1. Introduction
Sarcopenia is defined as a decrease in skeletal muscle mass, strength, or function. The most prevalent form of muscle atrophy in humans is the muscle loss associated with aging, which is characterized by a reduction in muscle mass and strength, as well as diminished mobility [
1]. The area and number of muscle fibers will decrease with age [
2,
3]. Additionally, aging may disrupt the interaction between myosatellite cells and their surrounding cells, leading to impaired muscle regeneration [
4]. At this stage, numerous studies have demonstrated that various natural polyphenols can effectively treat skeletal muscle diseases. The study by Chen et al. [
5] indicated that tea polyphenols can alleviate symptoms of muscle atrophy in mice through the mechanism of mitochondrial quality control. Tea polyphenols can reduce apoptosis of skeletal muscle cells and improve exercise ability [
6]. Polyphenols can activate the Kelch-like ECH-associated protein 1- Nuclear factor erythroid 2-related factor 2 (Keap1-Nrf2) signaling pathway, significantly improving the activity of antioxidant enzymes and the expression of antioxidant genes in mice [
7]. Human experiments have shown that polyphenols may have a positive impact on muscle damage through pathways such as regulating inflammatory factors [
8].
Endocrine hormones play a crucial role in regulating skeletal muscle metabolism. They help stabilize the balance between the synthesis and degradation of myocyte proteins, thereby maintaining skeletal muscle mass and function. The reduction in the secretion of various endocrine hormones such as growth hormone, sex hormones, and insulin is a direct cause of skeletal muscle atrophy. Although the mechanisms underlying sarcopenia are not yet fully understood, some studies suggest that a reduction in androgen levels is one of the contributing factors [
9]. Testosterone (T) is a steroid hormone secreted by the male testes or female ovaries. It helps maintain muscle strength and mass, supports bone density and strength, and can also boost energy and enhance physical performance. Testosterone is the primary androgen in males and can promote muscle regeneration and repair by increasing the number of muscle satellite cells [
10]. Testosterone promotes the expression of insulin-like growth factor-1 (IGF-I) in muscles, thereby activating the Akt-mTOR signaling pathway, which induces muscle hypertrophy [
11]. Testosterone can directly stimulate the Ras/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase (Ras/MEK/ERK) pathway in muscle cells, inhibiting the expression of myostatin [
12]. Testosterone also has a strong anti-apoptotic effect in muscle, leading to the inactivation of Forkhead box O (FOXO) and inhibiting the upregulation of pro-apoptotic genes [
13]. Numerous studies have shown that many natural polyphenolic substances can promote the secretion of steroid hormones. Daidzein can regulate the expression of genes such as
StAR,
P450scc, and
3β-HSD, promoting the synthesis of steroid hormones in granulosa cells [
14]. Cheng et al. [
15] found in their study on piglets that soy isoflavones can upregulate the serum testosterone levels.
Tea has a long history in China. Drinking tea not only refreshes the mind but also promotes health and wellness, making it highly popular among people. Catechins are the main components of tea polyphenols. Research shows that catechins have pharmacological effects such as scavenging free radicals in the body as well as anti-aging and anti-radiation effects. The beneficial properties of catechins on skeletal muscle are mainly reflected in enhancing SkM satellite cell differentiation, promoting muscle mitochondrial synthesis, enhancing capillary biogenesis, and combating age-related SkM degeneration [
16]. Catechins promote myoblast differentiation by stimulating the myogenic signaling pathway and the p38 mitogen-activated protein kinase (p38MAPK)/protein kinase B (AKT) pathway. Catechin can also positively regulate
MyoD-mediated muscle-specific gene expression (such as
MyHC and
MyoG), increasing the conversion of fibroblasts [
17]. To further explore the role of catechins in promoting skeletal muscle differentiation and their molecular mechanisms, the research team previously used four different catechins to culture C2C12 cells; measured the relative expression levels of muscle differentiation markers
MyoD,
MyoG, and
MyHC mRNAs; and assessed the number, length, and diameter of differentiated myotubes. The results indicated that epicatechin gallate (ECG) had a stronger pro-differentiation effect than other catechin monomers, and its pro-differentiation effect increased with concentration [
18]. The pro-differentiation effect of ECG suggests that it may have the potential to improve sarcopenia.
To investigate the impact and mechanism of ECG on the motor function and differentiation of skeletal muscle in aged mice, this study, based on previous research, utilized 54-week-old C57BL/6J mice as the research subjects. Animal behavioral experiments were conducted to assess changes in motor ability across different age groups of mice. Tissue sectioning and transcriptomic analysis were employed to preliminarily analyze the mechanistic effects of ECG on sarcopenia of aging. Additionally, this study verified the effect of ECG on steroid hormones using the TM3 cell model. It was found that ECG influenced the gene expression of testosterone synthetase, as demonstrated using qRT-PCR and Western blot experiments. Furthermore, the potential target of ECG in affecting skeletal muscle differentiation was predicted using network pharmacology. In summary, our study is the first to demonstrate that ECG can influence the function and differentiation of skeletal muscle in aged mice by regulating the synthesis and metabolism of testosterone, providing a theoretical basis for ECG as a potential dietary supplement for the protection of aging skeletal muscle.
2. Materials and Methods
2.1. Experimental Materials and Animals
ECG (purity 98%) was purchased from Hunan Sunfull Bio-tech Co., Ltd. (Changsha, China).
Sixty healthy male C57BL/6J mice of different ages were provided by Jicui Pharmaceutical Biotechnology Co., Ltd. (Nanjing, China). All animals were acclimatized for one week, with a rearing environment temperature of 25 ± 1 °C, relative humidity of 50 ± 1%, and a light/dark cycle of 12/12 h. All animal experiments were conducted in accordance with the animal husbandry regulations of Hunan Agricultural University (Approval No. 2025 No. 4). The subjects were divided into several groups: the juvenile control group (Y-C) consisting of 10 mice aged 3 weeks, the juvenile ECG group (Y-ECG) with 10 mice also aged 3 weeks, the middle-aged control group (M-C) comprising 10 mice aged 26 weeks, the middle-aged ECG group (M-ECG) with 10 mice aged 26 weeks, the elderly control group (O-C) containing 10 mice aged 54 weeks, and the elderly ECG group (O-ECG) with 10 mice aged 54 weeks.
