Concurrent Hydrolysis–Fermentation of Tenebrio molitor Protein by Lactobacillus plantarum KCCM13068P Attenuates Inflammation in RAW 264.7 Macrophages and Constipation in Loperamide-Induced Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of T. molitor Hydrolysate
2.2. SDS-PAGE Analysis
2.3. Protein Identification by LC-MS/MS
2.4. Amino Acid Composition Analysis
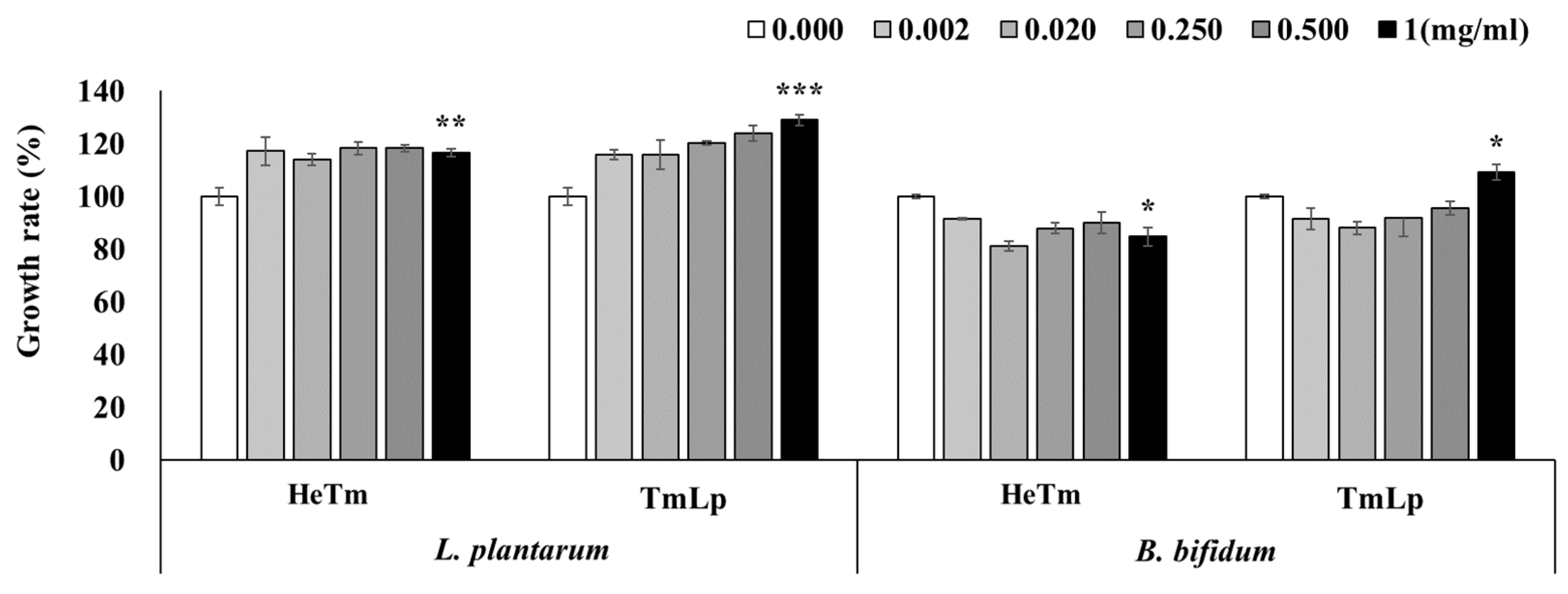
2.5. Growth-Promoting Effects on Intestinal Beneficial Bacteria
2.6. Cytotoxicity Analysis of RAW 264.7 Cells
2.7. Nitric Oxide (NO) Determination
2.8. Quantitative Real-Time PCR (qPCR) Analysis
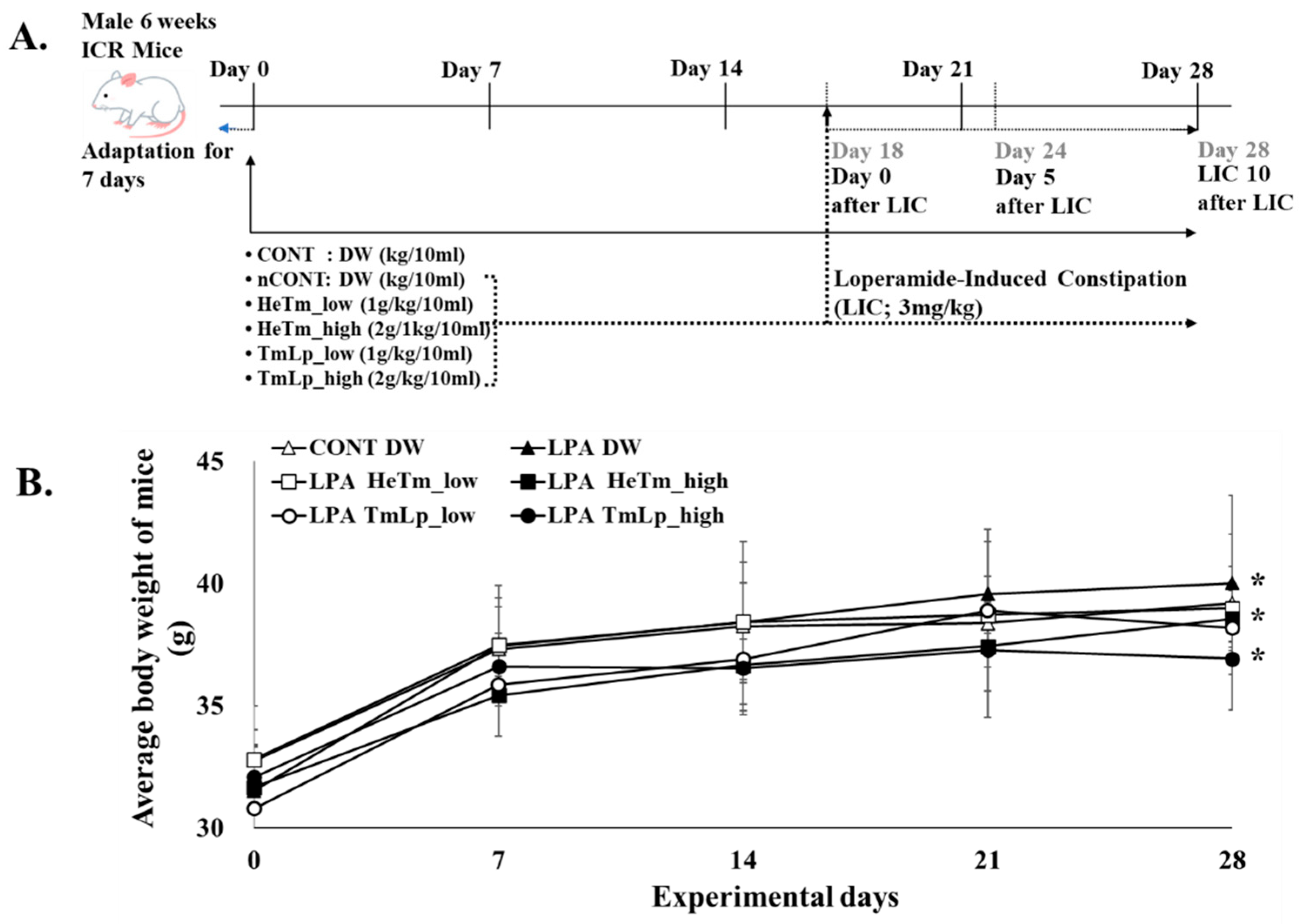
2.9. Animal Model and Experimental Design
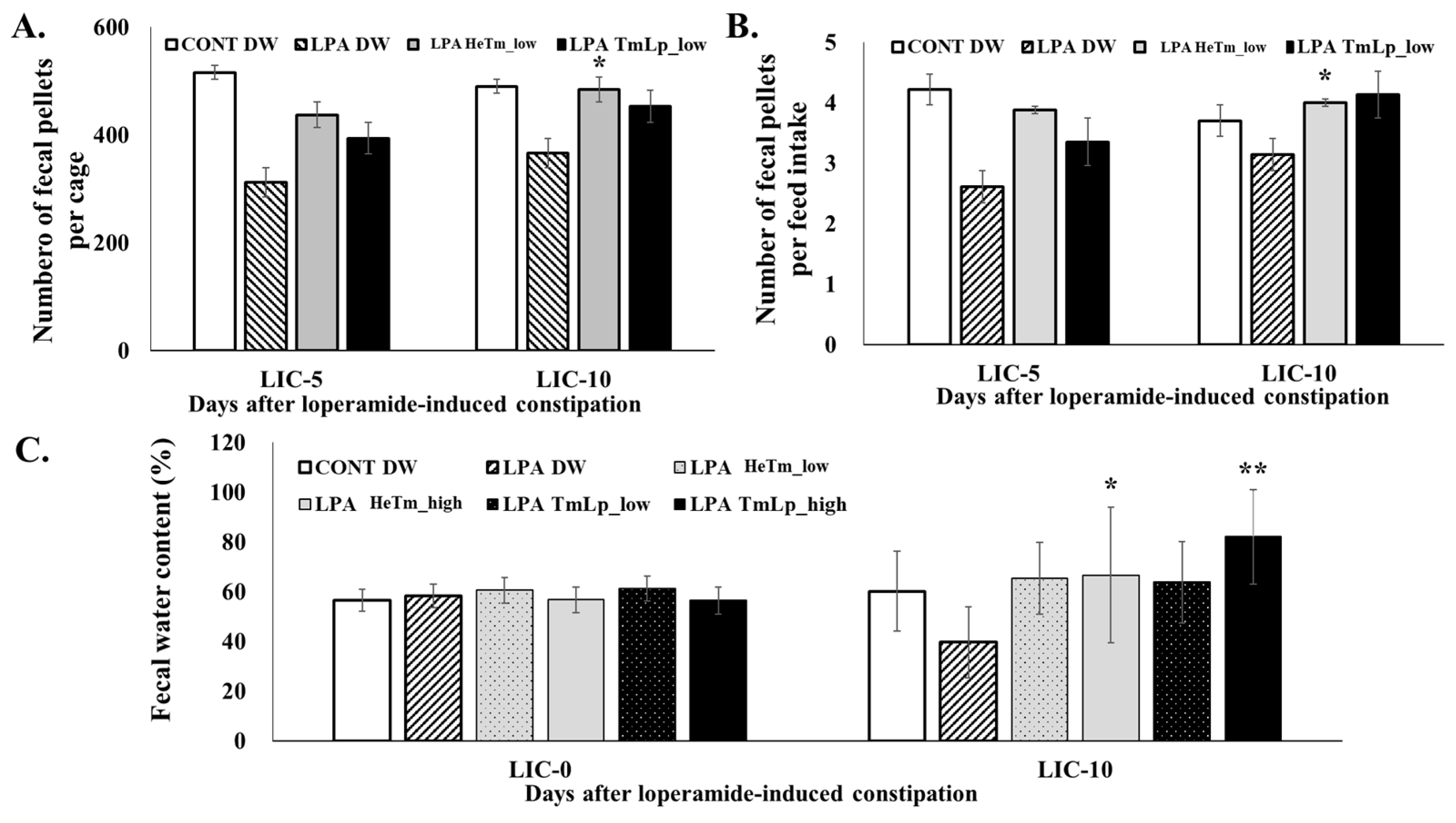
2.10. Measurement of Fecal Parameters and Dietary Intake in Mice
2.11. Measurement of Intestinal Transit and Fecal Retention in Mice
2.12. Data Analysis
3. Results and Discussion
3.1. Characterization of T. molitor Hydrolysate Produced by L. plantarum
3.2. Identification of Bioactive Peptides Using LC-MS/MS
3.3. Amino Acid Composition
