE 2013, 8, e56920. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, R.; Cancian, S.; Ghia, D.; Fea, G.; Coladonato, A. Color variation in signal crayfish Pacifastacus leniusculus. Curr. Zool. 2021, 67, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Price, T.D. Phenotypic plasticity, sexual selection and the evolution of colour patterns. J. Exp. Biol. 2006, 209, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Darnell, M.Z. Ecological physiology of the circadian pigmentation rhythm in the fiddler crab Uca panacea. J. Exp. Mar. Biol. Ecol. 2012, 426–427, 39–47. [Google Scholar] [CrossRef]
- Freire, A.S.; Pinheiro, M.A.A.; Karam-Silva, H.; Teschima, M.M. Biology of Grapsus grapsus (Linnaeus, 1758) (Brachyura, Grapsidae) in the Saint Peter and Saint Paul Archipelago, Equatorial Atlantic Ocean. Helgol. Mar. Res. 2011, 65, 263–273. [Google Scholar] [CrossRef]
- Kronstadt, S.M.; Darnell, M.Z.; Munguia, P. Background and temperature effects on Uca panacea color change. Mar. Biol. 2013, 160, 1373–1381. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Zhang, C.; Cheng, Y.; Wu, X. Can color-related traits in the Chinese mitten crab (Eriocheir sinensis) be improved through quantitative genetic inheritance? Aquaculture 2019, 512, 734355. [Google Scholar] [CrossRef]
- McNamara, J.; Milograna, S. Adaptive Color Change and the Molecular Endocrinology of Pigment Translocation in Crustacean Chromatophores. In The Natural History of the Crustacea; Oxford University Press: Oxford, UK, 2015; pp. 68–102. [Google Scholar]
- Lou, F.; Wang, Y.; Han, Z.; Shui, B. Comparative Transcriptome Reveals the Molecular Regulation Mechanism of Charybdis japonica to High- and Low-Temperature Stresses. Front. Mar. Sci. 2022, 9, 849485. [Google Scholar] [CrossRef]
- Gust, N.; Inglis, G.J. Adaptive Multi-scale Sampling to Determine an Invasive Crab’s Habitat Usage and Range in New Zealand. Biol. Invasions 2006, 8, 339–353. [Google Scholar] [CrossRef]
- Hong-Yu, L.; Lu-Qing, P.; Debin, Z. Effects of Salinity on Biogenic Amines, Hemolymph Osmotic Pressure, and Activity of Gill’s Na+/K+-ATPase in Charybdis japonica (Crustacea, Decapoda). J. World Aquac. Soc. 2008, 39, 812–820. [Google Scholar] [CrossRef]
- Yu, M.; Herrmann, B.; Cerbule, K.; Liu, C.; Zhang, L.; Tang, Y. Effect of gillnet mesh size on the capture probability and capture patterns in the Asian paddle crab (Charybdis japonica) fishery. Heliyon 2024, 10, e25771. [Google Scholar] [CrossRef] [PubMed]
- ELAmouri, R.; Tu, Z.; Abo-Raya, M.H.; Pang, X.; Hu, M.; Wang, Y. Nano-TiO2 impairs the health of crabs Charybdis japonica under warming conditions through waterborne and dietary exposures. J. Hazard. Mater. 2025, 487, 137092. [Google Scholar] [CrossRef]
- Sun, S.; He, Z.; Zhang, F.; Han, Z. Comparative transcriptome analysis of low- and high-latitude populations of Charybdis japonica under temperature stress. Front. Mar. Sci. 2024, 11, 1491685. [Google Scholar] [CrossRef]