According to the “Scientific opinion on the safety of green tea catechins” published by the European Food Safety Authority (EFSA) [
19], it is pointed out that the daily intake of EGCG in human body is safe under 800 mg. According to the dose conversion between human and experimental animals [
20], the maximum dose of ECG was 160 mg/kg/d (800 mg/60 kg/d ∗ 12.3 = 164 mg/kg/day). Following the grouping, the control group received a normal diet supplemented with gavage drinking water (160 mg/kg/d), whereas the experimental group was given a normal diet supplemented with gavage ECG (160 mg/kg/d). The experiment was conducted over a period of 8 weeks, during which the initial weight of the mice was recorded at the start and subsequently measured weekly. Animal behavioral tests were conducted after the completion of the gavage treatment.
2.2. Motor Ability Test of Mice
The grip strength of the mice was assessed using an electronic grip tester (Shanghai, China). The grip meter platform was positioned horizontally to allow the mice’s limbs to grasp the sensing rod of the grip meter. The mouse’s tail was then pulled horizontally backward until its forelimbs released the sensing rod. This procedure was repeated five times to collect measurement data. The maximum value of the median grip strength was recorded as the absolute grip of the mice (N). The relative grip (N/g) was calculated by dividing the absolute grip by the weight of the mouse (N).
An animal treadmill (Huaibei, China) was used for testing. The animal treadmill has a total of 8 lanes for mice to undergo exercise endurance tests. To achieve optimal results, varying speeds were set for different time segments: 0 to 5 min at 10 m/min, 5 to 10 min at 15 m/min, 10 to 15 min at 20 m/min, and 15 to 20 min at 25 m/min. This approach allows the animals to quickly acclimate to the training rhythm. Each training session lasted 20 min and was conducted once daily, with a total of three training sessions. During the formal test, the speed for the initial 5 min was set at 10 m/min, followed by an increase of 5 m/min every subsequent 5 min until the mice reached exhaustion. The speed at exhaustion (m/min) and the total distance traveled (m) were recorded.
Testing was conducted using a mouse rotarod apparatus (Shanghai, China). The instrument has 6 rotating rods for conducting experiments with mice. Mice were placed on the rotary rod, where the rotation speed was initially set to 10 r/min for 1 min, followed by a 5-min training period. Subsequently, the speed was increased to 20 r/min for an additional 5 min. During the initial training phase, the experimenter assisted the mice by gently grasping their tails to encourage movement in the opposite direction of the rotating rod. Once the mice stabilized, they were allowed to crawl freely. The timing commenced when a mouse fell due to errors or fatigue. Prior to the formal experimentation, the mice underwent a training period lasting 3 days. During the actual test, timing began at 4 r/min for 1 min, with a uniform acceleration from 4 r/min to 40 r/min over a duration of 10 min. The time until each mouse fell was meticulously recorded.
2.3. Animal Euthanasia and Tissue Collection
An intraperitoneal injection was administered using 2% sodium pentobarbital at a dosage of 40 mg/kg. Once the mice were fully anesthetized, blood was collected from the mouse’s eye into a 2 mL centrifuge tube, which was then stored at 4 °C for 1 h. Following this, the samples were centrifuged at 3000 r/min for 20 min to collect the supernatant, which was subsequently stored at −80 °C. After blood collection, the mice were positioned supine on a plate, and the chest cavity was exposed. A perfusion needle was inserted into the aorta, and the right atrium was quickly incised. Pre-cooled saline was then perfused until the liver appeared white and the effluent was clear, indicating that the perfusion was complete. Intact skeletal muscles from both sides of the mice were isolated on ice, and their wet weight (g) was measured using an analytical balance. Following this, a portion of the mice was fixed in 10% neutral formalin, and paraffin-embedded sections were prepared for subsequent pathological analysis. The tissues from the remaining mice were wrapped in tin foil, immediately placed in a liquid nitrogen tank, and subsequently transferred to −80 °C for storage in the refrigerator to facilitate future nucleic acid detection.
2.4. Organ Index
Following blood collection, each mouse was euthanized via cervical dislocation, and the spleen, thymus, heart, kidneys, and liver were excised. Excess adipose tissue was carefully removed, and the organs were rinsed with saline to eliminate surface blood. The organs were then gently blotted with filter paper to remove any excess moisture and weighed, and the organ index was calculated. The formula for calculating the organ index is as follows:
2.5. Muscle Histological Staining
Samples from the gastrocnemius muscle were fixed in 10% neutral buffered formalin (for at least 24 h) and transversely trimmed. After paraffin embedding using an automatic tissue processor (Wuhan, China) and embedding machine (Wuhan, China), nine continuous 5 μm sections were prepared using a microtome (Shanghai, China). Histopathological staining was performed with hematoxylin and eosin (H&E) (Wuhan, China). Detection was then carried out using an optical microscope (Tokyo, Japan) and an automatic image analyzer (Tokyo, Japan).
Staining was performed using the following method: take 1 mm3 of muscle tissue and place it in 2.5% glutaraldehyde fixative (Wuhan, China) at 4 °C for 24 h; rinse the tissue with 1×PBS (Wuhan, China) at 4 °C three times, 15 min each time; fix with 1% osmium tetroxide (Wuhan, China) at 4 °C overnight; rinse the tissue with 1× PBS at 4 °C three times, 15 min each time; stain with 2% uranyl acetate solution (Wuhan, China) for 2 h. After gradient dehydration with ethanol solutions (Wuhan, China) of different concentrations (30%, 50%, 70%, 80%, 90%, 95%, 100%), dehydration was performed with 100% acetone solution (containing anhydrous copper sulfate) (Wuhan, China) for 30 min. Embedding was performed with epoxy resin at 37 °C for 12 h. Trim the resin, cut thin sections at room temperature, control the thickness to 50–100 nm, and fix the tissue sections on copper grids. Next, stain with uranyl acetate in the dark for 30 min, rinse with deionized water, restain with lead citrate in the dark for 15 min, and rinse with deionized water. After drying, observe the ultrastructure of the tissue using TEM (Hillsboro, OR, USA).
The cross-sectional area (CSA) of myofibers and the quantity of muscle fibers were determined using ImageJ v.1.54g analysis software (Bethesda, MD, USA), and the average cross-sectional area of myofibers was subsequently calculated. The measurement method involved taking the same field of view for each slice while ensuring that the background lighting of each photograph remained consistent. ImageJ software was then utilized to analyze the images and obtain all relevant measurement data.