3.4. Analysis of the Growth-Promoting Effects of HeTm and TmLp on Intestinal Beneficial Bacteria
3.5. Analysis of Cytotoxicity in RAW 264.7 Cells
3.6. Anti-Inflammatory Effects of HeTm and TmLp
3.7. Body Weight Changes in Mice with Loperamide-Induced Constipation
3.8. Effects on Fecal Parameters and Dietary Intake in Mice
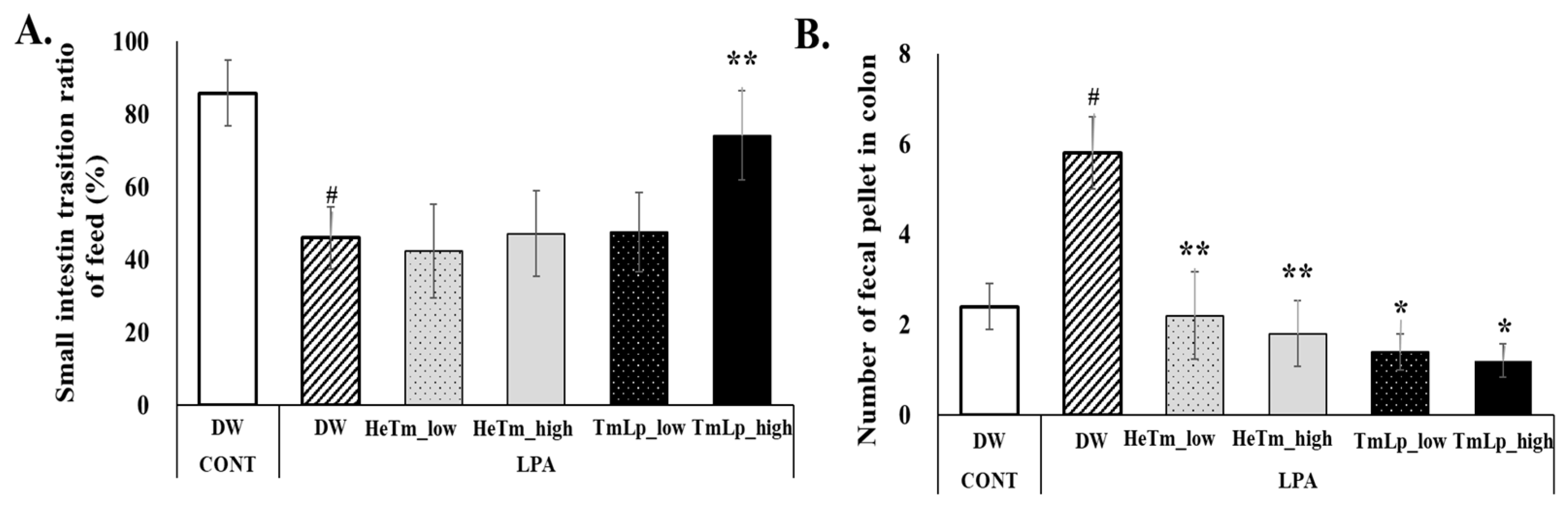
3.9. Effects of Intestinal Transit and Fecal Retention in Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DDA | data-dependent acquisition |
| DIW | deionized water |
| HCD | higher-energy collisional dissociation |
| ICR | Institute of Cancer Research |
| KCCM | Korean Culture Center of Microorganisms |
| LAB | lactic acid bacteria |
| NCE | normalized collision energy |
| PBS | phosphate-buffered saline |
| SEM | standard error of the mean |
References
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Hasnan, F.F.B.; Feng, Y.; Sun, T.; Parraga, K.; Schwarz, M.; Zarei, M. Insects as valuable sources of protein and peptides: Production, functional properties, and challenges. Foods 2023, 12, 4243. