Seabuckthorn Seed Meal Protein-Based Inhibitory Peptides Targeting Multiple Hyperglycemic Enzymes: Optimization of Process and Probing of Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of SSP
2.3. Preparation of SSPHs
2.4. Degree of Hydrolysis (DH)
2.5. Inhibitory Activities Against Hyperglycemic Enzymes
2.6. Process Optimization
2.7. Peptide Identification
2.8. Bioinformatics Analysis
2.9. Molecular Docking
2.10. Solid-Phase Synthesis of Peptides
2.11. Statistical Analysis
3. Results and Discussion
3.1. Screening of Proteases
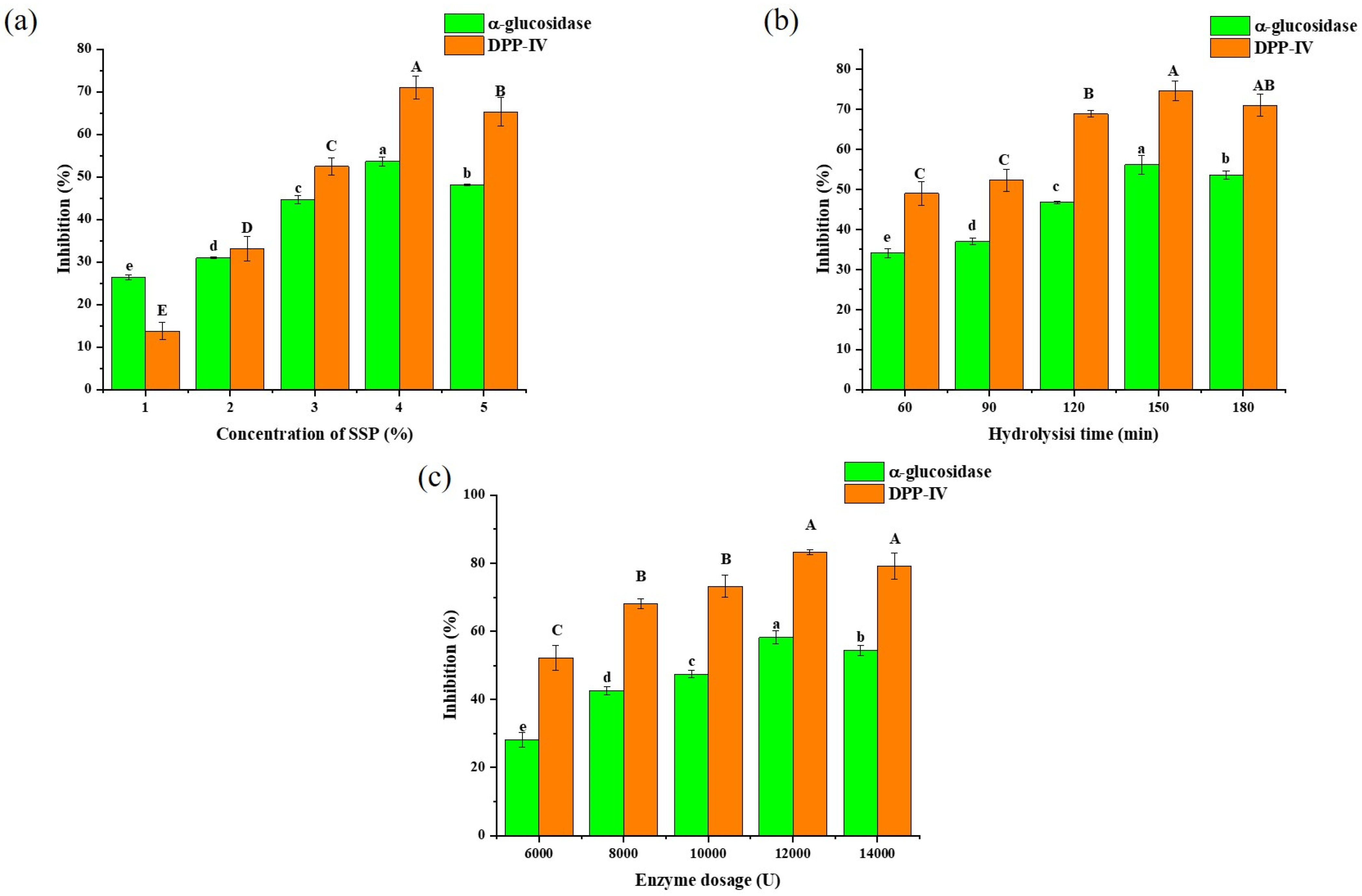
3.2. Single-Factor Experiments
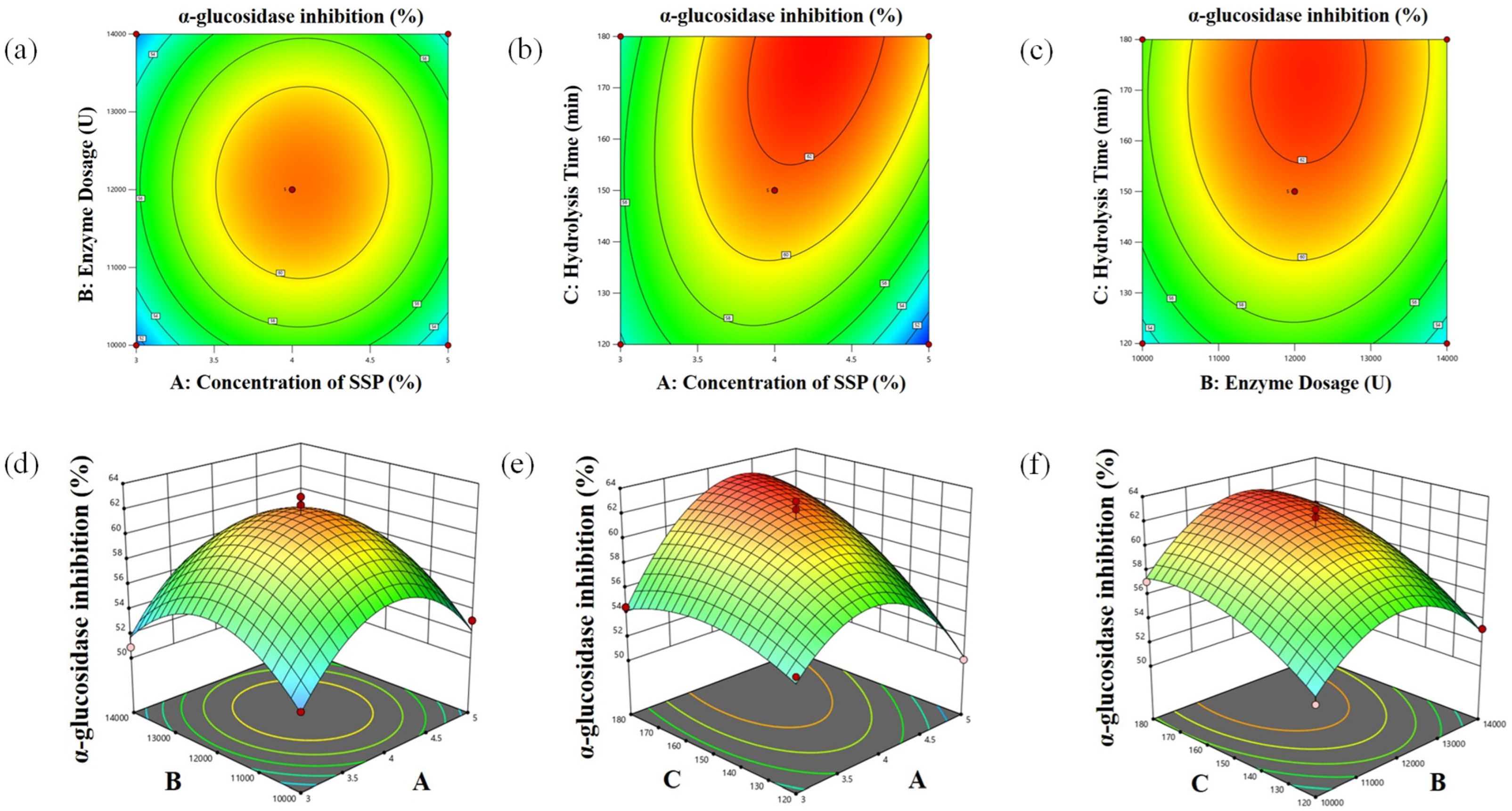
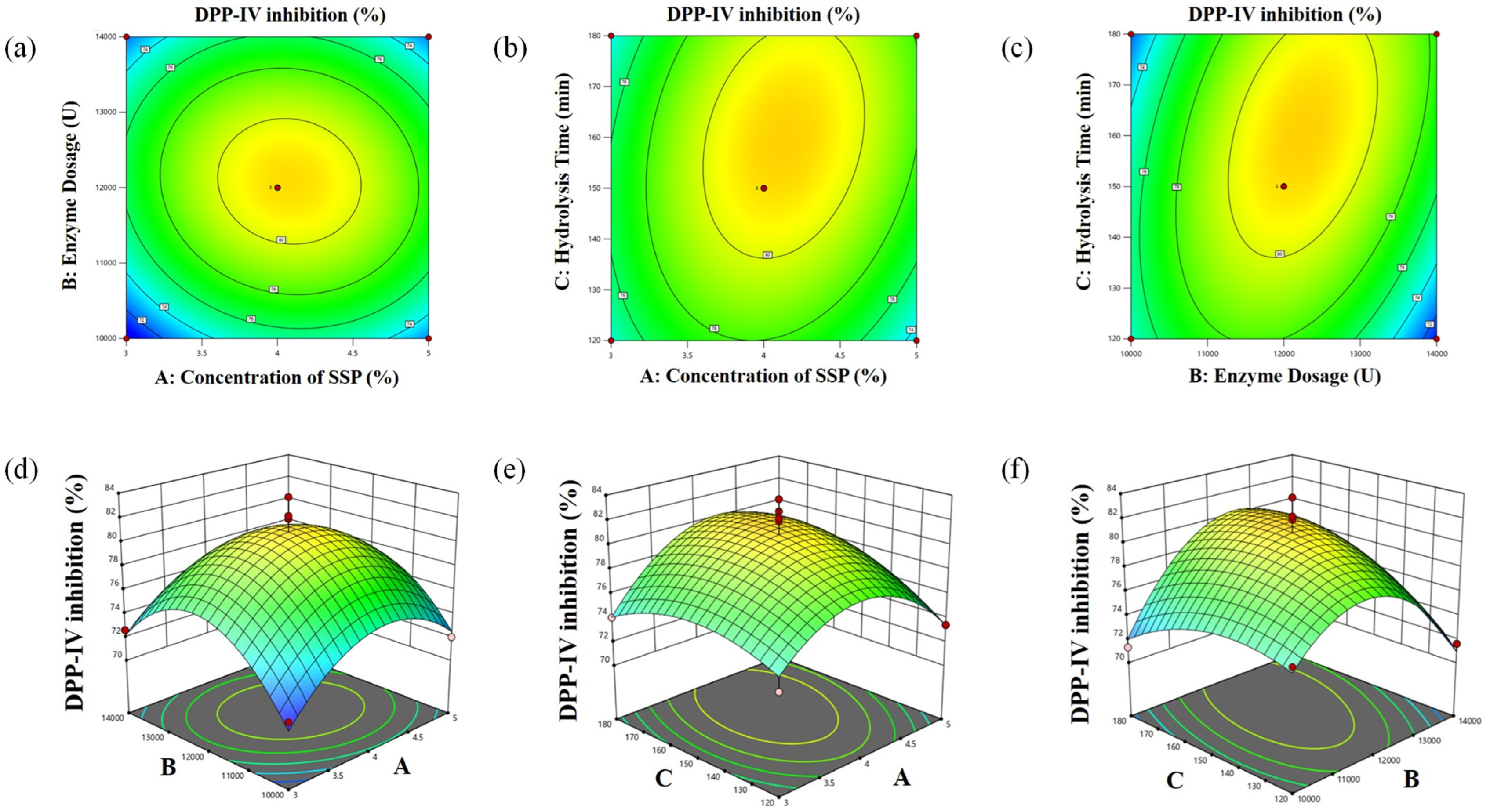
3.3. BBD-RSM Experiments
3.4. Identification by Peptidomics and Bioinformatics Analysis
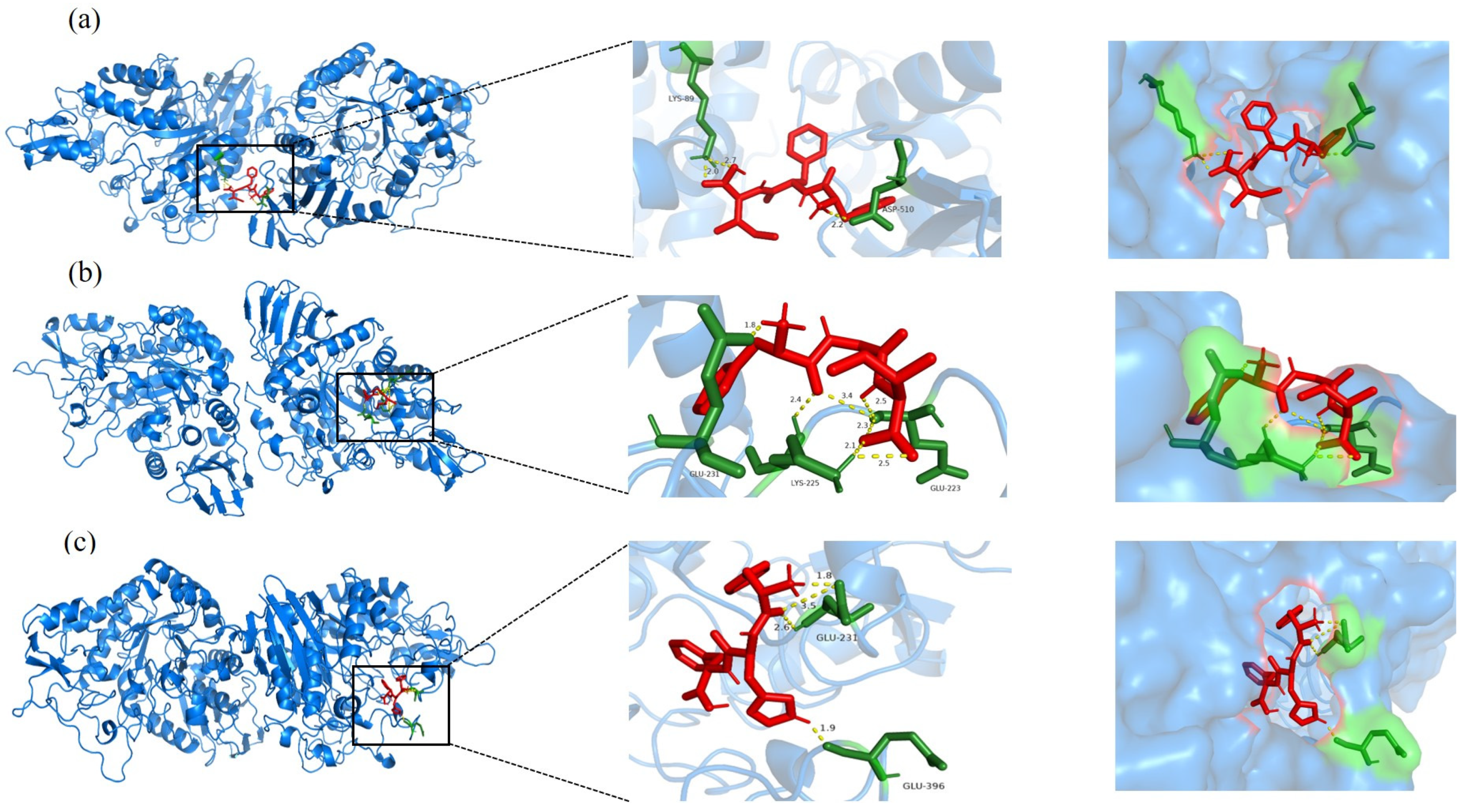
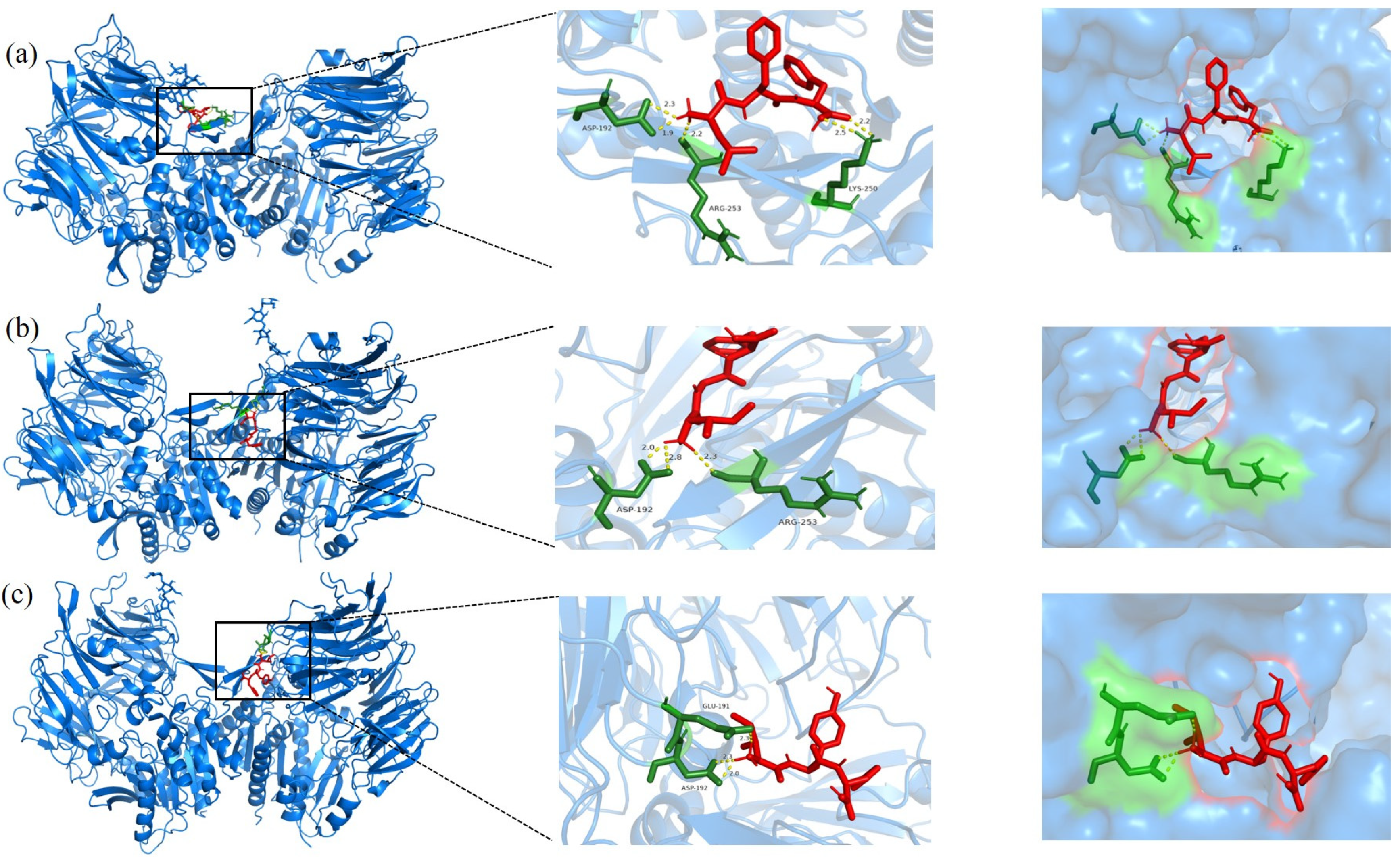
3.5. Molecular Docking Analysis
3.6. In Vitro Activity Verification of Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Yao, J.; Zhou, L.; Zhao, M.; Wang, W.; Liu, J.; Marchioni, E. Comprehensive untargeted lipidomic analysis of sea buckthorn using UHPLC-HR-AM/MS/MS combined with principal component analysis. Food Chem. 2024, 430, 136964. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Binosha Fernando, W.M.A.D.; Durham, R.; Stockmann, R.; Jayasena, V. Nutritional Value, Health-promoting Benefits and Food Application of SeaBuckthorn. Food Rev. Int. 2021, 39, 2122–2137. [Google Scholar] [CrossRef]
- Tkacz, K.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Microspheres as carriers of seabuckthorn carotenoids and tocols with antidiabetic potential: Effect of biopolymers, cross-linking and storage. Food Biosci. 2024, 59, 103995. [Google Scholar] [CrossRef]
