A Comprehensive Study on the Nutritional Profile and Shelf Life of a Custom-Formulated Protein Bar Versus a Market-Standard Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Source Selection and Soy Protein Extraction
- -
- Hydration:
- -
- Blending:
- -
- Filtration:
- -
- Protein precipitation:
- -
- Separation and storage:
2.2. Methods of Biochemical Analysis of Protein Extract
2.2.1. Preparation of Biuret Reagent
2.2.2. Assay Procedure
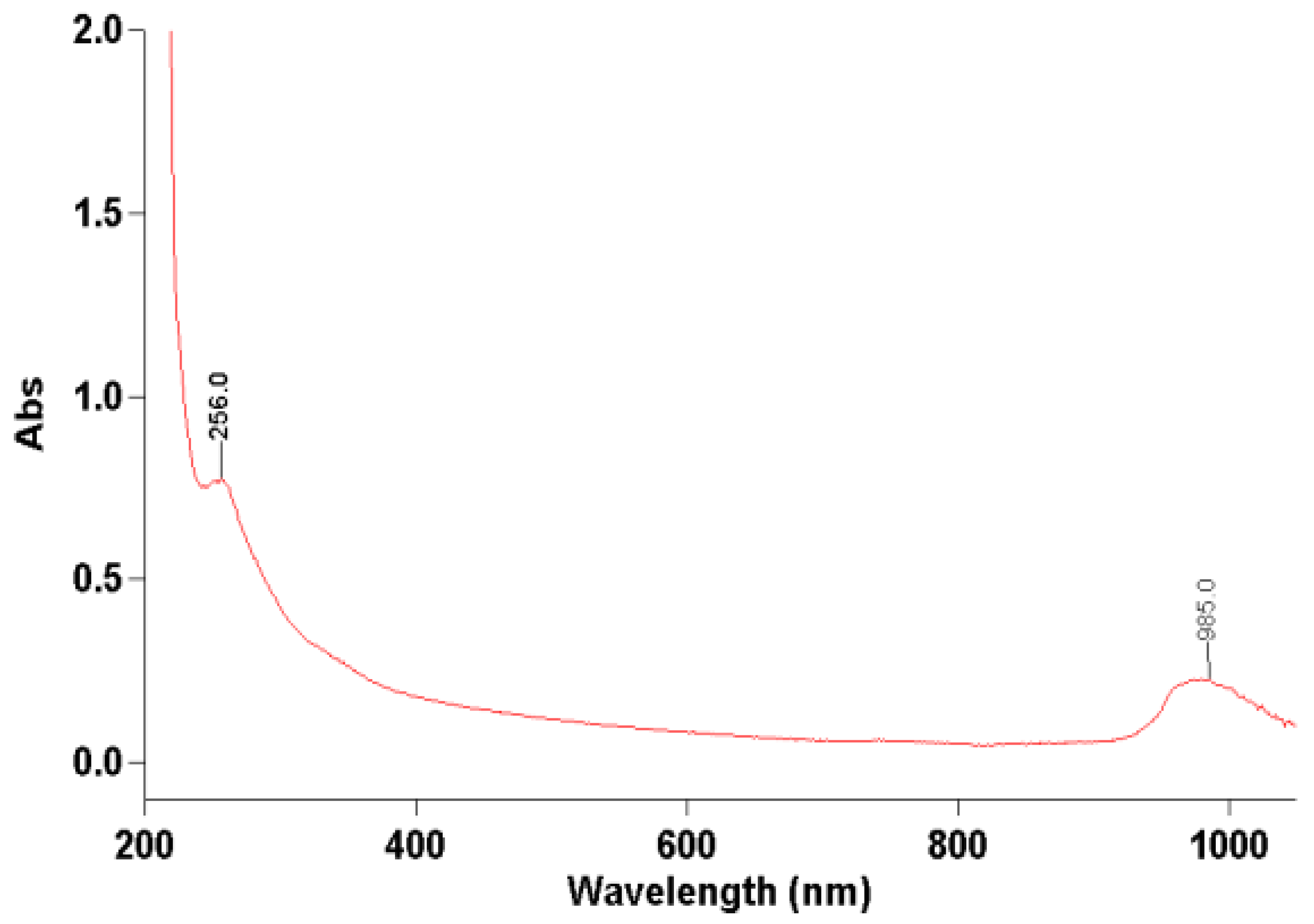
2.2.3. Quantitative and Structural Characterization
2.3. Selection of Ingredients and Protein Bar Formulation
- -
- Hydration of fiber sources:
- -
- Preparation of binding paste:
- -
- Incorporation of dry ingredients:
- -
- Shaping and setting:
- -
- Refrigeration and portioning:
- -
- Packaging and storage:
2.4. Selection of a Commercial Protein Bar for Benchmarking
2.5. Comparative Evaluation Methods
3. Results and Discussions
- -
- Soy protein extract served as the primary protein source, providing a complete amino acid profile essential for supporting muscle synthesis and metabolic health.