2.6. qRT-PCR Detects Differentiation-Related Genes
Gastrocnemius tissues from both the OC and O-ECG groups were extracted, and the mRNA levels of MyoD, MyoG, MRF4, and MCK were assessed using the Aikore qPCR kit (Changsha, China) for RNA extraction, reverse transcription, and real-time quantitative PCR. The relative expression levels were then analyzed.
2.7. Transcriptome Sequencing and Analysis
Randomly select gastrocnemius muscle tissue samples from mice in the O-C group and the O-ECG group, and commission Gidi BioTech Co., Ltd. (Guangzhou, China) to complete cDNA library construction, sequencing, and analysis. Using p < 0.05 and |log2FC| > 1.5 as criteria, differentially expressed genes (DEGs) were screened from the two groups, and then GO and KEGG enrichment analyses were performed on the screened DEGs.
2.8. Cellular Experiment Verification
The cell experiment was conducted by Shanghai Anwei Biotechnology Co., Ltd. (Shanghai, China). Testicular interstitial cells from mice (TM3) in the logarithmic growth phase were digested using trypsin and then prepared into a suspension at a concentration of 1 × 105 cells/mL. These cells were subsequently seeded into 96-well plates at a density of 1 × 104 cells per well. Following this, 100 μL of culture medium was added to each well, and the plates were placed in a CO2 incubator (5%) at 37 °C for 24 h, after which they were cultured without serum for an additional 24 h. After culturing, half of the cell sample culture medium was replaced with ECG at concentrations of 1, 5, 25, 50, 100, and 200 μg/mL for 24 h. The control group received a solvent-containing medium. Following the incubation period, CCK-8 solution was added to all wells, and the samples were incubated for an additional 2 h. The absorbance at 450 nm was measured using a microplate reader (Wuxi, China). The cell survival rate was calculated using the following formula: Survival rate% = (experimental group − blank group)/(control group − blank group).
2.9. Testosterone Concentration Measurement
The testosterone concentration in the supernatant of the cell culture medium was measured after 24 h using a Mouse Testo ELISA Kit (Wuhan, China). The analysis included a control group and an ECG treatment group (ECG 50 μm/mL). Absorbance values for each well were determined using a microplate reader (Shanghai, China) at a wavelength of 450 nm. The gastrocnemius muscle tissues from both the O-C group and the O-ECG group were collected, and the testosterone concentration was measured using the BCA Protein Quantification Kit (Changsha, China) and the Mouse Testo ELISA Kit (Wuhan, China).
2.10. qRT-PCR Detection
TM3 cells in the logarithmic growth phase were harvested and seeded into 6-well plates at a density of 50 × 10
4 cells per well. Once the cell density reached 70%, 2 mL of DMEM/F12 containing 1% antibody was added to each well in the control group. In the ECG treatment group, 2 mL of 50 μg/mL ECG was added per well, and the cells were treated for 24 h. Subsequently, cellular RNA was extracted, and reverse transcription followed by real-time quantitative PCR was performed to assess the mRNA expression levels of testosterone synthetase genes, including
StAR,
P450scc,
3β–HSD,
CYP17a1, and
17β–HSD. Gastrocnemius tissues from both the OC group and the O-ECG group were collected, and the relative expression level of the testosterone-metabolizing enzyme gene
UGT2A3 was assessed using the Aikore qPCR kit (Changsha, China) for RNA extraction, reverse transcription, and real-time quantitative PCR. Primers were designed and synthesized by Shenggong Bioengineering Co., Ltd. (Shanghai, China), with all primer sequences listed in
Table S1. The
β-actin gene served as the internal reference gene, and three replicates were conducted for each group. The relative expression levels of each gene were calculated using the 2
−ΔΔCt method.
2.11. Western Blot Detects Protein Expression Levels
Cultivate TM3 cells with 50 μg/mL ECG for 24 h, extract total cellular protein, and determine the sample protein concentration using the BCA protein concentration assay kit (Chengdu, China). According to the measured concentration, take the corresponding volume of sample to achieve 40 μg of protein per well, add 5× protein loading buffer, and incubate in a boiling water bath at 95–100 °C for 5 min. The following steps are performed: gel preparation, sample loading, electrophoresis, transfer, blocking for 1 h, incubation with primary antibody at 4 °C overnight, incubation with secondary antibody at room temperature for 30 min, ECL reaction, and darkroom exposure. Finally, the film is scanned and archived, and the AlphaEaseFC v.4.0 software processing system analyzes the optical density values of the target bands. Detect the protein expression levels of the testosterone synthesis enzyme genes StAR, P450scc, 3β-HSD, CYP17a1, and 17β-HSD, using β-actin as an internal reference.
2.12. Network Pharmacology Target Prediction and Validation
To conduct the analysis, enter the keywords “Epigallocatechin gallate,” “Testosterone,” or their structures into the TargetNet, CTD, TCMSP, Swiss Target Prediction, and Pharm Mapper databases. Additionally, input “Sarcopenia” into the Genecards, Drugbank, and CTD databases. After intersecting the target data, the effects of epigallocatechin gallate on potential targets related to sarcopenia through modulation of testosterone levels were assessed. The Venn diagram and protein–protein interaction (PPI) network were generated using Venny, String database tools, and Cytoscape version 3.10.2.
To validate the predicted results of network pharmacology targets, serum samples from the O-C group and the O-ECG group were taken and measured using Tumor Necrosis Factor-α (TNF-α) and Interleukin-6 (IL-6) detection kits (Wuhan, China). The concentration of the standard product was plotted on the vertical axis, while the optical density value was plotted on the horizontal axis. The professional curve production software “Curve Expert v.1.4” was employed for analysis, and standard curves were generated. The regression equation of the standard curve was calculated based on the concentration and OD values of the standard product. Subsequently, the OD values of the samples were substituted into the equation to determine the sample concentrations.
2.13. Statistical Analysis
SPSS 27.0.1 software (Armonk City, NY, USA) and Graphpad Prism 10.1.2 (San Diego City, CA, USA) were used for statistical analysis, and relevant data are expressed as mean ± standard deviation (x% ± s). An independent sample test was used for comparison between groups, and a non-parametric test was used if it did not conform to the normal distribution. For this study, p < 0.05 was considered statistically significant.