- Chen, A.; Feng, X.; Dorjsuren, B.; Chimedtseren, C.; Damda, T.-A.; Zhang, C. Traditional food, modern food and nutritional value of Seabuckthorn (Hippophae rhamnoides L.): A review. J. Future Foods 2023, 3, 191–205. [Google Scholar] [CrossRef]
- Lin, J.; Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Wang, W. Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: A comparison study. Food Sci. Hum. Wellness 2022, 11, 1028–1035. [Google Scholar] [CrossRef]
- Prandi, B.; Faccini, A.; Lambertini, F.; Bencivenni, M.; Jorba, M.; Van Droogenbroek, B.; Bruggeman, G.; Schober, J.; Petrusan, J.; Elst, K.; et al. Food wastes from agrifood industry as possible sources of proteins: A detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem. 2019, 286, 567–575. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, T.; Cao, Y.; Deng, B.; Zhang, J.; Zhao, J. Effects of dietary seabuckthorn pomace supplementation on skeletal muscle mass and meat quality in lambs. Meat Sci. 2020, 166, 108141. [Google Scholar] [CrossRef]
- Vaiserman, A.; Lushchak, O. Developmental origins of type 2 diabetes: Focus on epigenetics. Ageing Res. Rev. 2019, 55, 100957. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Yu, Y.; Liu, B.; Liu, J.; Chen, F. Novel peptides derived from egg white protein inhibiting alpha-glucosidase. Food Chem. 2011, 129, 1376–1382. [Google Scholar] [CrossRef]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Food-derived dipeptidyl-peptidase IV inhibitors as a potential approach for glycemic regulation-Current knowledge and future research considerations. Trends Food Sci. Technol. 2016, 54, 863–880. