Informative Wavelength Selection for Evaluation of Bacterial Spoilage in Raw Salmon (Salmo salar) Fillet Using FT-NIR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Procurement and Preparation
2.2. NIR Measurements
2.3. Microbiological Analysis
2.4. Data Analysis
3. Results and Discussion
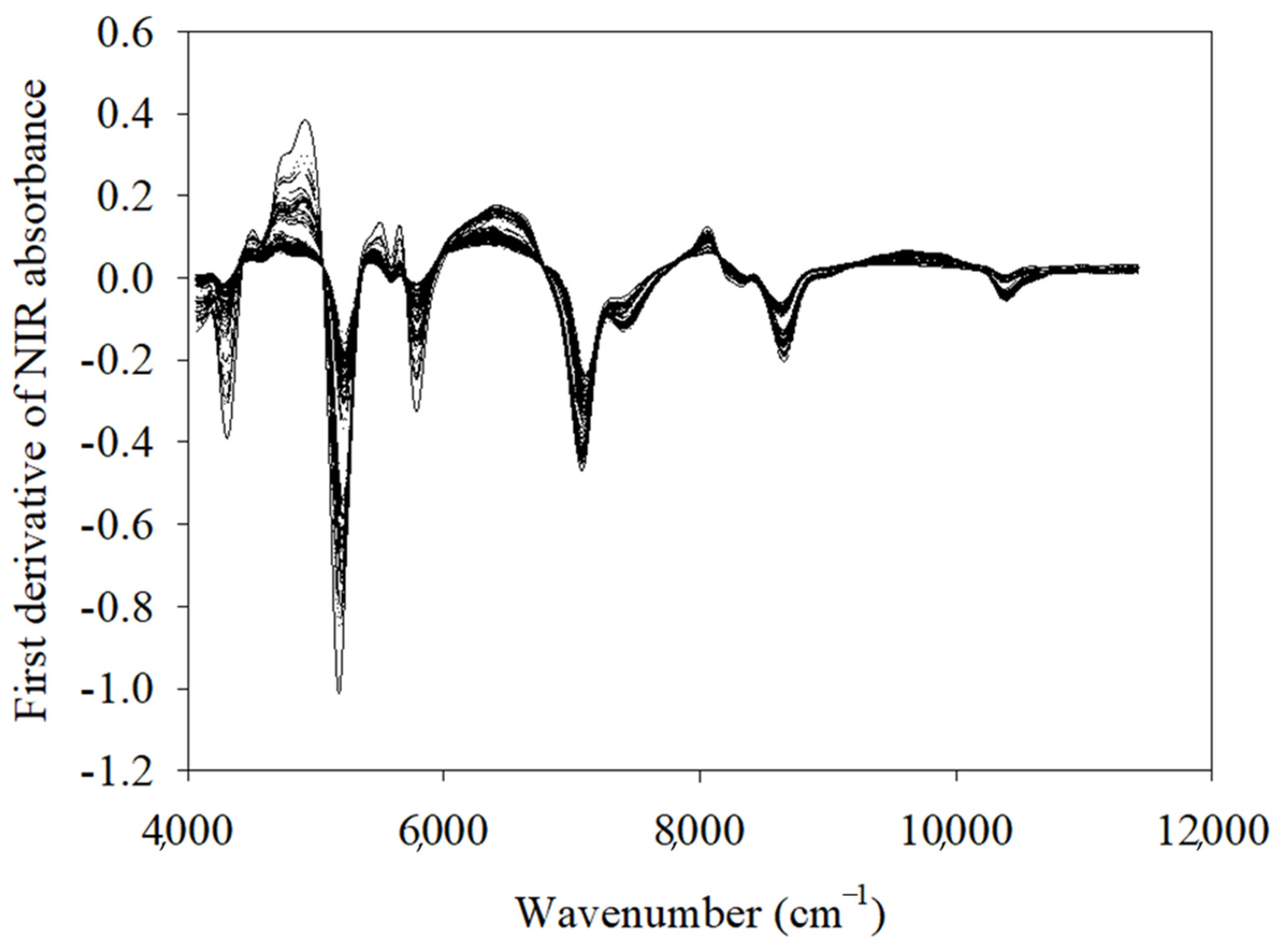
3.1. NIR Spectroscopy and Bacterial Characteristics of Raw Salmon Fillets
3.2. Optimization of Spoilage Identification Method
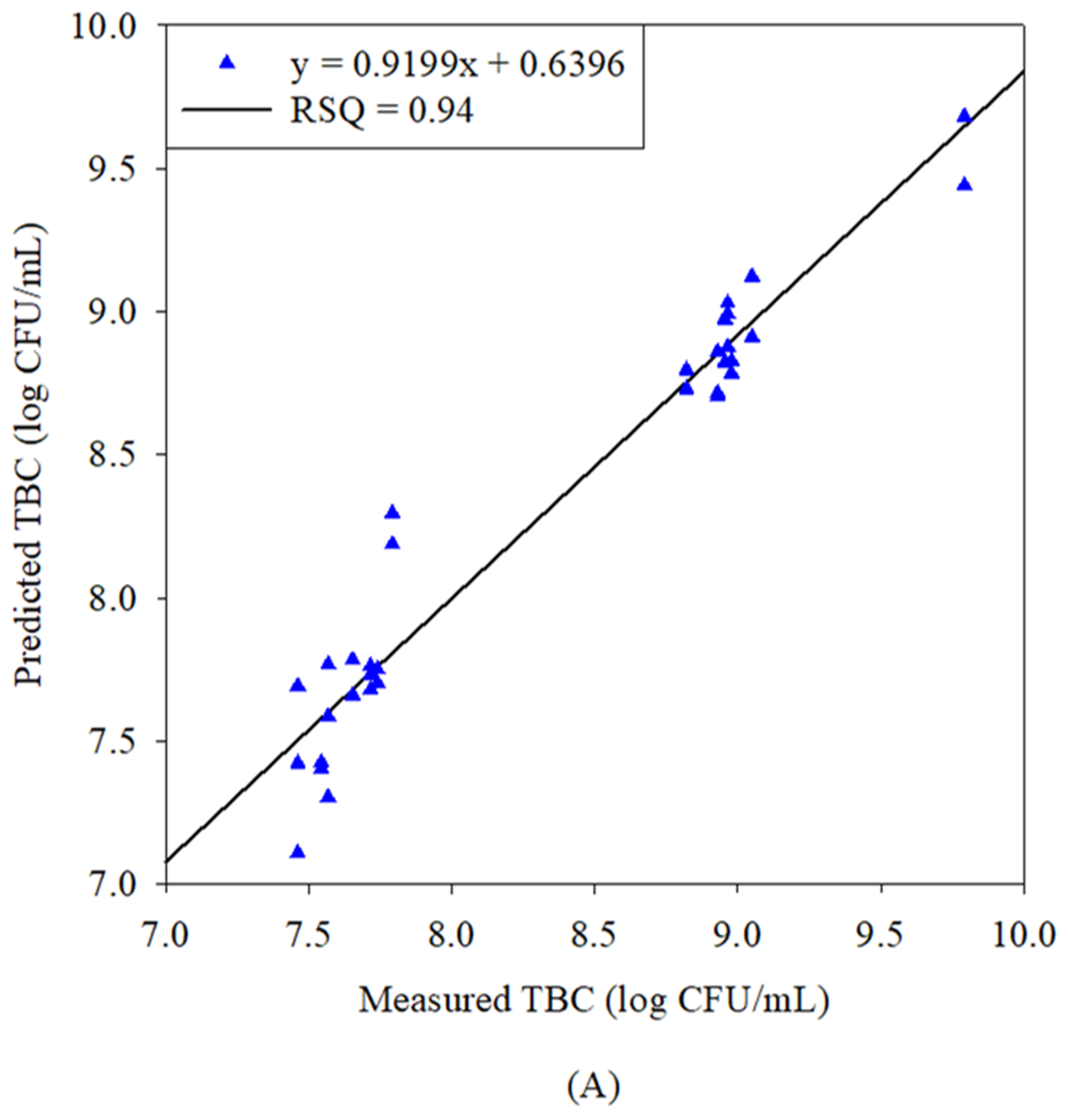
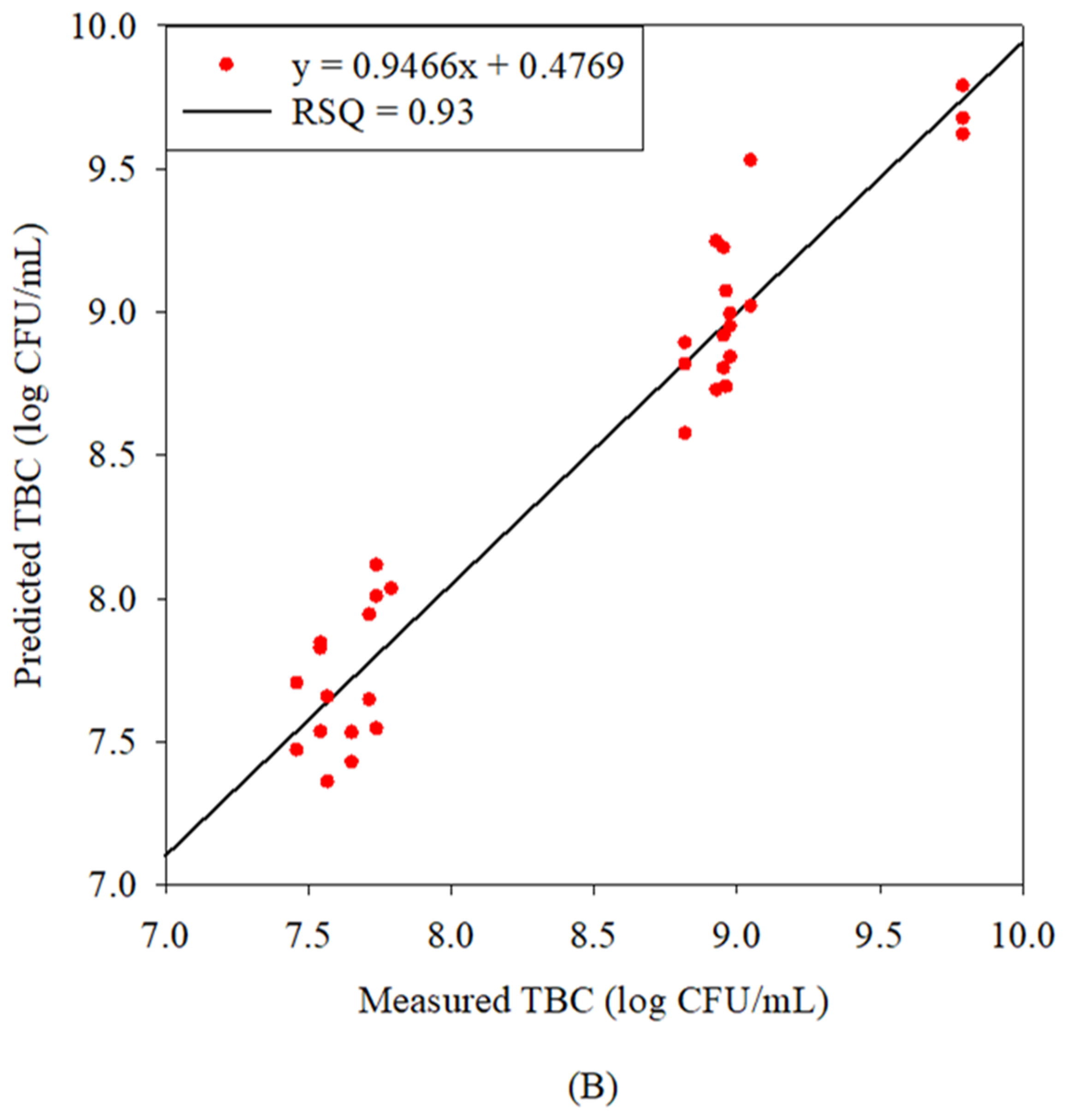
3.3. Correlation Between Predicted and Laboratory Concentrations of TBC
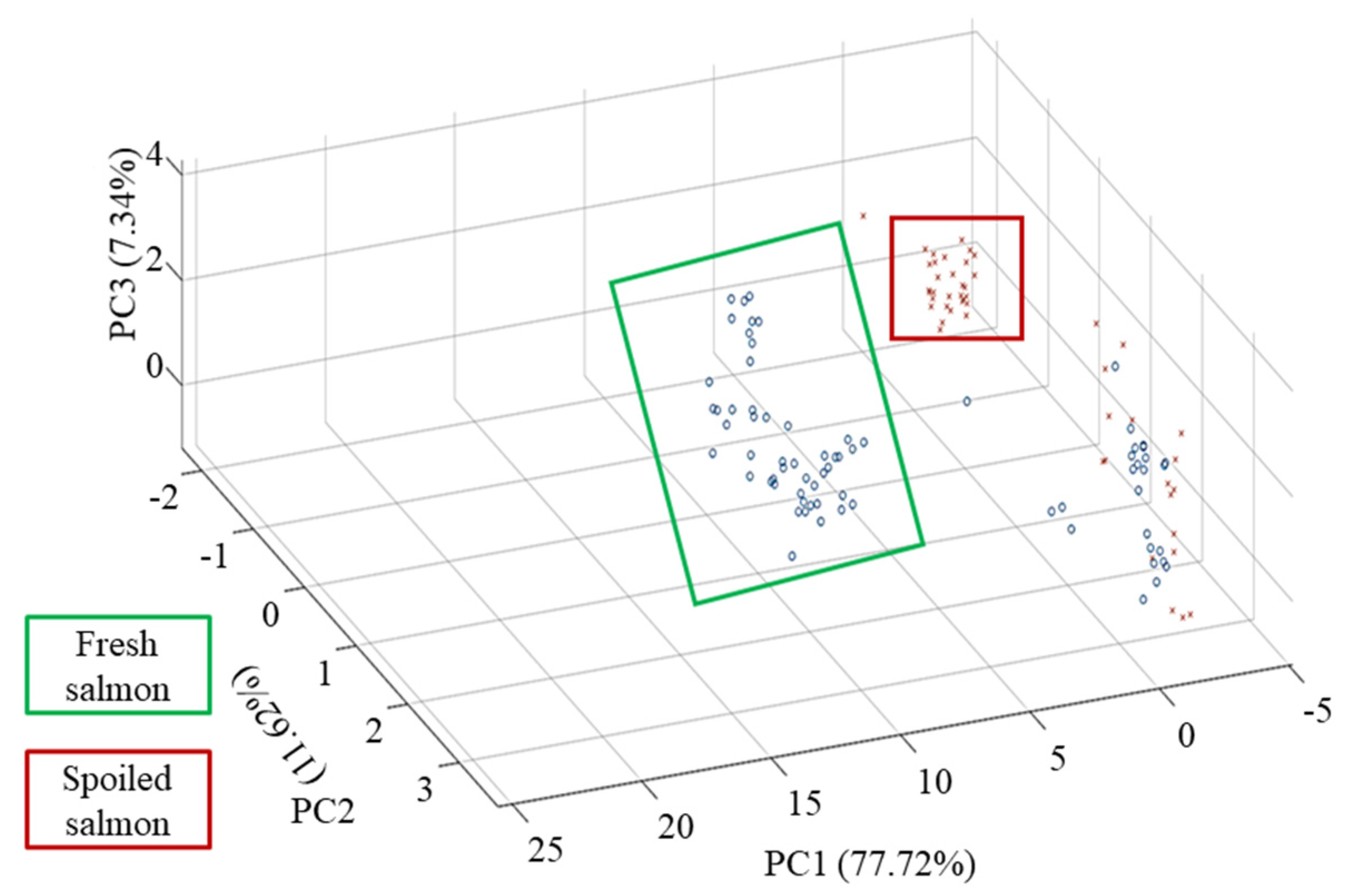
3.4. Analysis of Principal Components
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cen, H.; He, Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, O.; Panagou, E.Z.; Tassou, C.C.; Nychas, G.J.E. Contribution of Fourier transform infrared (FTIR) spectroscopy data on the quantitative determination of minced pork meat spoilage. Food Res. Int. 2011, 44, 3264–3271. [Google Scholar] [CrossRef]
- Asche, F.; Cojocaru, A.L.; Roth, B. The development of large scale aquaculture production: A comparison of the supply chains for chicken and salmon. Aquaculture 2018, 493, 446–455. [Google Scholar] [CrossRef]
- Asche, F.; Roll, K.H.; Sandvold, H.N.; Sorvig, A.; Zhang, D.J. Salmon aquaculture: Larger companies and increased production. Aquac. Econ. Manag. 2013, 17, 322–339. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef]
- Wu, L.L.; Pu, H.B.; Sun, D.W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Maillet, A.; Denojean, P.; Bouju-Albert, A.; Scaon, E.; Leuillet, S.; Dousset, X.; Jaffres, E.; Combrisson, J.; Prevost, H. Characterization of Bacterial Communities of Cold-Smoked Salmon during Storage. Foods 2021, 10, 362. [Google Scholar] [CrossRef]