- -
- Oatmeal and chia seeds were selected for their high content of soluble dietary fiber, promoting gastrointestinal health, improving glycemic control, and contributing to the final texture and structural stability of the bars.
- -
- Dates performed a dual function: acting as a natural sweetener by providing intrinsic sugars and serving as an effective natural binding agent due to their viscous texture, helping to hold the ingredients together without the need for synthetic binders.
- -
- Peanut butter was incorporated as a source of healthy monounsaturated and polyunsaturated fatty acids, contributing both to the creamy mouthfeel of the bars and to the overall energy density required for a functional snack.
- -
- Cinnamon was added to enhance flavor naturally and to potentially provide antioxidant benefits.
3.1. Biochemical Analysis of Protein Extract
- -
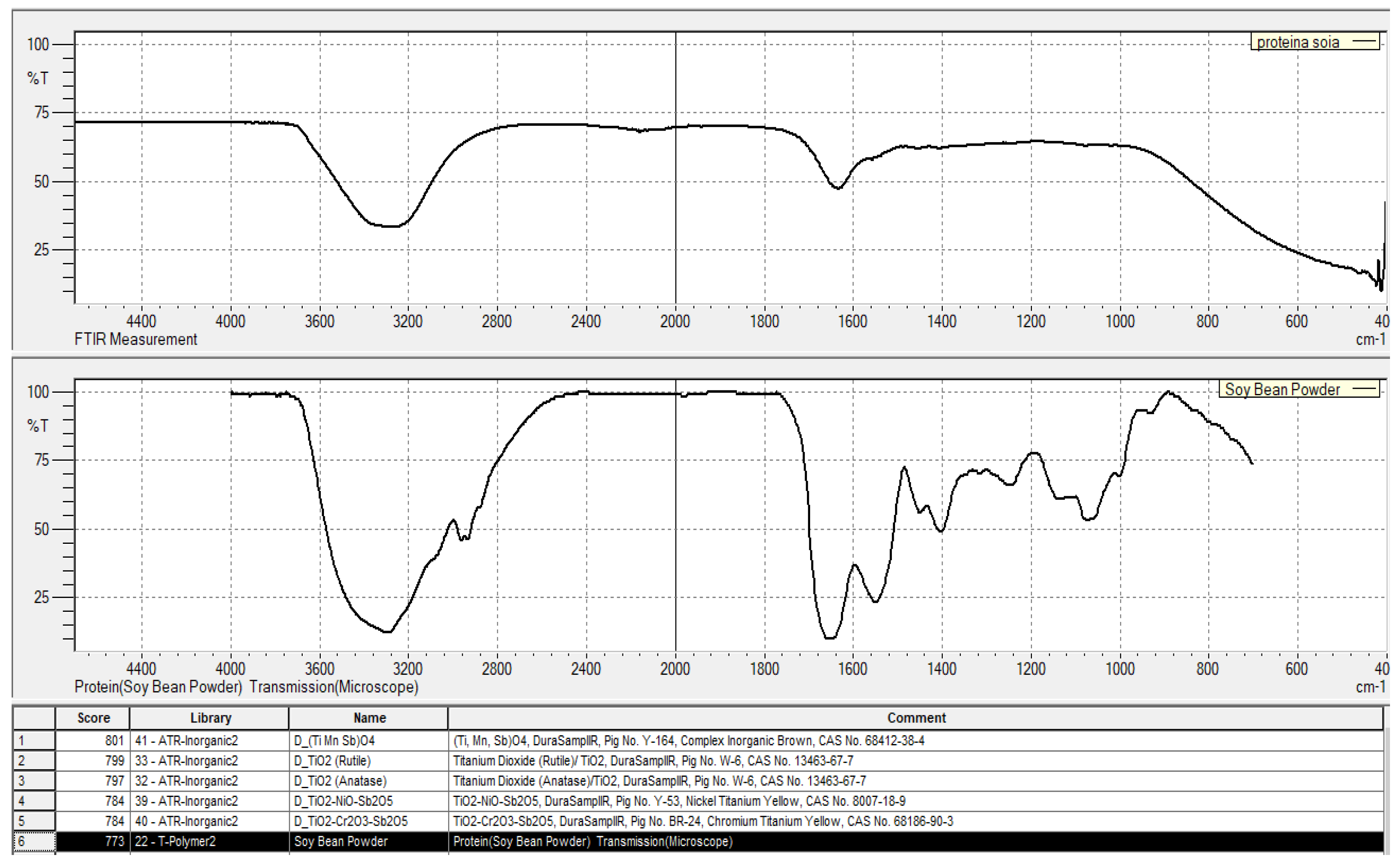
- A broad, intense band was observed in the region between 3500 and 3200 cm−1, attributed to O–H stretching vibrations from hydroxyl groups and N–H stretching vibrations from amine groups. These functional groups are abundant in proteins, polysaccharides, and other biomolecules, indicating the presence of hydrophilic structures within the soy matrix.