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Wang, F.; Yu, G.; Zhang, Y.; Zhang, B.; Fan, J. Dipeptidyl Peptidase IV Inhibitory Peptides Derived from Oat (Avena sativa L.), Buckwheat (Fagopyrum esculentum), and Highland Barley (Hordeum vulgare trifurcatum (L.) Trofim) Proteins. J. Agric. Food Chem. 2015, 63, 9543–9549. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Waterhouse, D.-S.; Liu, P.; Waterhouse, G.I.N.; Li, J.; Cui, C. Pancreatic lipase-inhibiting protein hydrolysate and peptides from seabuckthorn seed meal: Preparation optimization and inhibitory mechanism. LWT-Food Sci. Technol. 2020, 134, 109870. [Google Scholar] [CrossRef]
- Leeb, E.; Gotz, A.; Letzel, T.; Cheison, S.C.; Kulozik, U. Influence of denaturation and aggregation of beta-lactoglobulin on its tryptic hydrolysis and the release of functional peptides. Food Chem. 2015, 187, 545–554. [Google Scholar] [CrossRef]
- Zan, R.; Zhu, L.; Wu, G.; Zhang, H. Identification of Novel Peptides with Alcohol Dehydrogenase (ADH) Activating Ability in Chickpea Protein Hydrolysates. Foods 2023, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- Padial-Dominguez, M.; Espejo-Carpio, F.J.; Garcia-Moreno, P.J.; Jacobsen, C.; Guadix, E.M. Protein derived emulsifiers with antioxidant activity for stabilization of omega-3 emulsions. Food Chem. 2020, 329, 127148. [Google Scholar] [CrossRef]
- Sadeghi, M.; Miroliaei, M.; Ghanadian, M.; Szumny, A.; Rahimmalek, M. Exploring the inhibitory properties of biflavonoids on alpha-glucosidase; computational and experimental approaches. Int. J. Biol. Macromol. 2023, 253 Pt 7, 127380. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Du, Z.; Yu, Z.; Liu, J. Hydrolysis and Transport of Egg White-Derived Peptides in Caco-2 Cell Monolayers and Everted Rat Sacs. J. Agric. Food Chem. 2019, 67, 4839–4848. [Google Scholar] [CrossRef]