4. Discussion
Catechins, a natural active polyphenol found in tea, comprise 12% to 24% of the dry weight of tea and are known to alleviate apoptosis [
19] and delay muscle aging [
14]. Additionally, natural polyphenols can enhance the number of skeletal muscle capillaries, promote the biosynthesis of mitochondria within muscle fibers [
21], and increase the cross-sectional area of muscle fibers by slowing the rate of protein degradation associated with muscle atrophy [
22]. This may explain why ECG in this experiment contributes to increased muscle mass and promotes a larger cross-sectional area of muscle fibers.
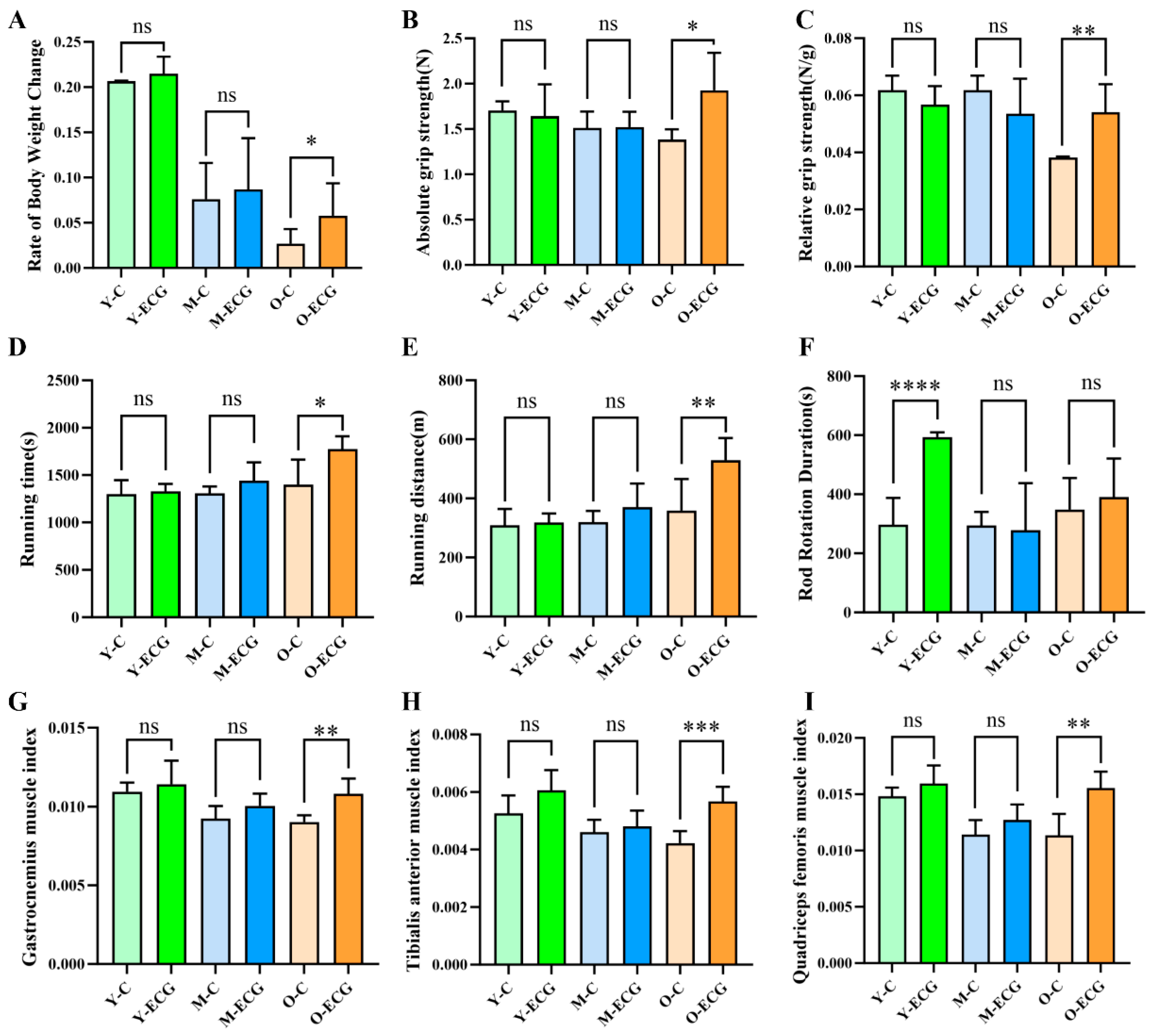
Behavioral experiments are significantly related to muscle anti-fatigue and motor ability. Grip strength evaluation is one of the most widely utilized methods. Treadmill locomotion is regarded as a measure of overall muscle function. Rod fatigue experiments are employed to assess the coordination of movement in animals. This experiment demonstrated that in vivo ECG supplementation could increase the absolute grip strength and relative grip strength of aged mice by 38.8% and 42.1%, respectively, and increase the running time and distance of the mice by 26.6% and 47.5%, respectively, indicating that ECG could significantly enhance the muscle endurance and muscle strength of aged mice (
Figure 1B,E). It has been established that phenolic compounds, such as naringenin, can improve the motility of mice by enhancing oxidase activity, oxygen uptake, and PGC-1α expression, thereby augmenting endurance and exercise capacity [
23]. Additionally, purple sweet potato anthocyanins enhance the body’s anti-fatigue and exercise capabilities by increasing the activity of skeletal muscle antioxidant enzymes and cell membrane enzymes [
24]. Similarly, resveratrol improves anti-fatigue and exercise performance through the enhancement of skeletal muscle antioxidant enzyme activity and cell membrane enzyme activity. Furthermore, the expression of genes associated with synaptic function promotes synaptic vesicle circulation in the skeletal muscles of elderly rats [
25], suggesting that ECG may operate through analogous regulatory mechanisms to enhance skeletal muscle motor performance.
Skeletal muscle mass is regarded as a critical indicator for assessing skeletal muscle atrophy [
26]. In the present study, ECG supplementation significantly increased skeletal muscle CSA, body weight, skeletal muscle volume, and skeletal muscle index in aged mice compared with the control group, indicating that ECG intervention can significantly improve muscle atrophy in aged mice. As we age, the reduction in muscle mass is manifested through a decrease in the number of muscle fibers or the size of the cross-sectional area of muscle fibers, ultimately leading to muscle fiber atrophy [
27]. Hematoxylin and eosin staining results showed that the cross-sectional area and number of muscle fibers in aged mice in the O-C group were significantly decreased, while ECG intervention increased the cross-sectional area and number of muscle fibers in aged mice by 55.2% and 41.4%, respectively (
Figure 2D,E).