- -
- A prominent absorption peak near 1650 cm−1 corresponds to C=O stretching vibrations of amide linkages, known as the Amide I band. This band is a hallmark of peptide bonds and provides strong evidence for the presence of proteins in the sample. The Amide I region is often used to assess protein secondary structures, including α-helices and β-sheets.
- -
- Absorption bands around 1400 cm−1 were assigned to the bending vibrations of aliphatic C–H groups. These signals reflect the presence of nonpolar hydrocarbon chains, likely originating from fatty acid residues or aliphatic side chains of amino acids embedded within the soy proteins or associated lipids.
- -
- Additional medium-intensity bands detected near 1100 cm−1 are associated with C–O and C–N stretching vibrations. These bands are characteristic of glycosidic linkages in carbohydrates and peptide bonds in proteins, further confirming the composite nature of the soy material, consisting of both protein and polysaccharide constituents.
3.2. Comparative Analysis of the Experimental Protein Bar and a Commercial Counterpart
3.2.1. Nutritional Value of Protein Bars
3.2.2. Shelf Life of Protein Bars
3.2.3. Qualitative Assessment of Ingredients and Their Nutritional Relevance
3.2.4. Cost Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jovanov, P.; Sakač, M.; Jurdana, M.; Pražnikar, Z.J.; Kenig, S.; Hadnađev, M.; Jakus, T.; Petelin, A.; Škrobot, D.; Marić, A. High-protein bar as a meal replacement in elite sports nutrition: A pilot study. Foods 2021, 10, 2628. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, R.; Sabapathy, S.N.; Bawa, A.S. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008, 7, 14–28. [Google Scholar] [CrossRef]
- Géci, A.; Krivošíková, A.; Nagyová, L.; Cagáňová, D. The influence of lifestyle on consumer behavior and decision making in research aimed at protein bars. Potr. Slovak J. Food Sci. 2020, 14, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Constantin, O.E.; Istrati, D.I. Chapter 4. Functional properties of snack bars. In Functional Foods; Lagouri, V., Ed.; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, K.; Zhao, X.; Li, J.; Yu, R.; Fu, R.; He, Y.; Zhao, P.; Oh, K.-C.; Hou, J. High-protein nutrition bars: Hardening mechanisms and anti-hardening methods during storage. Food Control 2021, 127, 108127. [Google Scholar] [CrossRef]
- Abdel-salam, F.F.; Ibrahim, R.M.; Ali, M.I. Formulation and evaluation of high energy-protein bars as a nutritional supplement for sports athletics. Am. J. Food Sci. Technol. 2022, 10, 53–65. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Li, Y.; Szlachetka, K.; Chen, P.; Lin, X.; Ruan, R. Ingredient characterization and hardening of high-protein food bars: An NMR state diagram approach. Cereal Chem. 2008, 85, 780–786. [Google Scholar] [CrossRef]
- Greaves, K.A.; Wilson, M.D.; Rudel, L.L.; Williams, J.K.; Wagner, J.D. Consumption of soy protein reduces cholesterol absorption compared to casein protein alone or supplemented with an isoflavone extract or conjugated equine estrogen in ovariectomized cynomolgus monkeys. J. Nutr. 2000, 130, 820–826. [Google Scholar] [CrossRef]
- Małecki, J.; Terpilowski, K.; Nastaj, M.; Sołowiej, B. Physicochemical, nutritional, microstructural, surface and sensory properties of a model high-protein bars intended for athletes depending on the type of protein and syrup used. Int. J. Environ. Res. Public Health 2022, 19, 3923. [Google Scholar] [CrossRef]