- Huang, Y.; Richardson, S.J.; Brennan, C.S.; Kasapis, S. Mechanistic insights into α-amylase inhibition, binding affinity and structural changes upon interaction with gallic acid. Food Hydrocoll. 2024, 148, 109467. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Ashraf, Z.U.; Gani, A.; Shah, A.; Gani, A. Identification of antidiabetic peptides from broad bean protein: Sequencing using LC-MS-QTOF and in-vitro confirmative studies. Food Biosci. 2024, 61, 104903. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D.J.; Wu, L. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016, 61, 251–260. [Google Scholar] [CrossRef]
- Yuan, G.; Li, W.; Pan, Y.; Wang, C.; Chen, H. Shrimp shell wastes: Optimization of peptide hydrolysis and peptide inhibition of α-amylase. Food Biosci. 2018, 25, 52–60. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Ni, Y.; Zhang, Y.; Liu, Y.; Du, B.; Luo, H.; Lin, D. Production of novel mung bean peptides-based zinc supplement: Process optimization, chelation mechanism, and stability assessment in vitro. Food Biosci. 2024, 59, 104188. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- Zan, R.; Wu, Q.; Chen, Y.; Wu, G.; Zhang, H.; Zhu, L. Identification of Novel Dipeptidyl Peptidase-IV Inhibitory Peptides in Chickpea Protein Hydrolysates. J. Agric. Food Chem. 2023, 71, 8211–8219. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Cadamuro, C.; Le Gouic, A.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of a camel whey protein enriched hydrolysate preparation. Food Chem. 2019, 279, 70–79. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef]