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.V. Nutritional composition of five commercial edible insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Qian, L.; Deng, P.; Chen, F.; Cao, Y.; Sun, H.; Liao, H. The exploration and utilization of functional substances in edible insects: A review. Food Prod. Process. Nutr. 2022, 4, 6101. [Google Scholar] [CrossRef]
- D’Antonio, V.; Serafini, M.; Battista, N. Dietary modulation of oxidative stress from edible insects: A mini-review. Front. Nutr. 2021, 8, 642551. [Google Scholar] [CrossRef]
- Aiello, D.; Barbera, M.; Bongiorno, D.; Cammarata, M.; Censi, V.; Indelicato, S.; Mazzotti, F.; Napoli, A.; Piazzese, D.; Saiano, F. Edible insects as an alternative nutritional source of bioactive compounds: A review. Molecules 2023, 18, 699. [Google Scholar] [CrossRef]
- Choi, R.-Y.; Ji, M.; Lee, M.-K.; Paik, M.-J. Metabolomics study of serum from a chronic alcohol-fed rat model following administration of defatted Tenebrio molitor larva fermentation extract. Metabolites 2020, 10, 436. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive molecules released in food by lactic acid bacteria: Encrypted peptides and biogenic amines. Front. Microbiol. 2016, 7, 876. [Google Scholar] [CrossRef]