- Wu, F.; Wang, T.; Cui, S.; Xie, Z.; Dupont, S.; Zeng, J.; Gu, H.; Kong, H.; Hu, M.; Lu, W.; et al. Effects of seawater pH and temperature on foraging behavior of the Japanese stone crab Charybdis japonica. Mar. Pollut. Bull. 2017, 120, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes Method for the Optimal Superimposition of Landmarks. Syst. Biol. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Parkes, L.; Quinitio, E.T.; Le Vay, L. Phenotypic differences between hatchery-reared and wild mud crabs, Scylla serrata, and the effects of conditioning. Aquac. Int. 2011, 19, 361–380. [Google Scholar] [CrossRef]
- Olesen, J. Crustacean Life Cycles—Developmental Strategies and Environmental Adaptations. In Life Histories; Oxford Academic: New York, NY, USA, 2018; pp. 1–34. [Google Scholar]
- Zhao, Y.; Zhu, X.; Jiang, Y.; Li, Z.; Li, X.; Xu, W.; Wei, H.; Li, Y.; Li, X. Genetic diversity and variation of seven Chinese grass shrimp (Palaemonetes sinensis) populations based on the mitochondrial COI gene. BMC Ecol. Evol. 2021, 21, 167. [Google Scholar] [CrossRef]
- Wang, W.; Ma, C.; Chen, W.; Jin, Z.; Zhao, M.; Zhang, F.; Liu, Z.; Ma, L. Population genetic diversity of mud crab (Scylla paramamosain) from southeast coastal regions of China based on mitochondrial COI gene sequence. Gene 2020, 751, 144763. [Google Scholar] [CrossRef]
- Ren, G.; Miao, G.; Ma, C.; Lu, J.; Yang, X.; Ma, H. Genetic structure and historical demography of the blue swimming crab (Portunus pelagicus) from southeastern sea of China based on mitochondrial COI gene. Mitochondrial DNA Part A 2016, 29, 192–198. [Google Scholar] [CrossRef]
- Choubert, G.; Mendes-Pinto, M.M.; Morais, R. Pigmenting efficacy of astaxanthin fed to rainbow trout Oncorhynchus mykiss: Effect of dietary astaxanthin and lipid sources. Aquaculture 2006, 257, 429–436. [Google Scholar] [CrossRef]
- Yanar, Y.; Büyükçapar, H.; Yanar, M.; Göcer, M. Effect of carotenoids from red pepper and marigold flower on pigmentation, sensory properties and fatty acid composition of rainbow trout. Food Chem. 2007, 100, 326–330. [Google Scholar] [CrossRef]
- Li, T.; Kuang, S.; Hu, L.; Nie, P.; Ramaswamy, H.S.; Yu, Y. Influence of the pressure shift freezing and thawing on the microstructure of largemouth bass. Innov. Food Sci. Emerg. Technol. 2022, 82, 103176. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Wang, Y.; Tang, R.; Li, D. The growth performance, antioxidative status and muscle quality of grass carp (Ctenopharyngodon Idellus) cultured in the recirculating pond aquaculture system (RPAS). Aquaculture 2023, 562, 738829. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Cheng, Y.; Wu, X. Growth performance, gonad development and nutritional composition of Chinese mitten crab Eriocheir sinensis selected for growth and different maturity time. Aquaculture 2020, 523, 735194. [Google Scholar] [CrossRef]
- Hidayani, A.A.; Trijuno, D.D.; Fujaya, Y.; Umar, M.T. The morphology and morphometric characteristics of the male swimming crab (Portunus pelagicus) from the East Sahul Shelf, Indonesia. Aquac. Aquar. Conserv. Legis. 2018, 11, 1724–1736. [Google Scholar]