- Kawakami, K.; Li, P.; Uraji, M.; Hatanaka, T.; Ito, H. Inhibitory effects of pomegranate extracts on recombinant human maltase-glucoamylase. J. Food Sci. 2014, 79, H1848–H1853. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xian, M.; Qu, C.; Peng, P.; Yongo, E.; Guo, Z.; Du, Z.; Xiao, J. Novel Se-enriched α-glucosidase inhibitory peptide derived from tuna dark meat: Preparation, identification and effects on IR-HepG2 cells. Food Biosci. 2024, 60, 104357. [Google Scholar] [CrossRef]
- Ortizo, R.G.G.; Sharma, V.; Tsai, M.-L.; Nargotra, P.; Sun, P.-P.; Chen, C.-W.; Dong, C.-D. A novel deep eutectic solvent-based green extraction and purification of DPP-IV inhibitory peptides from tilapia (Oreochromis niloticus) viscera hydrolysate. Food Biosci. 2024, 61, 104658. [Google Scholar] [CrossRef]

| Factor | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: Concentration of SSP (%) | 3 | 4 | 5 |
| B: Enzyme dosage (U) | 10,000 | 12,000 | 14,000 |
| C: Hydrolysis time (min) | 120 | 150 | 180 |
| Std | Run | A: Concentration of SSP (%) | B: Enzyme Dosage (U) | C: Hydrolysis Time (min) | α-Glucosidase Inhibition (%) |
|---|---|---|---|---|---|
| 15 | 1 | 0 | 0 | 0 | 62.98 ± 0.96 |
| 9 | 2 | 0 | −1 | −1 | 52.54 ± 0.97 |
| 8 | 3 | 1 | 0 | 1 | 59.80 ± 1.66 |
| 2 | 4 | 1 | −1 | 0 | 53.12 ± 1.12 |
| 6 | 5 | 1 | 0 | −1 | 50.07 ± 0.85 |
| 14 | 6 | 0 | 0 | 0 | 62.35 ± 1.11 |
| 3 | 7 | −1 | 1 | 0 | 50.89 ± 0.82 |
| 4 | 8 | 1 | 1 | 0 | 53.37 ± 1.41 |
| 11 | 9 | 0 | −1 | 1 | 57.12 ± 1.73 |
| 16 | 10 | 0 | 0 | 0 | 61.46 ± 2.44 |
| 1 | 11 | −1 | −1 | 0 | 51.50 ± 0.74 |
| 7 | 12 | −1 | 0 | 1 | 54.48 ± 1.37 |
| 5 | 13 | −1 | 0 | −1 | 54.25 ± 1.57 |