- Hakim, B.N.A.; Xuan, N.J.; Oslan, S.N.H. A comprehensive review of bioactive compounds from lactic acid bacteria: Potential functions as functional food in dietetics and the food industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–nomad and ideal probiotic. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.H.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Abdullah, N. Comparative studies of versatile extracellular proteolytic activities of lactic acid bacteria and their potential for extracellular amino acid productions as feed supplements. J. Anim. Sci. Biotechnol. 2019, 10, 15. [Google Scholar] [CrossRef]
- Fadda, S.; Sanz, Y.; Vignolo, G.; Aristoy, M.-C.; Oliver, G.; Toldrá, F. Characterization of muscle sarcoplasmic and myofibrillar protein hydrolysis caused by Lactobacillus plantarum. Appl. Environ. Microbiol. 1999, 65, 3540–3546. [Google Scholar] [CrossRef]
- Li, C.; Nie, S.-P.; Zhu, K.-X.; Xiong, T.; Li, C.; Gong, J.; Xie, M.Y. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int. J. Food Sci. Nutr. 2015, 66, 533–538. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hou, Y.; Yin, Y.; Qiu, Y.; Wu, G.; Hu, C.-A.A. Roles of amino acids in preventing and treating intestinal diseases: Recent studies with pig models. Amino Acids 2017, 49, 1277–1291. [Google Scholar] [CrossRef]
- Abduh, M.Y.; Firmansyah, M.A.; Nugroho, R.A. Effects of enzymatic hydrolysis on the antioxidant activity of protein hydrolysate from black soldier fly (Hermetia illucens L.) larvae. J. Appl. Biol. Biotechnol. 2023, 11, 151–157. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Anti-inflammatory effects of Epimedium koreanum extract on LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2012, 139, 626–633. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). Amino Acid–Identification and Quantification by Automatic Amino Acid Analyzer. In Food Code; Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2016. Available online: https://various.foodsafetykorea.go.kr/fsd/?idx=11010#/ext/Document/FC?searchNm=아미노산&itemCode=FC0A090004005A093 (accessed on 21 February 2025).