- Moretro, T.; Moen, B.; Heir, E.; Hansen, A.A.; Langsrud, S. Contamination of salmon fillets and processing plants with spoilage bacteria. Int. J. Food Microbiol. 2016, 237, 98–108. [Google Scholar] [CrossRef]
- Dalgaard, P. Freshness, Quality and Safety in Seafoods; Danish Institute for Fisheries Research Silkeborg: Silkeborg, Denmark, 2000. [Google Scholar]
- Zhou, J.; Liu, C.; Zhong, Y.; Luo, Z. Applications of Near-Infrared Spectroscopy for Nondestructive Quality Analysis of Fish and Fishery Products. Foods 2024, 13, 3992. [Google Scholar] [CrossRef]
- Cozzolino, D. Use of infrared spectroscopy for in-field measurement and phenotyping of plant properties: Instrumentation, data analysis, and examples. Appl. Spectrosc. Rev. 2014, 49, 564–584. [Google Scholar] [CrossRef]
- Alexandrakis, D.; Downey, G.; Scannell, A.G.M. Rapid Non-destructive Detection of Spoilage of Intact Chicken Breast Muscle Using Near-infrared and Fourier Transform Mid-infrared Spectroscopy and Multivariate Statistics. Food Bioprocess Technol. 2012, 5, 338–347. [Google Scholar] [CrossRef]
- Moscetti, R.; Monarca, D.; Cecchini, M.; Haff, R.P.; Contini, M.; Massantini, R. Detection of mold-damaged chestnuts by near-infrared spectroscopy. Postharvest Biol. Technol. 2014, 93, 83–90. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Potential of time series-hyperspectral imaging (TS-HSI) for non-invasive determination of microbial spoilage of salmon flesh. Talanta 2013, 111, 39–46. [Google Scholar] [CrossRef]