Skeletal muscle is a highly heterogeneous tissue composed of muscle fibers, each of which is proliferated, differentiated, and fused by myoblasts. Muscle progenitor cells (MPCs) are derived from dermal sarcoma and subsequently enter the myogenesis process under the regulation of
Myf5 and
Mrf4 [
28]. The development of skeletal muscle is contingent upon the expression of myogenic regulators, with
MYOG,
MYOD,
MyHC, and others collectively governing this process [
29]. Myotubules continue to elongate and fuse to repair damaged myofibers or to form new multinucleated myofibers. Creatine kinase, encoded by the
MCK gene, is widely expressed in skeletal muscle and serves as a significant marker of skeletal muscle development and differentiation [
30]. In this study, qRT-PCR detection revealed that the relative expression levels of mRNA for skeletal muscle differentiation marker genes
MyoD,
MyoG,
MRF4, and
MCK in ECG-treated elderly mice increased significantly (
Figure 2F). This finding demonstrates that the dietary addition of ECG may enhance the potential for skeletal muscle hyperplasia in elderly rats. Recent studies have indicated that natural polyphenols can promote muscle repair and regeneration by enhancing muscle differentiation. Catechins can directly promote the differentiation of myoblasts by upregulating the expression of myogenic regulators (MyoD, MyoG, Myf5) and myogenesis enhancers (MEF2) [
17,
31]. Additionally, they can indirectly support bone health by inhibiting the activity associated with muscle development [
31].
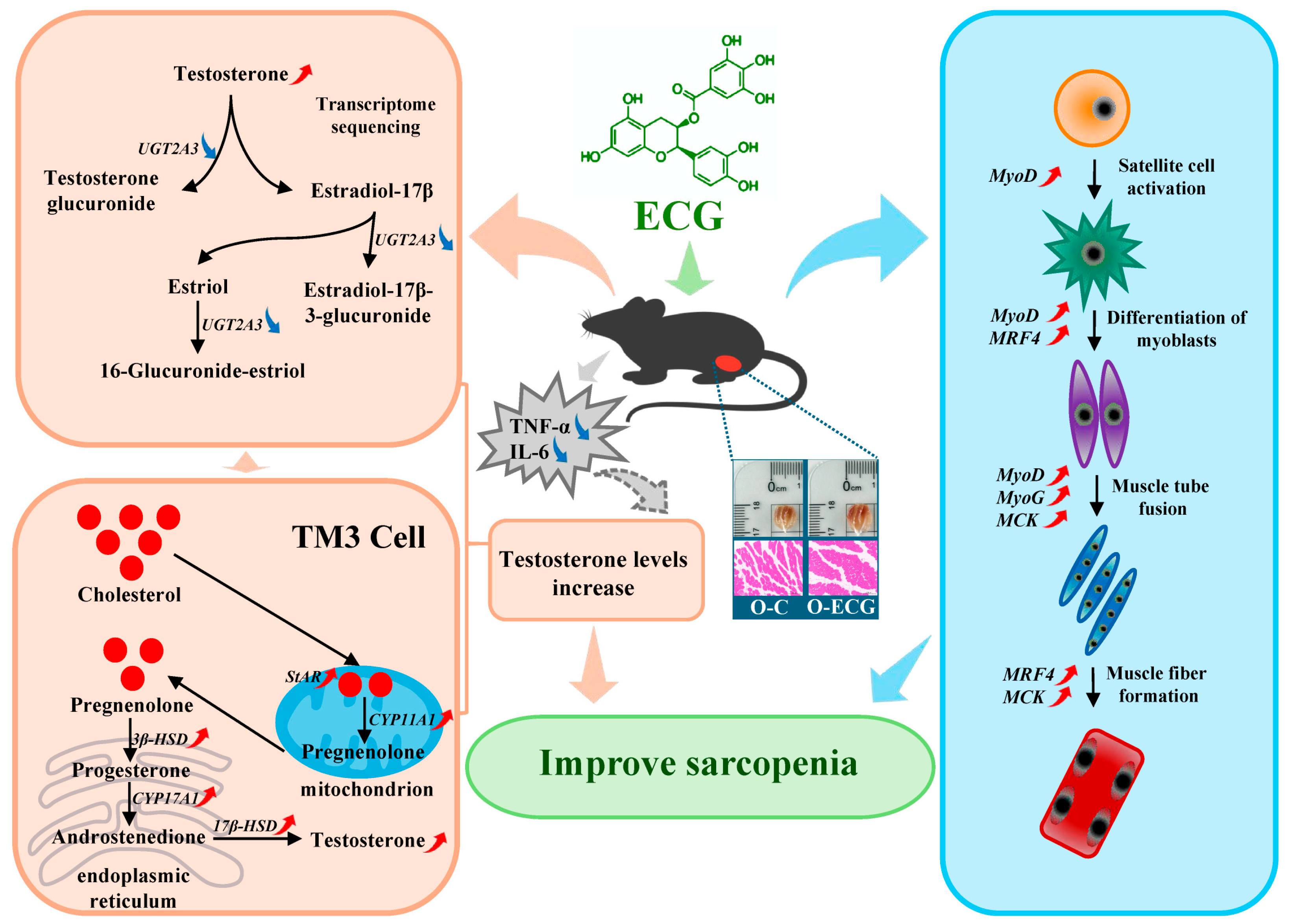
Glucuronosyltransferases (UGTs) catalyze the transfer of glucuronic acid from uridine diphosphate glucuronic acid (UDPGA) to various endogenous substances, including bilirubin, steroid hormones, and fat-soluble vitamins, resulting in the formation of the corresponding glucuronide products [
32]. Glucuronylation enhances water solubility and reduces the activity of the resulting products, thereby facilitating the clearance of metabolites through the kidneys, bile, or intestines. This process plays a crucial regulatory role in maintaining the balance of endogenous substances and in the elimination of exogenous substances from the body. The UGT family is primarily studied in the context of drug metabolism, but it also significantly contributes to the metabolism of toxic carcinogens and the breakdown of endogenous carcinogenic molecules in tumors [
33]. For instance, bladder cancer and prostate cancer exhibit a deficiency in UGTs, which impairs androgen metabolism, consequently promoting the onset and progression of cancer [
34].