- Zhang, J.; Wu, Y.; Wang, L.; Lu, C.; Zhao, Y.; Jin, Y.; Jiang, Y. Effect of Lactobacillus plantarum on constipation in mice induced by a high-fat diet. J. Funct. Foods 2016, 22, 75–84. [Google Scholar] [CrossRef]
- Purohit, K.; Reddy, N.; Sunna, A. Exploring the potential of bioactive peptides: From natural sources to therapeutics. Int. J. Mol. Sci. 2024, 25, 1391. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in intestinal mucosal defense and inflammation: Learning from clinical and experimental studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, N.I.M.; Shahabudin, N.A.; Razif, R.A.M.; Sanyal, A.; Ghosh, A.; Baharin, K.I.; Ahmad, H.; Maarof, M.; Motta, A.; Fauzi, M.B. Discovery of bioactive peptides as therapeutic agents for skin wound repair. J. Tissue Eng. 2024, 15, 20417314241280359. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Merchant, K.S.; Zlotnik, A.; Boyko, M. Gut microbiome modulation of glutamate dynamics: Implications for brain health and neurotoxicity. Nutrients 2024, 16, 4405. [Google Scholar] [CrossRef]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of potentially probiotic lactic acid bacteria and bifidobacteria isolated from human colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Boekel, M.A.J.S.; van der Linden, E. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Fusco, W.; Turco, F.; Palumbo, D.; Russo, M.; D’Alessandro, A.; Colicchio, R.; Nardone, G. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Hang, S.; Zhu, W.; Wu, G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 2015, 21, 389–409. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kang, C.-H. Immunostimulatory activity of lactic acid bacteria cell-free supernatants through the activation of NF-κB and MAPK signaling pathways in RAW 264.7 cells. Microorganisms 2022, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Wang, J.; Chen, H.; Liu, Y.; Xu, Q.; Zhou, M. The anti-inflammatory mechanism of flaxseed linusorbs on lipopolysaccharide-induced RAW 264.7 macrophages by modulating TLR4/NF-κB/MAPK pathway. Foods 2023, 12, 2398. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways. J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Shimizu, M. Food-derived peptides and intestinal functions. BioFactors 2004, 21, 43–47. [Google Scholar] [CrossRef]