- Hogan, S.A.; Chaurin, V.; O’Kennedy, B.T.; Kelly, P.M. Influence of dairy proteins on textural changes in high-protein bars. Int. Dairy J. 2012, 26, 58–65. [Google Scholar] [CrossRef]
- Veggi, N.; Faria, W.C.S.; Ãvila, E.T.; de Rosa Lima, T.; Almeida, P.C.; Simoni, T.; Navalta, J.W.; Voltarelli, F.A.; Converti, A.; Barros, W.M. High-protein bar supplemented with chia seed improves lipidemic parameters in Wistar rats. Int. J. Adv. Eng. Res. Sci. 2022, 9, 152–163. [Google Scholar] [CrossRef]
- Williams, G.; Noakes, M.; Keogh, J.; Foster, P.; Clifton, P. High protein high fibre snack bars reduce food intake and improve short term glucose and insulin profiles compared with high fat snack bars. Asia Pac. J. Clin. Nutr. 2006, 15, 443–450. [Google Scholar] [PubMed]
- Gentile, L. Protein-polysaccharide interactions and aggregates in food formulations. Curr. Opin. Colloid. Interface Sci. 2020, 48, 18–27. [Google Scholar] [CrossRef]
- Diaz, J.; Lila, M.A.; Foegeding, E.A. Whey protein-polyphenol aggregate particles mitigate bar hardening reactions in high protein bars. LWT 2021, 138, 110747. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J.C. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- FAO. Dietary protein quality evaluation in human nutrition. Report of an FAQ Expert Consultation. FAO Food. Nutr. Pap. 2013, 92, 1–66. [Google Scholar] [PubMed]
- Tufa, M. Development and nutritional assessment of complementary foods from fermented cereals and soybean. Food Sci. Nutr. 2016, 2, 014. [Google Scholar] [CrossRef]
- Xipsiti, M. Protein quality evaluation: FAO perspective. Front. Nutr. 2024, 11, 1446879. [Google Scholar] [CrossRef]
- Hayes, M. Measuring protein content in food: An overview of methods. Foods 2020, 9, 1340. [Google Scholar] [CrossRef]
- More, T.A.; Sheikh, Z.; Ali, A. Artificial sweeteners and their health implications: A review. Biosci. Biotech. Res. Asia 2021, 18, 227–237. [Google Scholar] [CrossRef]
- Delimaris, I. Adverse effects associated with protein intake above the recommended dietary allowance for adults. Int. Sch. Res. Not. 2013, 2013, 126929. [Google Scholar] [CrossRef] [PubMed]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for life: Review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiong, Y.L. Processing, nutrition, and functionality of hempseed protein: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Małecki, J.; Tomasevic, I.; Djekic, I.; Sołowiej, B.G. The effect of protein source on the physicochemical, nutritional properties and microstructure of high-protein bars intended for physically active people. Foods 2020, 9, 1467. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Passi, S.J.; Misra, A. Overview of trans fatty acids: Biochemistry and health effects. Diabetes Metab. Syndr. Clin. Res. Rev. 2011, 5, 161–164. [Google Scholar] [CrossRef]
- Mehta, B.M.; Cheung, P.C.K. Overview of food chemistry. In Handbook of Food Chemistry; Cheung, P., Mehta, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–13. [Google Scholar] [CrossRef]
- Biter, A.B.; Pollet, J.; Chen, W.H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. A method to probe protein structure from UV absorbance spectra. Anal. Biochem. 2019, 587, 113450. [Google Scholar] [CrossRef]
- Food Safety—European Commission. Available online: https://food.ec.europa.eu/index_en (accessed on 10 May 2024).