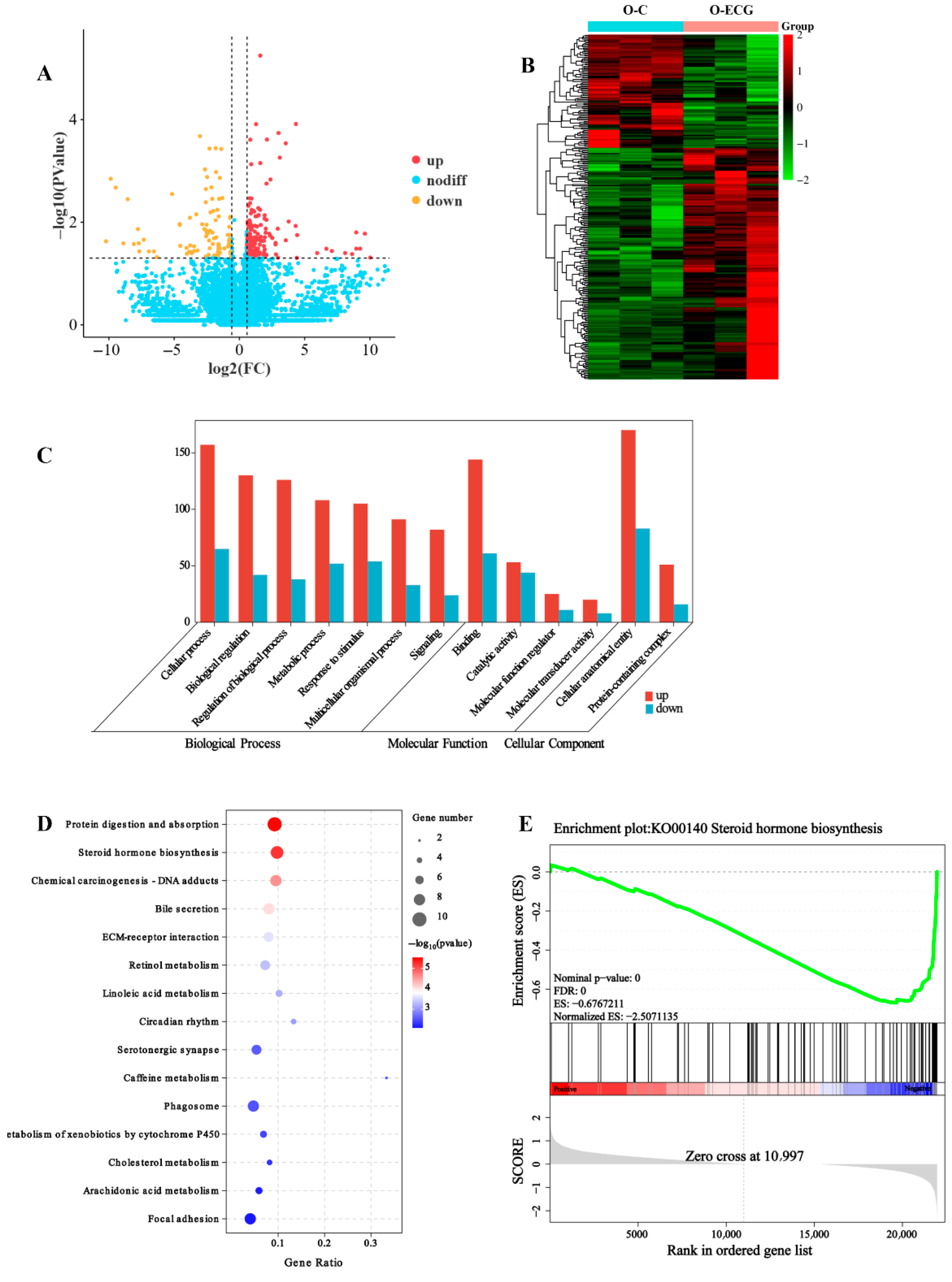
UGT2A3 (UDP-glucuronosyltransferase 2A3) is a protein belonging to the UGT family. Transcriptomic analyses revealed that
UGT2A3 is the most significantly downregulated gene among the major enrichment signal pathways of differentially expressed genes (
Figure 3E) and is involved in the metabolic process of testosterone within the steroid hormone biosynthesis pathway. After testosterone exerts its effects on mouse skeletal muscle, it may be directly converted into testosterone glucuronide by the UGT2A3 enzyme, or it may be transformed into estradiol-17β through the action of the CYP19A enzyme. Estradiol-17β can then be directly converted into estradiol-17β-3-glucuronide by the UGT2A3 enzyme, or it may be further metabolized into estriol via the CYP1A1 enzyme. Subsequently, estriol can also be converted by the UGT2A3 enzyme into 16-glucuronidine triol. The metabolic enzyme UGT2A3 plays a crucial role in testosterone metabolism. In mice administered ECG over an extended period, there was a highly significant reduction in the expression of the UGT2A3 gene in skeletal muscle (
Figure 4F), resulting in sustained high levels of testosterone in the gastrocnemius muscle (
Figure 4C). This phenomenon can regulate the expression of differentiation-related genes in skeletal muscle, leading to increased muscle fiber size and delayed muscle loss.
Testosterone is a crucial pleiotropic sex hormone that significantly contributes to muscle growth and maintenance in both men and women [
35]. It reduces myocyte protein degradation by supporting myocyte metabolism and inhibiting the ubiquitin-proteasome pathway. Additionally, testosterone promotes skeletal muscle protein synthesis [
36,
37] and increases the number of muscle satellite cells, thereby facilitating muscle regeneration and repair [
10]. Testosterone deficiency reduces the phosphorylation levels of the mammalian target of rapamycin (mTOR) and impairs Akt activation, which downregulates protein synthesis and results in a decrease in skeletal muscle mass [
38]. Testosterone is primarily synthesized and secreted by testicular interstitial cells, with its synthesis regulated by the hypothalamus–pituitary–testis axis (HPT), transcription factors, and coenzyme factors. Cholesterol serves as the raw material for testosterone synthesis in these interstitial cells. The steroidogenic acute regulatory [
39] protein, located on the outer membrane of mitochondria, facilitates the transport of cholesterol to the inner mitochondrial membrane [
40]. Once there, cholesterol side-chain cleavage cytochrome P450scc catalyzes the conversion of cholesterol into pregnenolone. Following its transport to the endoplasmic reticulum, pregnenolone is enzymatically converted into progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD). Subsequently, both progesterone and pregnenolone undergo multiple enzymatic transformations via CYP17A1, leading to the formation of androstenedione. Finally, androstenedione is converted into testosterone through the action of 17β-hydroxysteroid dehydrogenase (17β-HSD) [
41]. The rate-limiting step of this process significantly influences testosterone synthesis and secretion.