- He, H.-J.; Sun, D.-W. Selection of Informative Spectral Wavelength for Evaluating and Visualising Enterobacteriaceae Contamination of Salmon Flesh. Food Anal. Methods 2015, 8, 2427–2436. [Google Scholar] [CrossRef]
- He, H.-J.; Sun, D.-W. Inspection of harmful microbial contamination occurred in edible salmon flesh using imaging technology. J. Food Eng. 2015, 150, 82–89. [Google Scholar] [CrossRef]
- Tito, N.B.; Rodernann, T.; Powell, S.M. Use of near infrared spectroscopy to predict microbial numbers on Atlantic salmon. Food Microbiol. 2012, 32, 431–436. [Google Scholar] [CrossRef]
- Varrà, M.O.; Ghidini, S.; Ianieri, A.; Zanardi, E. Near infrared spectral fingerprinting: A tool against origin-related fraud in the sector of processed anchovies. Food Control 2021, 123, 107778. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; You, J.; Xiong, S. Rapid determination of the textural properties of silver carp (Hypophthalmichthys molitrix) using near-infrared reflectance spectroscopy and chemometrics. LWT 2020, 129, 109545. [Google Scholar] [CrossRef]
- Pauline, O.; Chang, H.-T.; Tsai, I.L.; Lin, C.-H.; Chen, S.; Chuang, Y.-K. Intelligent assessment of the histamine level in mackerel (Scomber australasicus) using near-infrared spectroscopy coupled with a hybrid variable selection strategy. LWT 2021, 145, 111524. [Google Scholar] [CrossRef]
- Cao, L.; Huang, Z.; Wu, D.; Ruan, R.; Liu, Y. Rapid and nondestructive determination of qualities in vacuum-packaged catfish (Clarias leather) fillets during slurry ice storage. J. Food Process. Preserv. 2021, 45, e15262. [Google Scholar] [CrossRef]