| 12 | 14 | 0 | 1 | 1 | 59.77 ± 1.48 |
| 10 | 15 | 0 | 1 | −1 | 53.18 ± 0.35 |
| 13 | 16 | 0 | 0 | 0 | 59.94 ± 1.05 |
| 17 | 17 | 0 | 0 | 0 | 61.05 ± 1.37 |
| Std | Run | A: Concentration of SSP (%) | B: Enzyme Dosage (U) | C: Hydrolysis Time (min) | DPP-IV Inhibition (%) |
|---|---|---|---|---|---|
| 15 | 1 | 0 | 0 | 0 | 78.25 ± 1.62 |
| 9 | 2 | 0 | −1 | −1 | 75.13 ± 1.01 |
| 8 | 3 | 1 | 0 | 1 | 79.05 ± 2.33 |
| 2 | 4 | 1 | −1 | 0 | 72.01 ± 1.44 |
| 6 | 5 | 1 | 0 | −1 | 73.44 ± 1.26 |
| 14 | 6 | 0 | 0 | 0 | 81.92 ± 1.73 |
| 3 | 7 | −1 | 1 | 0 | 72.62 ± 0.64 |
| 4 | 8 | 1 | 1 | 0 | 71.58 ± 2.02 |
| 11 | 9 | 0 | −1 | 1 | 71.31 ± 2.26 |
| 16 | 10 | 0 | 0 | 0 | 83.69 ± 2.76 |
| 1 | 11 | −1 | −1 | 0 | 70.72 ± 0.96 |
| 7 | 12 | −1 | 0 | 1 | 74.06 ± 2.13 |
| 5 | 13 | −1 | 0 | −1 | 73.47 ± 2.21 |
| 12 | 14 | 0 | 1 | 1 | 77.20 ± 1.80 |
| 10 | 15 | 0 | 1 | −1 | 71.62 ± 1.12 |
| 13 | 16 | 0 | 0 | 0 | 78.25 ± 2.46 |
| 17 | 17 | 0 | 0 | 0 | 82.17 ± 1.71 |
| Database | Web Link |
|---|---|
| BIOPEP | https://biochemia.uwm.edu.pl/ (accessed on 17 May 2024) |
| PeptideRanker | http://distilldeep.ucd.ie/PeptideRanker/ (accessed on 18 May 2024). |
| UniProt | https://www.uniprot.org/ (accessed on 10 May 2024). |
| ToxinPred | https://webs.iiitd.edu.in/raghava/toxinpred/index.html (accessed on 19 May 2024). |
| AllerTOP | https://www.ddg-pharmfac.net/allertop_test (accessed on 19 May 2024). |
| PDB | https://www.rcsb.org (accessed on 3 June 2024). |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 301.32 | 9 | 33.48 | 27.64 | 0.0001 | significant |
| A: Concentration of SSP | 3.43 | 1 | 3.43 | 2.83 | 0.1362 | |
| B: Enzyme dosage | 1.07 | 1 | 1.07 | 0.8860 | 0.3779 | |