- Miner-Williams, W.; Stevens, B.R.; Moughan, P.J. Are intact peptides absorbed from the healthy gut in the adult human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2016, 57, 1–9. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition and biologically active compounds. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, M.; Jung, J.H.; Kim, H.Y.; Yu, J. Evaluation of immunomodulatory activity of fermented Protaetia brevitarsis extract in macrophage cells. Food Sci. Anim. Resour. 2020, 40, 636–646. [Google Scholar] [CrossRef]
- Pihlanto, A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006, 16, 1306–1314. [Google Scholar] [CrossRef]
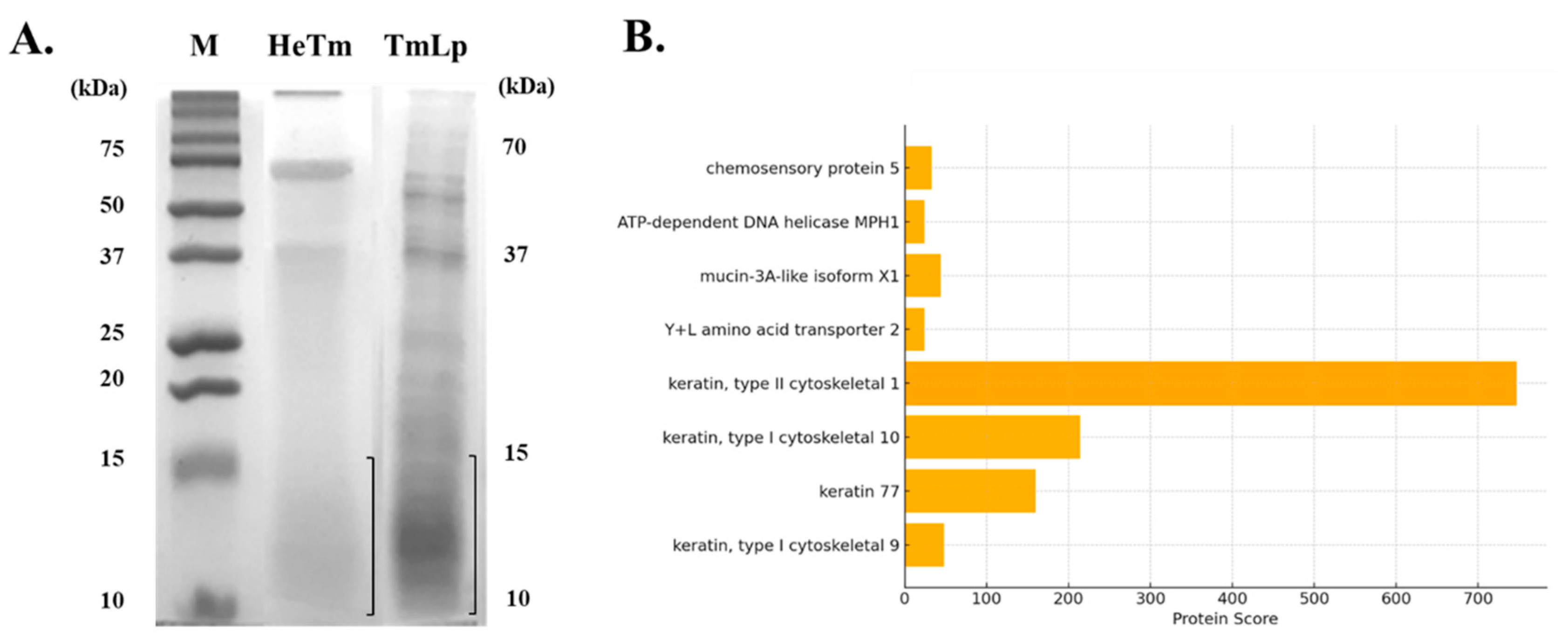

| Acc No | Protein Name | Species | Score | |
|---|---|---|---|---|
| HeTm | XP_033230134.1 | zinc finger protein 853-like isoform X1 | Belonocnema kinseyi | 15 |
| NP_002284.3 | laminin subunit gamma-1 precursor | Homo sapiens | 20 | |
| ATO91254.1 | immunoglobulin heavy chain variable region | Homo sapiens | 18 | |
| AAH04300.1 | VILL protein | Homo sapiens | 19 | |
| TmLp | UMT69257.1 | chemosensory protein 5 | Ophraella communa | 33 |
| XP_002066172.2 | ATP-dependent DNA helicase MPH1 | Drosophila willistoni | 24 | |
| XP_047519532.1 | mucin-3A-like isoform X1 | Pieris napi | 44 | |
| XP_002070402.1 | Y+L amino acid transporter | Drosophila willistoni | 24 | |
| UMT69257.1 | chemosensory protein 5 | Ophraella communa | 43 | |
| XP_047519532.1 | mucin-3A-like isoform X1 | Pieris napi | 39 | |
| NP_006112.3 | keratin, type II cytoskeletal 1 | Homo sapiens | 193 | |
| AAG41947.1 | keratin 1 | Homo sapiens | 138 | |
| NP_000217.2 | keratin, type I cytoskeletal 9 | Homo sapiens | 29 | |
| AAF28956.1 | HSPC278, partial | Homo sapiens | 14 | |
| BAS02858.1 | T cell receptor alpha chain V-J-region | Homo sapiens | 38 | |
| NP_002572.2 | pappalysin-1 preproprotein | Homo sapiens | 18 | |
| NP_006112.3 | keratin, type II cytoskeletal 1 | Homo sapiens | 746 | |
| AFA52006.1 | keratin 1 | Homo sapiens | 225 | |
| NP_000412.4 | keratin, type I cytoskeletal 10 isoform 1 | Homo sapiens | 214 | |
| aai22559.1 | Keratin 77 | Homo sapiens | 160 | |
| BAS02858.1 | T cell receptor alpha chain V-J-region | Homo sapiens | 76 | |
| NP_000217.2 | keratin, type I cytoskeletal 9 | Homo sapiens | 48 |
| Amino Acid (mg/100 g) | HeTm | TmLp | |||
|---|---|---|---|---|---|
| Free Amino Acids | Bound Amino Acids | Free Amino Acids | Bound Amino Acids | ||
| Essential | Threonine (T) | 0.67 ± 0.10 | 4.30 ± 0.65 | 8.49 ± 1.02 | 48.99 ± 5.88 |
| Valine (V) | 3.15 ± 0.47 | 7.18 ± 1.08 | 23.68 ± 2.84 | 64.25 ± 7.71 | |
| Methionine (M) | - | 1.99 ± 0.29 | 0.35 ± 0.04 | 14.36 ± 1.72 | |
| Isoleucine (I) | 1.83 ± 0.27 | 3.21 ± 0.48 | 12.99 ± 1.56 | 40.27 ± 4.89 | |