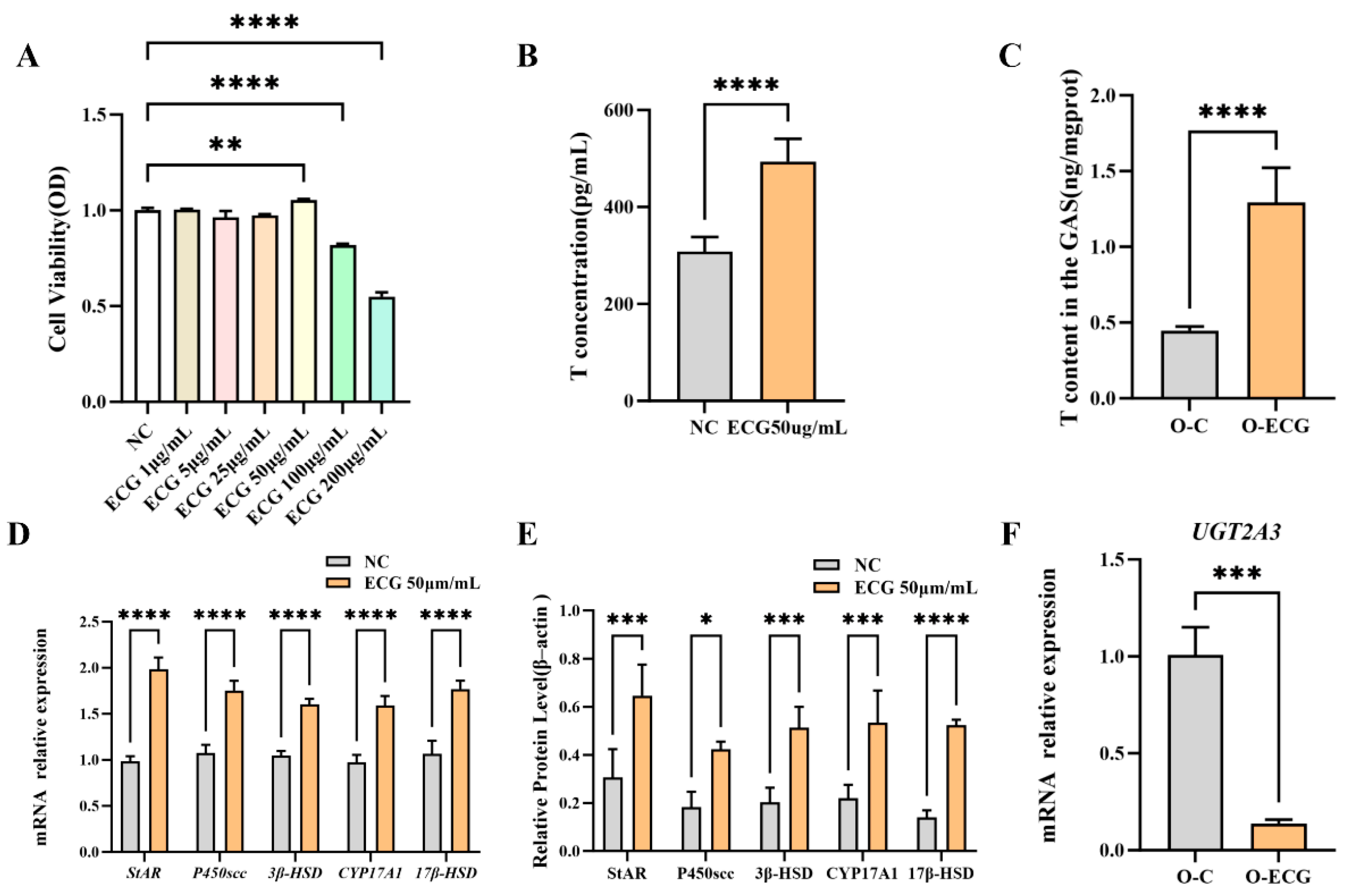
In this cell experiment, treatment of TM3 cells with 50 μg/mL ECG significantly increased the testosterone content and the expression of testosterone synthetase genes, including
StAR,
P450scc,
3β–HSD,
CYP17a1, and
17β–HSD, as well as their corresponding protein levels in TM3 cells (
Figure 4D,E). These findings suggest that appropriate concentrations of ECG can enhance testosterone synthesis by upregulating the expression of genes related to testosterone synthesis enzymes. Interestingly, the levels of muscle steroid hormones were significantly correlated with muscle strength and CSA [
42]. This experiment demonstrated that testosterone levels in the gastrocnemius muscle of mice administered ECG significantly increased (
Figure 4C). The exogenous supplementation of ECG appears to enhance the levels of skeletal muscle steroid hormones and may contribute, to some extent, to increases in muscle strength and CSA. Related studies have demonstrated that supplementing with Qiang Juice Tablets can regulate the expression of CYP11A1 and CYP17A1, thereby alleviating the compensatory expression of StAR and 3β-HSD in rat testicles. This regulation helps restore and maintain testosterone secretion levels to normal in elderly rats [
43]. Furthermore, natural plant extracts have shown significant potential in enhancing the viability of testicular interstitial cells [
44]. Both in vitro and in vivo tests indicate that catechins promote testosterone secretion in rats by increasing the enzyme activity of 17β-HSD [
45]. This study further confirmed that ECG upregulates the expression of genes related to testosterone synthesis, thereby enhancing testosterone secretion and influencing the differentiation of skeletal muscles in male mice. However, female individuals lack testicular interstitial cells and possess a limited capacity to synthesize testosterone. Consequently, it remains to be verified whether ECG produces a similar effect in elderly females.