- Gao, B.-Q.; Ping, L.; Jian, L.; Dai, F.-Y.; Shen, M.; Liu, P.; Li, J.; Ma, S.; Dai, F.; Gao, B. Analysis of morphological variations among four wild populations of Portunus trituberculatus. J. Fish. Sci. China 2007, 14, 223–228. [Google Scholar]
- Mayr, E.; Ashlock, P.D. Principles of Systematic Zoology; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Li, Y.L.; Kong, X.Y.; Yu, Z.N.; Kong, J.; Ma, S.; Chen, L.M. Genetic diversity and historical demography of Chinese shrimp Feneropenaeus chinensis in Yellow Sea and Bohai Sea based on mitochondrial DNA analysis. Afr. J. Biotechnol. 2009, 8, 1193–1202. [Google Scholar]
- Ren, G.; Ma, H.; Ma, C.; Wang, W.; Chen, W.; Ma, L. Genetic diversity and population structure of Portunus sanguinolentus (Herbst, 1783) revealed by mtDNA COI sequences. Mitochondrial DNA Part A 2017, 28, 740–746. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Shang, M.; Wu, X.; Cheng, Y. Genetic diversity and population structure of native mitten crab (Eriocheir sensu stricto) by microsatellite markers and mitochondrial COI gene sequence. Gene 2019, 693, 101–113. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Chi, D.; Gao, H.; Shen, S.; Yan, B. Phylogenetic Relationships Between Purple and Tea-Green Individuals of the Blue Swimming Crab Portunus trituberculatus by PCR-SSCP Analysis. Mar. Sci. Bull. 2009, 2, 79–88. [Google Scholar]
- Calo-Mata, P.; Pascoal, A.; Fernández-No, I.; Böhme, K.; Gallardo, J.M.; Barros-Velázquez, J. Evaluation of a novel 16S rRNA/tRNAVal mitochondrial marker for the identification and phylogenetic analysis of shrimp species belonging to the superfamily Penaeoidea. Anal. Biochem. 2009, 391, 127–134. [Google Scholar] [CrossRef]
- Yu, J.; Han, Y.; Jiang, Y.; Chen, Y.; Li, X.; Liu, X.; Liu, Q. Ovarian development and mating choice in Asian swimming crab Charybdis japonica. J. Dalian Ocean. Univ. 2016, 31, 477–481. [Google Scholar]
- Silva, D.J.A.; Erickson, M.F.; Dos Santos Guidi, R.; Pessoa, D.M.A. Thin-fingered fiddler crabs display a natural preference for UV light cues but show no sensory bias to other hypertrophied claw coloration. Behav. Process. 2022, 200, 104667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, Z.; Jiang, X.; Liu, Y.; Chen, X.; Wu, X. The comparison of morphology and transcriptome in the inner membrane reveals the potential mechanism of the heritable carapace color of the Chinese mitten crab Eriocheir sinensis. Gene 2024, 897, 148058. [Google Scholar] [CrossRef]
- de Coursey, P.J.; O’Hara, J. Multiple environmental factor effects on physiology and behavior of the fiddler crab, Uca pugilator. In Pollution and Physiology of Marine Organisms; Academic Press: New York, NY, USA, 2013; p. 381. [Google Scholar]
- Silbiger, N.; Munguia, P. Carapace color change in Uca pugilator as a response to temperature. J. Exp. Mar. Biol. Ecol. 2008, 355, 41–46. [Google Scholar] [CrossRef]
- Cronin, T.W.; Caldwell, R.L.; Erdmann, M.V. Tuning of photoreceptor function in three mantis shrimp species that inhabit a range of depths. I. Visual pigments. J. Comp. Physiol. A 2002, 188, 179–186. [Google Scholar] [CrossRef]