- Yu, H.-D.; Qing, L.-W.; Yan, D.-T.; Xia, G.; Zhang, C.; Yun, Y.-H.; Zhang, W. Hyperspectral imaging in combination with data fusion for rapid evaluation of tilapia fillet freshness. Food Chem. 2021, 348, 129129. [Google Scholar] [CrossRef] [PubMed]
- Moosavi-Nasab, M.; Khoshnoudi-Nia, S.; Azimifar, Z.; Kamyab, S. Evaluation of the total volatile basic nitrogen (TVB-N) content in fish fillets using hyperspectral imaging coupled with deep learning neural network and meta-analysis. Sci. Rep. 2021, 11, 5094. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhu, H.; Wang, J.; Yuan, H.; Zhao, J.; Chen, Q. Prediction of black tea fermentation quality indices using NIRS and nonlinear tools. Food Sci. Biotechnol. 2017, 26, 853–860. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, H.-l.; Chen, L.; Huang, J.-h. An improved weighted partial least squares method coupled with near infrared spectroscopy for rapid determination of multiple components and anti-oxidant activity of Pu-Erh tea. Molecules 2018, 23, 1058. [Google Scholar] [CrossRef]
- Chen, S.M.; Wang, C.Y.; Tsai, C.Y.; Yang, I.C.; Luo, S.J.; Chuang, Y.K. Fermentation quality evaluation of tea by estimating total catechins and theanine using near-infrared spectroscopy. Vib. Spectrosc. 2021, 115, 103278. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W. Rapid and non-invasive detection of fish microbial spoilage by visible and near infrared hyperspectral imaging and multivariate analysis. LWT Food Sci. Technol. 2015, 62, 1060–1068. [Google Scholar] [CrossRef]
- Guillén-Casla, V.; Rosales-Conrado, N.; León-González, M.E.; Pérez-Arribas, L.V.; Polo-Díez, L.M. Principal component analysis (PCA) and multiple linear regression (MLR) statistical tools to evaluate the effect of E-beam irradiation on ready-to-eat food. J. Food Compos. Anal. 2011, 24, 456–464. [Google Scholar] [CrossRef]