| C: Hydrolysis time | 55.81 | 1 | 55.81 | 46.80 | 0.0003 | |
| AB | 0.1849 | 1 | 0.1849 | 0.1527 | 0.7076 | |
| AC | 22.56 | 1 | 22.56 | 18.63 | 0.0035 | |
| BC | 1.01 | 1 | 1.01 | 0.8339 | 0.3915 | |
| A2 | 112.51 | 1 | 112.51 | 92.89 | < 0.0001 | |
| B2 | 73.10 | 1 | 73.10 | 60.35 | 0.0001 | |
| C2 | 12.70 | 1 | 12.70 | 10.49 | 0.0143 | |
| Residual | 8.48 | 7 | 1.21 | |||
| Lack of Fit | 2.94 | 3 | 0.9812 | 0.7091 | 0.5951 | not significant |
| Pure Error | 5.53 | 4 | 1.38 | |||
| Cor Total | 309.80 | 16 | ||||
| R2 | 0.9726 | |||||
| Adjusted R2 | 0.9374 | |||||
| Predicted R2 | 0.8201 | |||||
| Adeq Precision | 13.3485 | |||||
| Std. Dev. | 1.10 | |||||
| Mean | 56.35 | |||||
| C.V. % | 1.95 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 254.93 | 9 | 28.33 | 6.65 | 0.0103 | significant |
| A: Concentration of SSP | 3.39 | 1 | 3.39 | 0.7967 | 0.4017 | |
| B: Enzyme dosage | 1.85 | 1 | 1.85 | 0.4350 | 0.5306 | |
| C: Hydrolysis time | 7.92 | 1 | 7.92 | 1.86 | 0.2149 | |
| AB | 1.36 | 1 | 1.36 | 0.3187 | 0.5900 | |
| AC | 6.30 | 1 | 6.30 | 1.48 | 0.2633 | |
| BC | 22.09 | 1 | 22.09 | 5.19 | 0.0569 | |
| A2 | 66.25 | 1 | 66.25 | 15.56 | 0.0056 | |
| B2 | 111.97 | 1 | 111.97 | 26.29 | 0.0014 | |
| C2 | 14.95 | 1 | 14.95 | 3.51 | 0.1031 | |
| Residual | 29.81 | 7 | 4.26 | |||
| Lack of Fit | 5.34 | 3 | 1.78 | 0.2910 | 0.8308 | not significant |
| Pure Error | 24.47 | 4 | 6.12 | |||
| Cor Total | 284.75 | 16 | ||||
| R2 | 0.8953 | |||||
| Adjusted R2 | 0.7607 | |||||
| Predicted R2 | 0.5656 | |||||
| Adeq Precision | 6.8476 | |||||
| Std. Dev. | 2.06 | |||||
| Mean | 75.68 | |||||
| C.V. % | 2.73 |
| Sequence/Name | α-Glucosidase Inhibition IC50 a (mg/mL) | α-Glucosidase Inhibition IC50 a (mM) |