- Cabezas, D.; Madoery, R.; Diehl, B.; Tomás, M. Emulsifying properties of different modified sunflower lecithins. J. Am. Oil Chem. Soc. 2012, 89, 355–361. [Google Scholar] [CrossRef]
- Pan, Y.; Tikekar, R.V.; Nitin, N. Effect of antioxidant properties of lecithin emulsifier on oxidative stability of encapsulated bioactive compounds. Int. J. Pharm. 2013, 450, 129–137. [Google Scholar] [CrossRef]
- Judde, A.; Villeneuve, P.; Rossignol-Castera, A.; Le Guillou, A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J. Am. Oil Chem. Soc. 2003, 80, 1209–1215. [Google Scholar] [CrossRef]
- Mazaletskaya, L.I.; Sheludchenko, N.I.; Kasaikina, O.T. Kinetics of soy lecithin oxidation at high concentrations: The effect of antioxidants. Russ. J. Phys. Chem. B 2024, 18, 1496–1500. [Google Scholar] [CrossRef]
- Rao, Q.; Klaassen Kamdar, A.; Labuza, T.P. Storage stability of food protein hydrolysates—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1169–1192. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mitra, A. Health Effects of Palm Oil. J. Hum. Ecol. 2009, 26, 197–203. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.-R.; Xue, A.-N.; Ge, K.-Y. Effects of red palm oil on serum lipids and plasma carotenoids level in Chinese male adults. Biomed. Environ. Sci. 2003, 16, 348–354. [Google Scholar] [PubMed]
- Bonku, R.; Yu, J. Health aspects of peanuts as an outcome of its chemical composition. Food Sci. Hum. Well. 2020, 9, 21–30. [Google Scholar] [CrossRef]
- Blomhoff, R.; Carlsen, M.H.; Andersen, L.F.; Jacobs, D.R. Health benefits of nuts: Potential role of antioxidants. Br. J. Nutr. 2006, 96 (Suppl. S2), S52–S60. [Google Scholar] [CrossRef]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef]
- Ahmad, M.; Han, Z.; Kong, Q. Aflatoxin in peanuts and maize: An overview on occurrence, regulations, prevention, and control methods. World Mycotoxin J. 2023, 16, 99–114. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef]
- Yang, J.; Shen, H.; Mi, M.; Qin, Y. Isoflavone consumption and risk of breast cancer: An updated systematic review with meta-analysis of observational studies. Nutrients 2023, 15, 2402. [Google Scholar] [CrossRef]
- Messina, M.; Nechuta, S. A review of the clinical and epidemiologic evidence relevant to the impact of postdiagnosis isoflavone intake on breast cancer outcomes. Curr. Nutr. Rep. 2025, 14, 50. [Google Scholar] [CrossRef]