- Haus, F.; Boissel, O.; Junter, G.A. Multiple regression modelling of mineral base oil biodegradability based on their physical properties and overall chemical composition. Chemosphere 2003, 50, 939–948. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W. Recent applications of spectroscopic and hyperspectral imaging techniques with chemometric analysis for rapid inspection of microbial spoilage in muscle foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 478–490. [Google Scholar] [CrossRef]
- Mahesh, S.; Jayas, D.; Paliwal, J.; White, N. Comparison of partial least squares regression (PLSR) and principal components regression (PCR) methods for protein and hardness predictions using the near-infrared (NIR) hyperspectral images of bulk samples of Canadian wheat. Food Bioprocess Technol. 2015, 8, 31–40. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Rajalahti, T.; Kvalheim, O.M. Multivariate data analysis in pharmaceutics: A tutorial review. Int. J. Pharm. 2011, 417, 280–290. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Khambaty, F.M.; Fry, F.S.; Calvey, E.M. Rapid detection and identification of bacterial strains by Fourier transform near-infrared spectroscopy. J. Agric. Food Chem. 2001, 49, 574–579. [Google Scholar] [CrossRef]
- He, H.-J.; Sun, D.-W.; Wu, D. Rapid and real-time prediction of lactic acid bacteria (LAB) in farmed salmon flesh using near-infrared (NIR) hyperspectral imaging combined with chemometric analysis. Food Res. Int. 2014, 62, 476–483. [Google Scholar] [CrossRef]