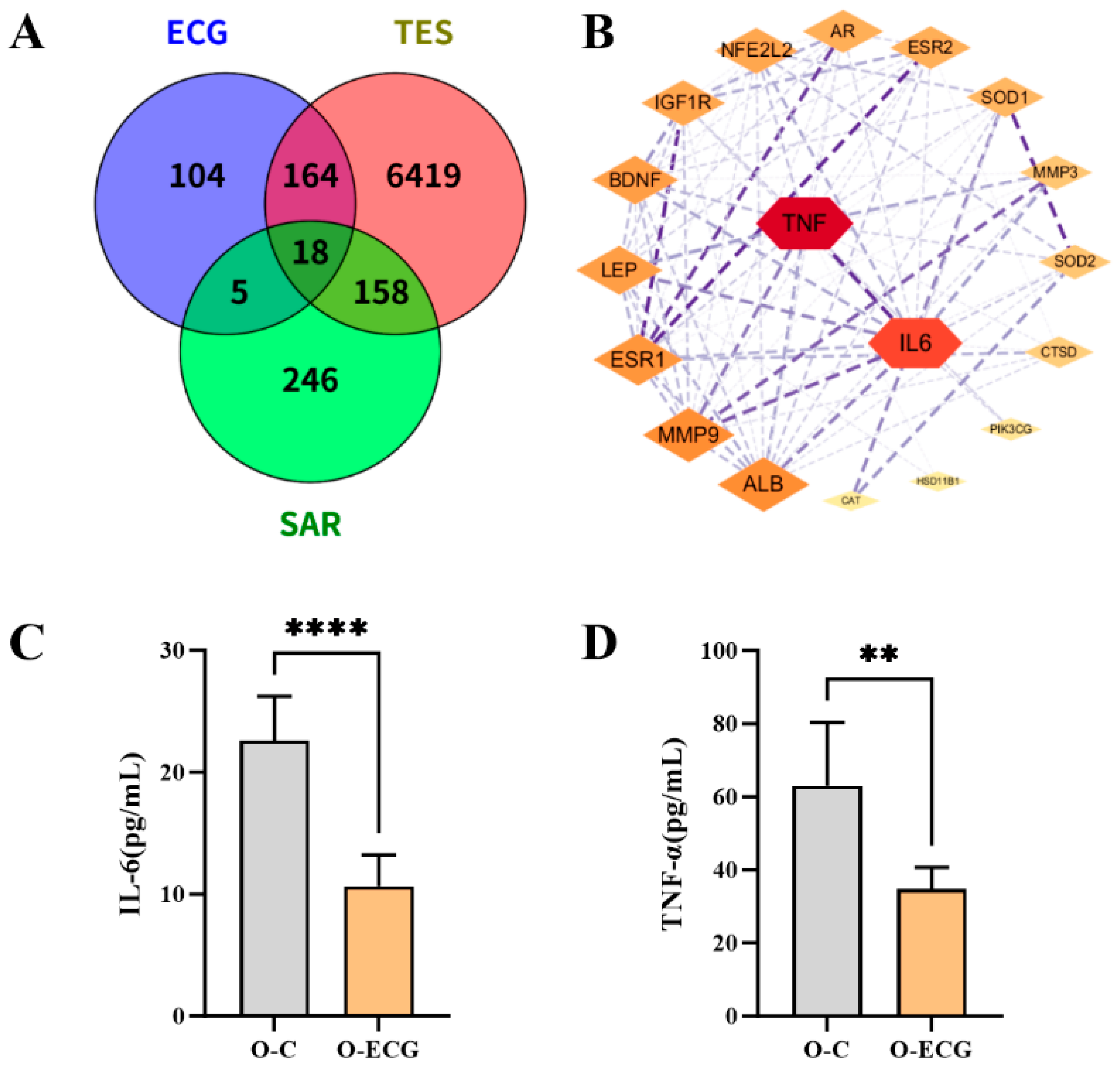
Network pharmacology can establish a network of interactions between drugs and targets, based on the multi-component and multi-target characteristics of natural materials. This approach enables a comprehensive analysis of the network, revealing potential drug-effective components and targets for disease treatment, as well as mechanisms of action that provide important references for screening active ingredients and understanding drug mechanisms. Furthermore, network pharmacological analysis indicates that ECG plays a significant role in regulating cytokines. In this experiment, the serum proinflammatory factor content in mice administered ECG was significantly reduced (
Figure 5C,D), indicating that ECG can regulate cytokines, promote steroid hormone synthesis, and subsequently modulate the expression of genes associated with skeletal muscle differentiation. Cytokines are synthesized and secreted by various histocellular cells and are primarily categorized into growth factors, interleukins, and interferons. Several cytokines play a role in regulating the synthesis of testosterone, with their mechanisms of action potentially linked to the condition of testicular interstitial cells. For instance, TNF-α and IL-6 exert significant inhibitory effects on steroid production [
46]. NUR77 serves as a crucial regulator of multiple genes involved in steroid hormone production. Additionally, TNF-α influences this process by interfering with the nuclear receptor NUR77 [
47]. In vitro studies have demonstrated that steroid hormone deficiency is correlated with elevated expression and secretion of IL-6 and TNF-α [
48].
Finally, this study evaluated mouse motor capacity using a combination of limb grip strength tests, rotarod fatigue tests, and treadmill exercise tests, visually demonstrating the effects of ECG on muscular function. These findings were corroborated by transcriptomic analysis and in vitro cell experiments, which revealed that ECG ameliorates sarcopenia in aged mice by regulating testosterone synthesis and metabolism. Notably, due to the absence of Leydig cells in female individuals—resulting in significantly lower testosterone production capacity than males—it remains to be determined whether ECG exerts similar effects in aged females. This question warrants further investigation to validate potential gender-specific responses.
In addition, the accuracy of ECG dosage is also an important issue to be considered in the experiment. In this experimental protocol, the maximum dosage of ECG was set at 160 mg/kg/d, with the solution concentration reaching 16 mg/mL—exceeding its room-temperature solubility of 5 mg/mL. To address this, we applied short-term ultrasonic treatment to facilitate dissolution. Notably, as a polyphenolic antioxidant, ECG is highly sensitive to thermal and oxidative degradation. Uncontrolled temperature during processing could significantly reduce its antioxidant activity, thereby compromising the compound’s intended efficacy. To avoid the increase in temperature during the ultrasound process that may affect the ECG activity, we recommend future studies maintain a low-temperature environment by adding ice cubes to the ultrasonic solution, thus preserving ECG’s biological activity to the greatest extent. Additionally, while the actual gavage dosage for mice was lower than the theoretical value due to solubility constraints, we ensured consistent total ECG intake across all subjects. The findings from this study are expected to provide valuable references for similar research.