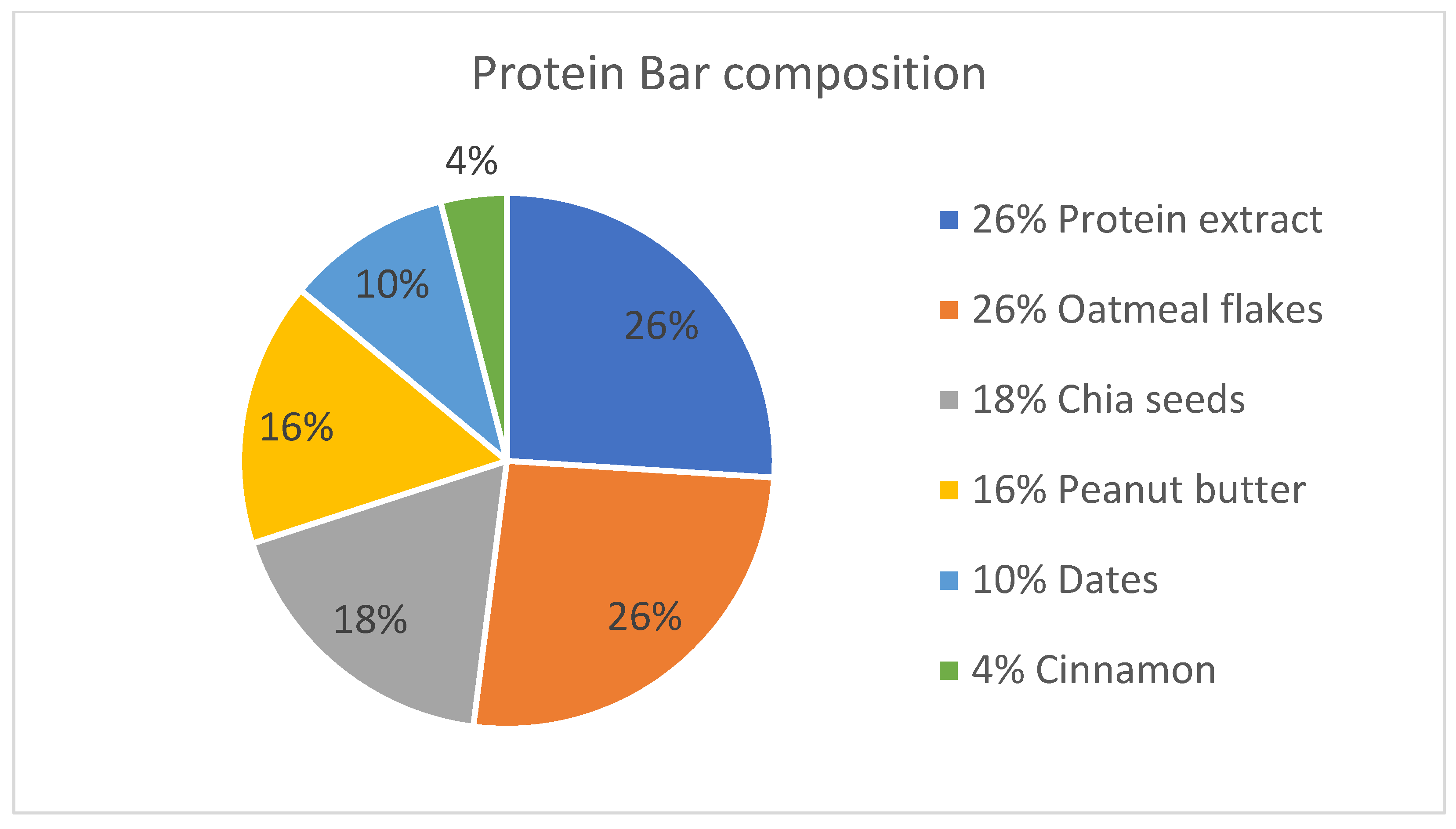
| Ingredient | Content per 100 g (g) | Content per 50 g (g) | Cost per 100 g (RON) |
|---|---|---|---|
| Protein extract from soy flakes | 25.97 | 12.98 | 2.45 |
| Oatmeal | 25.97 | 12.98 | 0.99 |
| Peanut butter | 15.58 | 7.79 | 6.39 |
| Dates | 10.38 | 5.19 | 2.19 |
| Chia seeds | 18.18 | 9.09 | 4.30 |
| Cinnamon | 3.89 | 1.94 | 15.46 |
| Experimental Protein Bar | Commercial Protein Bar | |||
|---|---|---|---|---|
| Macronutrient | 100 g | 50 g | 100 g | 50 g |
| Proteins (g) | 25.52 ± 0.34 | 12.76 ± 0.17 | 18.5 (label) | 9.25 (label) |
| Carbohydrates (g) | 42.41 ± 0.45 | 21.20 ± 0.23 | 45.8 (label) | 22.9 (label) |
| Fibre (g) | 13.46 ± 0.29 | 6.73 ± 0.14 | 3.7 (label) | 1.85 (label) |
| Fats (g) | 15.46 ± 0.37 | 7.73 ± 0.18 | 20.4 (label) | 10.2 (label) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Seiman, C.; Mititelu-Tartau, L.; Biriescu, S.; Almășan, A.-L.; Bitu, B.-O.; Bucur, A.-I.; Luca, A.; Hoinoiu, B.; Hoinoiu, T. A Comprehensive Study on the Nutritional Profile and Shelf Life of a Custom-Formulated Protein Bar Versus a Market-Standard Product. Foods 2025, 14, 2141. https://doi.org/10.3390/foods14122141
Duda-Seiman C, Mititelu-Tartau L, Biriescu S, Almășan A-L, Bitu B-O, Bucur A-I, Luca A, Hoinoiu B, Hoinoiu T. A Comprehensive Study on the Nutritional Profile and Shelf Life of a Custom-Formulated Protein Bar Versus a Market-Standard Product. Foods. 2025; 14(12):2141. https://doi.org/10.3390/foods14122141
Chicago/Turabian StyleDuda-Seiman, Corina, Liliana Mititelu-Tartau, Simona Biriescu, Alexandra-Loredana Almășan, Bianca-Oana Bitu, Adina-Ioana Bucur, Andrei Luca, Bogdan Hoinoiu, and Teodora Hoinoiu. 2025. "A Comprehensive Study on the Nutritional Profile and Shelf Life of a Custom-Formulated Protein Bar Versus a Market-Standard Product" Foods 14, no. 12: 2141. https://doi.org/10.3390/foods14122141
APA StyleDuda-Seiman, C., Mititelu-Tartau, L., Biriescu, S., Almășan, A.-L., Bitu, B.-O., Bucur, A.-I., Luca, A., Hoinoiu, B., & Hoinoiu, T. (2025). A Comprehensive Study on the Nutritional Profile and Shelf Life of a Custom-Formulated Protein Bar Versus a Market-Standard Product. Foods, 14(12), 2141. https://doi.org/10.3390/foods14122141