- Grau, R.; Sánchez, A.J.; Girón, J.; Iborra, E.; Fuentes, A.; Barat, J.M. Nondestructive assessment of freshness in packaged sliced chicken breasts using SW-NIR spectroscopy. Food Res. Int. 2011, 44, 331–337. [Google Scholar] [CrossRef]
- Arruda de Brito, A.; Campos, F.; dos Reis Nascimento, A.; Damiani, C.; Alves da Silva, F.; de Almeida Teixeira, G.H.; Cunha Júnior, L.C. Non-destructive determination of color, titratable acidity, and dry matter in intact tomatoes using a portable Vis-NIR spectrometer. J. Food Compos. Anal. 2022, 107, 104288. [Google Scholar] [CrossRef]
- Bodor, Z.; Kovacs, Z.; Benedek, C.; Hitka, G.; Behling, H. Origin Identification of Hungarian Honey Using Melissopalynology, Physicochemical Analysis, and Near Infrared Spectroscopy. Molecules 2021, 26, 7274. [Google Scholar] [CrossRef]
- Allende-Prieto, C.; Fernández, L.; Rodríguez-Gonzálvez, P.; García, P.; Rodríguez, A.; Recondo, C.; Martínez, B. Advances in the Detection and Identification of Bacterial Biofilms Through NIR Spectroscopy. Foods 2025, 14, 913. [Google Scholar] [CrossRef]
- He, H.J.; Sun, D.W. Microbial evaluation of raw and processed food products by Visible/Infrared, Raman and Fluorescence spectroscopy. Trends Food Sci. Technol. 2015, 46, 199–210. [Google Scholar] [CrossRef]
- Lin, M.; Al-Holy, M.; Mousavi-Hesary, M.; Al-Qadiri, H.; Cavinato, A.; Rasco, B. Rapid and quantitative detection of the microbial spoilage in chicken meat by diffuse reflectance spectroscopy (600–1100 nm). Lett. Appl. Microbiol. 2004, 39, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Mousavi, M.; Al-Holy, M.; Cavinato, A.G.; Rasco, B.A. Rapid near infrared spectroscopic method for the detection of spoilage in rainbow trout (Oncorhynchus mykiss) fillet. J. Food Sci. 2006, 71, S18–S23. [Google Scholar] [CrossRef]