| Leucine (L) | 1.10 ± 0.16 | 4.28 ± 0.64 | 12.04 ± 0.91 | 58.79 ± 7.05 | |
| Phenylalanine (F) | 0.70 ± 0.11 | 4.15 ± 1.87 | 6.90 ± 0.73 | 33.56 ± 4.02 | |
| Histidine (H) | 4.33 ± 0.65 | 6.00 ± 0.90 | 26.68 ± 4.01 | 51.98 ± 6.24 | |
| Lysine (K) | 1.22 ± 0.18 | 4.22 ± 0.63 | 12.04 ± 1.44 | 69.78 ± 8.37 | |
| Tryptophan (W) | 1.95 ± 0.29 | 16.73 ± 2.01 | - | ||
| Conditionally Essential | Arginine (R) | 6.78 ± 1.02 | 7.11 ± 1.07 | - | 57.17 ± 6.86 |
| Cysteine (C) | - | - | |||
| Glycine (G) | 0.94 ± 0.10 | 3.78 ± 0.57 | 7.97 ± 0.96 | 67.44 ± 8.09 | |
| Proline (P) | 17.67 ± 2.65 | 12.90 ± 1.94 | 162.91 ± 19.55 | 204.55 ± 24.55 | |
| Tyrosine (Y) | 5.08 ± 0.76 | 5.19 ± 0.78 | 48.28 ± 5.79 | 48.28 ± 5.79 | |
| Non-Essential | Aspartic acid (D) | 0.51 ± 0.08 | 5.30 ± 0.79 | 2.98 ± 0.36 | 103.84 ± 12.46 |
| Serine (S) | 0.59 ± 0.09 | 5.06 ± 0.76 | 4.94 ± 0.59 | 54.77 ± 6.57 | |
| Glutamic acid (E) | 3.60 ± 0.54 | 18.77 ± 2.82 | 21.88 ± 2.63 | 207.13 ± 24.86 | |
| Alanine (A) | 6.78 ± 1.02 | 4.77 ± 0.72 | 36.66 ± 4.39 | 87.22 ± 10.47 | |
| Total A.A | 56.9 ± 8.53 | 98.21 ± 4.91 | 405.52 ± 20.28 | 1212.38 ± 60.62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-S.; Song, D.-B.; Lee, S.-H.; Lee, K.-S.; Yang, J.; Hong, S.-M. Concurrent Hydrolysis–Fermentation of Tenebrio molitor Protein by Lactobacillus plantarum KCCM13068P Attenuates Inflammation in RAW 264.7 Macrophages and Constipation in Loperamide-Induced Mice. Foods 2025, 14, 1886. https://doi.org/10.3390/foods14111886
Jo H-S, Song D-B, Lee S-H, Lee K-S, Yang J, Hong S-M. Concurrent Hydrolysis–Fermentation of Tenebrio molitor Protein by Lactobacillus plantarum KCCM13068P Attenuates Inflammation in RAW 264.7 Macrophages and Constipation in Loperamide-Induced Mice. Foods. 2025; 14(11):1886. https://doi.org/10.3390/foods14111886
Chicago/Turabian StyleJo, Hyun-Sol, Da-Bin Song, Sang-Hee Lee, Kyu-Shik Lee, Jungwoo Yang, and Sun-Mee Hong. 2025. "Concurrent Hydrolysis–Fermentation of Tenebrio molitor Protein by Lactobacillus plantarum KCCM13068P Attenuates Inflammation in RAW 264.7 Macrophages and Constipation in Loperamide-Induced Mice" Foods 14, no. 11: 1886. https://doi.org/10.3390/foods14111886
APA StyleJo, H.-S., Song, D.-B., Lee, S.-H., Lee, K.-S., Yang, J., & Hong, S.-M. (2025). Concurrent Hydrolysis–Fermentation of Tenebrio molitor Protein by Lactobacillus plantarum KCCM13068P Attenuates Inflammation in RAW 264.7 Macrophages and Constipation in Loperamide-Induced Mice. Foods, 14(11), 1886. https://doi.org/10.3390/foods14111886