- Fukuda, A.; Sato, K.; Fujimori, C.; Yamashita, T.; Takeuchi, A.; Ohuchi, H.; Umatani, C.; Kanda, S. Direct photoreception by pituitary endocrine cells regulates hormone release and pigmentation. Science 2025, 387, 43–48. [Google Scholar] [CrossRef]
- Sun, X.; Chang, Y.; Ye, Y.; Ma, Z.; Liang, Y.; Li, T.; Jiang, N.; Xing, W.; Luo, L. The effect of dietary pigments on the coloration of Japanese ornamental carp (koi, Cyprinus carpio L.). Aquaculture 2012, 342–343, 62–68. [Google Scholar] [CrossRef]
- Aguilera Barraza, F.A.; León, R.A.Q.; Álvarez, P.X.L. Kinetics of protein and textural changes in Atlantic salmon under frozen storage. Food Chem. 2015, 182, 120–127. [Google Scholar] [CrossRef]
- Archile-Contreras, A.C.; Purslow, P.P. Oxidative stress may affect meat quality by interfering with collagen turnover by muscle fibroblasts. Food Res. Int. 2011, 44, 582–588. [Google Scholar] [CrossRef]
- He, C.; Geng, H.; Qin, Y.; Yang, P.; Wang, W.; Mai, K.; Song, F. Dietary xanthophyll improved growth, antioxidant, pigmentation and meat quality in the southern catfish (Silurus soldatovi meridionalis Chen). Anim. Nutr. 2023, 13, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, K. Umami the Fifth Basic Taste: History of Studies on Receptor Mechanisms and Role as a Food Flavor. Biomed Res. Int. 2015, 2015, 189402. [Google Scholar] [CrossRef] [PubMed]




| Species | Genebank Accession Number | Length of Fragments (bp) |
|---|---|---|
| Charybdis feriatus | EU284140 (COI) | 709 |
| Charybdis acuta | EU284143 (COI) | 709 |
| Portunus pelagicus | DQ889124 (COI) | 657 |
| Portunus trituberculatus | GU321231 (COI) | 658 |
| Scylla serrata | JN085429 (COI) | 659 |
| AY181979 (ITS-1) | 653 | |
| Eriocheir sinensis | HQ534047 (COI) | 686 |
| AY181978 (ITS-1) | 612 | |
| Callinectes saqidus | AY682078 (COI) | 1534 |
| AY781436 (ITS-1) | 1864 | |
| Macrobrachium nipponense | EU346851 (ITS-1) | 1725 |
| Morphologic Parameters | ♂ Red Morph (n = 32) | ♂ Green Morph (n = 40) | ♀ Red Morph (n = 33) | ♀ Green Morph (n = 31) |
|---|---|---|---|---|
| CL | 51.72 ± 4.54 a | 47.96 ± 5.03 b | 46.29 ± 4.54 b | 44.31 ± 2.87 b |
| CW | 77.91 ± 7.50 a | 71.27 ± 8.25 b | 68.84 ± 6.45 b | 66.44 ± 4.39 b |
| CH | 29.50 ± 2.98 a | 28.57 ± 9.01 a | 25.86 ± 2.39 a | 25.82 ± 3.45 a |
| BW | 111.57 ± 36.16 a | 80.49 ± 29.07 b | 66.21 ± 18.98 b | 58.06 ± 10.77 b |
| FOMW | 38.99 ± 2.46 a | 36.41 ± 3.12 b | 35.68 ± 3.19 b | 34.38 ± 1.87 b |
| FFLC | 26.93 ± 3.99 a | 24.25 ± 3.58 b | 19.01 ± 1.87 c | 18.50 ± 1.65 c |
| MLC | 30.40 ± 3.75 a | 27.48 ± 4.08 b | 22.47 ± 2.66 c | 21.51 ± 1.57 c |
| PW | 22.84 ± 3.84 a | 17.59 ± 2.81 b | 16.66 ± 2.73 b | 15.53 ± 1.83 b |
| ST | 0.71 ± 0.12 a | 0.62 ± 0.09 b | 0.63 ± 0.07 b | 0.60 ± 0.07 b |
| Morphologic Parameters Ratio | ♂ Red Morph (n = 32) | ♂ Green Morph (n = 40) | ♀ Red Morph (n = 33) | ♀ Green Morph (n = 31) |
|---|---|---|---|---|
| CL/CW | 0.66 ± 0.01 b | 0.67 ± 0.02 a | 0.67 ± 0.01 a | 0.67 ± 0.01 a |
| CH/CW | 0.38 ± 0.01 a | 0.40 ± 0.10 a | 0.38 ± 0.01 a | 0.39 ± 0.05 a |
| FOMW/CW | 0.50 ± 0.02 a | 0.51 ± 0.02 a | 0.52 ± 0.01 a | 0.52 ± 0.01 a |