- Suthiluk, P.; Saranwong, S.; Kawano, S.; Numthuam, S.; Satake, T. Possibility of using near infrared spectroscopy for evaluation of bacterial contamination in shredded cabbage. Int. J. Food Sci. Technol. 2008, 43, 160–165. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Khambaty, F.M.; Fry, F.S.; Dubois, J.; Calvey, E.M. Detection and identification of bacteria in a juice matrix with Fourier transform-near infrared spectroscopy and multivariate analysis. J. Food Prot. 2004, 67, 2555–2559. [Google Scholar] [CrossRef]
- Durmuş, E.; Güneş, A.; Kalkan, H. Detection of aflatoxin and surface mould contaminated figs by using Fourier transform near-infrared reflectance spectroscopy. J. Sci. Food Agric. 2017, 97, 317–323. [Google Scholar] [CrossRef]
| Constituent | Model | Data Processing | Spectral Range (nm) | Segment | Derivative Gap | RC | RV |
|---|---|---|---|---|---|---|---|
| TBC | SMLR | SNV and Detrend-d1 | 867–2505 | 2 | 2 | 0.97 | 0.97 |
| TBC | PLSR | SNV and Detrend-d1 | 867–2505 | 2 | 2 | 0.99 | 0.99 |
| Model | Sample Set | n | Range (TBC log CFU/mL) Predicted | Mean (log CFU/mL) | STD (log CFU/mL) | RMSE (log CFU/mL) |
|---|---|---|---|---|---|---|
| SMLR | Calibration | 34 | 7.69–9.68 | 8.31 | 0.72 | 0.18 |
| SMLR | Validation | 34 | 7.70–9.79 | 8.43 | 0.76 | 0.20 |
| PLSR | Calibration | 34 | 7.44–9.82 | 8.34 | 0.75 | 0.10 |
| PLSR | Validation | 34 | 7.48–9.81 | 8.40 | 0.77 | 0.06 |