|---|---|---|
| FHF | 1.79 ± 0.07 c | 3.98 ± 0.16 c |
| FFI | 3.50 ± 0.09 b | 8.21 ± 0.21 b |
| FGI | 3.88 ± 0.07 a | 11.57 ± 0.20 a |
| SSPD-purification | 3.45 ± 0.18 b | - |
| Acarbose | 0.52 ± 0.03 d | 0.80 ± 0.04 d |
| Sequence/Name | DPP-IV Inhibition IC50 a (mg/mL) | DPP-IV Inhibition IC50 a (mM) |
|---|---|---|
| IYF | 2.35 ± 0.07 c | 5.32 ± 0.15 c |
| IGF | 2.40 ± 0.05 c | 7.17 ± 0.14 b |
| LFF | 3.24 ± 0.08 b | 7.62 ± 0.19 a |
| SSPD-purification | 5.01 ± 0.21 a | - |
| IPI | 3.91 × 10−3 ± 0.18 d | 8.57 × 10−3 ± 0.40 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Q.; Jia, Y.; Wu, T.; Zhang, J.; Shan, L. Seabuckthorn Seed Meal Protein-Based Inhibitory Peptides Targeting Multiple Hyperglycemic Enzymes: Optimization of Process and Probing of Mechanisms. Foods 2025, 14, 1876. https://doi.org/10.3390/foods14111876
Shan Q, Jia Y, Wu T, Zhang J, Shan L. Seabuckthorn Seed Meal Protein-Based Inhibitory Peptides Targeting Multiple Hyperglycemic Enzymes: Optimization of Process and Probing of Mechanisms. Foods. 2025; 14(11):1876. https://doi.org/10.3390/foods14111876
Chicago/Turabian StyleShan, Qi, Yeping Jia, Tonghua Wu, Jun Zhang, and Liang Shan. 2025. "Seabuckthorn Seed Meal Protein-Based Inhibitory Peptides Targeting Multiple Hyperglycemic Enzymes: Optimization of Process and Probing of Mechanisms" Foods 14, no. 11: 1876. https://doi.org/10.3390/foods14111876
APA StyleShan, Q., Jia, Y., Wu, T., Zhang, J., & Shan, L. (2025). Seabuckthorn Seed Meal Protein-Based Inhibitory Peptides Targeting Multiple Hyperglycemic Enzymes: Optimization of Process and Probing of Mechanisms. Foods, 14(11), 1876. https://doi.org/10.3390/foods14111876