| FFLC/CW | 0.34 ± 0.02 a | 0.34 ± 0.02 a | 0.28 ± 0.01 b | 0.28 ± 0.01 b |
| MLC/CW | 0.39 ± 0.02 a | 0.39 ± 0.02 a | 0.33 ± 0.02 b | 0.32 ± 0.01 b |
| PW/CW | 0.29 ± 0.03 a | 0.25 ± 0.02 b | 0.24 ± 0.02 b | 0.23 ± 0.02 b |
| BW/CW | 1.40 ± 0.33 a | 1.10 ± 0.25 b | 0.95 ± 0.19 c | 0.87 ± 0.11 c |
| ST/CW | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Morphologic Parameters Ratio | ♂ Red Morph | ♂ Green Morph | Coefficient of Variation |
|---|---|---|---|
| CL/CW | 0.66 ± 0.10 | 0.67 ± 0.02 | 0.37 |
| BW/CW | 1.40 ± 0.33 | 1.10 ± 0.25 | 0.52 |
| PW/CW | 0.29 ± 0.03 | 0.25 ± 0.02 | 1.02 |
| Amino Acids (g/100 g Dry Matter) | Content | ||
|---|---|---|---|
| Red Morph | Green Morph | p-Value | |
| Essential amino acid (EAA) | |||
| Leucine | 0.93 ± 0.23 | 0.89 ± 0.21 | 0.827 |
| Isoleucine | 0.53 ± 0.14 | 0.48 ± 0.12 | 0.662 |
| Phenylalanine * | 0.48 ± 0.08 | 0.47 ± 0.09 | 0.807 |
| Lysine | 0.88 ± 0.29 | 0.85 ± 0.28 | 0.905 |
| Methionine | 0.18 ± 0.11 | 0.21 ± 0.08 | 0.732 |
| Threonine | 0.52 ± 0.06 | 0.51 ± 0.05 | 0.847 |
| Valine | 0.59 ± 0.15 | 0.54 ± 0.14 | 0.695 |
| Semi essential amino acid (SEAA) | |||
| Histidine | 0.26 ± 0.06 | 0.25 ± 0.07 | 0.841 |
| Arginine | 1.29 ± 0.35 | 1.04 ± 0.11 | 0.307 |
| Non-essential amino acid (NEAA) | |||
| Glutamic Acid * | 1.60 ± 0.46 | 1.50 ± 0.50 | 0.807 |
| Aspartic Acid * | 0.87 ± 0.31 | 0.84 ± 0.31 | 0.929 |
| Glycine | 1.08 ± 0.24 | 1.03 ± 0.18 | 0.754 |
| Alanine * | 0.91 ± 0.22 | 0.83 ± 0.21 | 0.660 |
| Serine | 0.37 ± 0.10 | 0.36 ± 0.10 | 0.886 |
| Proline | 0.74 ± 0.07 | 0.78 ± 0.04 | 0.497 |
| Tyrosine * | 0.43 ± 0.05 | 0.45 ± 0.13 | 0.799 |
| ∑AA | 11.66 ± 2.84 | 11.01 ± 2.52 | 0.779 |
| ∑EAA | 4.11 ± 1.03 | 3.94 ± 0.96 | 0.845 |
| ∑DAA | 4.29 ± 1.11 | 4.08 ± 1.22 | 0.839 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; He, Y.; Meenu, M.; Liu, Y.; Jiang, Y. Genetic, Diversity, and Muscle Quality Among Red and Green Color Morphs of Asian Swimming Crab (Charybdis japonica): Implications for Accurate Species Recognition and Sustainable Management. Foods 2025, 14, 2516. https://doi.org/10.3390/foods14142516
Zhang B, He Y, Meenu M, Liu Y, Jiang Y. Genetic, Diversity, and Muscle Quality Among Red and Green Color Morphs of Asian Swimming Crab (Charybdis japonica): Implications for Accurate Species Recognition and Sustainable Management. Foods. 2025; 14(14):2516. https://doi.org/10.3390/foods14142516
Chicago/Turabian StyleZhang, Bingqian, Yuhang He, Maninder Meenu, Ying Liu, and Yusheng Jiang. 2025. "Genetic, Diversity, and Muscle Quality Among Red and Green Color Morphs of Asian Swimming Crab (Charybdis japonica): Implications for Accurate Species Recognition and Sustainable Management" Foods 14, no. 14: 2516. https://doi.org/10.3390/foods14142516
APA StyleZhang, B., He, Y., Meenu, M., Liu, Y., & Jiang, Y. (2025). Genetic, Diversity, and Muscle Quality Among Red and Green Color Morphs of Asian Swimming Crab (Charybdis japonica): Implications for Accurate Species Recognition and Sustainable Management. Foods, 14(14), 2516. https://doi.org/10.3390/foods14142516