| Wavelength | 885.27 nm (11,294.2 cm−1) | 1026.27 nm (9740.3 cm−1) | 1039.93 nm (9616.8 cm−1) | 1068.38 nm (9360.9 cm−1) | 1257.55 nm (7953.0 cm−1) | 1267.75 nm (7886.2 cm−1) | 1453.49 nm (6878.6 cm−1) |
|---|---|---|---|---|---|---|---|
| FT-NIR absorption band region | Third overtone | Second overtone | Second overtone | Second overtone | Second overtone | Second overtone | First overtone |
| Indication | ArCH, CH3 and CH2 | NH | RNH2, ArCH | ArCH | CH, CH2, CH3 | CH | CONHR, CH2, CH, ROH, H2 |
| Significance | C–H | N–H | N–H, C–H | C–H | C–H | C–H | O–H, N–H, C–H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panwar, R.; Lin, S.-P.; Lin, S.-H.; Lin, J.-A.; Wang, Y.-J.; Chuang, Y.-K. Informative Wavelength Selection for Evaluation of Bacterial Spoilage in Raw Salmon (Salmo salar) Fillet Using FT-NIR Spectroscopy. Foods 2025, 14, 2074. https://doi.org/10.3390/foods14122074
Panwar R, Lin S-P, Lin S-H, Lin J-A, Wang Y-J, Chuang Y-K. Informative Wavelength Selection for Evaluation of Bacterial Spoilage in Raw Salmon (Salmo salar) Fillet Using FT-NIR Spectroscopy. Foods. 2025; 14(12):2074. https://doi.org/10.3390/foods14122074
Chicago/Turabian StylePanwar, Roma, Shin-Ping Lin, Shyh-Hsiang Lin, Jer-An Lin, Yu-Jen Wang, and Yung-Kun Chuang. 2025. "Informative Wavelength Selection for Evaluation of Bacterial Spoilage in Raw Salmon (Salmo salar) Fillet Using FT-NIR Spectroscopy" Foods 14, no. 12: 2074. https://doi.org/10.3390/foods14122074
APA StylePanwar, R., Lin, S.-P., Lin, S.-H., Lin, J.-A., Wang, Y.-J., & Chuang, Y.-K. (2025). Informative Wavelength Selection for Evaluation of Bacterial Spoilage in Raw Salmon (Salmo salar) Fillet Using FT-NIR Spectroscopy. Foods, 14(12), 2074. https://doi.org/10.3390/foods14122074








