Proteomic Analysis of Listeria monocytogenes Subjected to Pulsed Magnetic Field
Abstract
1. Introduction
2. Materials and Methods
2.1. L. monocytogenes Sample Preparation
2.2. PMF Treatment
2.3. Determination of L. monocytogenes Inactivation
2.4. Protein Extraction and Quantitation
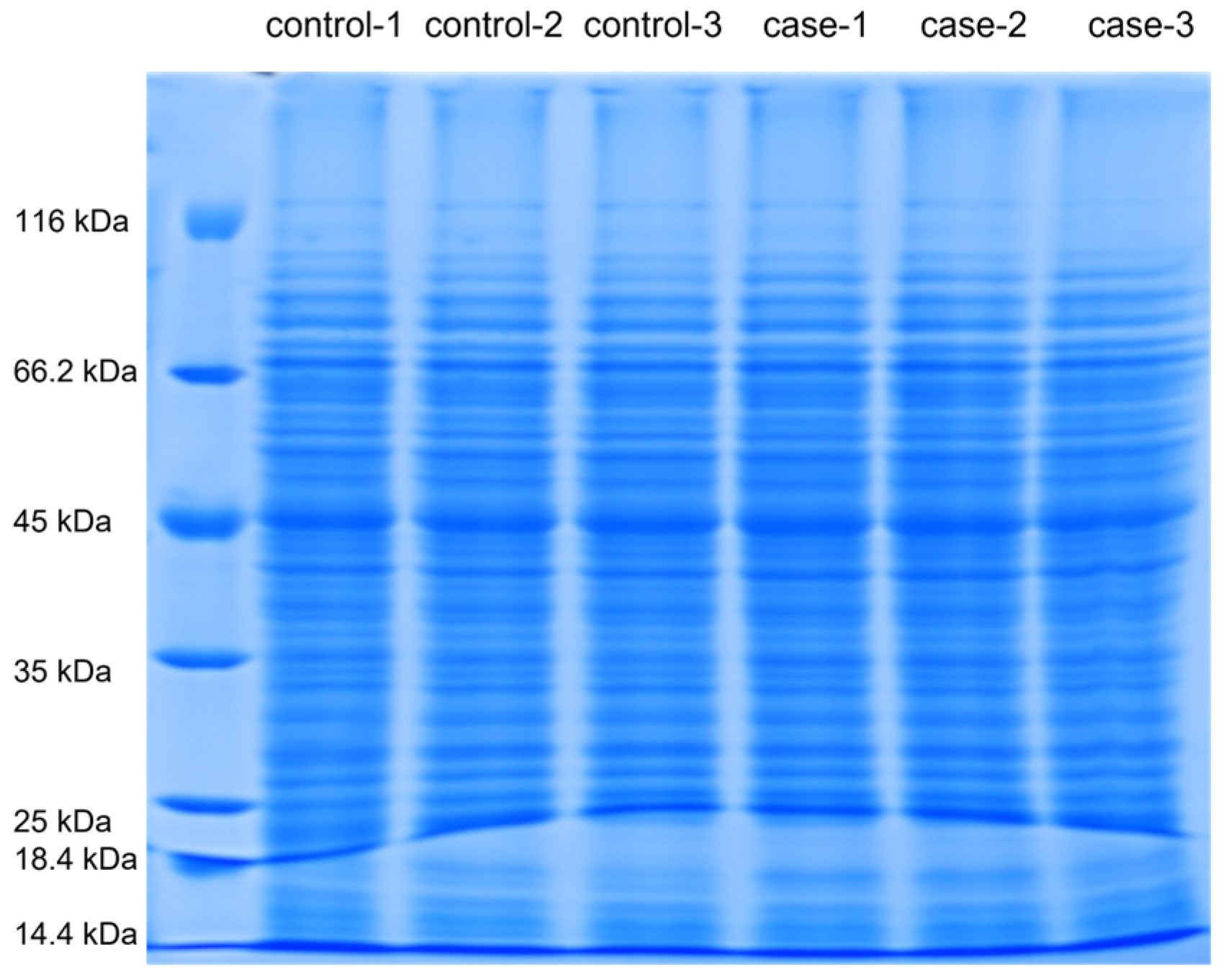
2.5. SDS-PAGE Electrophoresis
2.6. Protein Digestion and TMT Labeling
2.7. Reverse-Phase Liquid Chromatography (RPLC) Analysis
2.8. MS Analysis
2.9. Database Search
2.10. Bioinformatic Analysis
3. Results
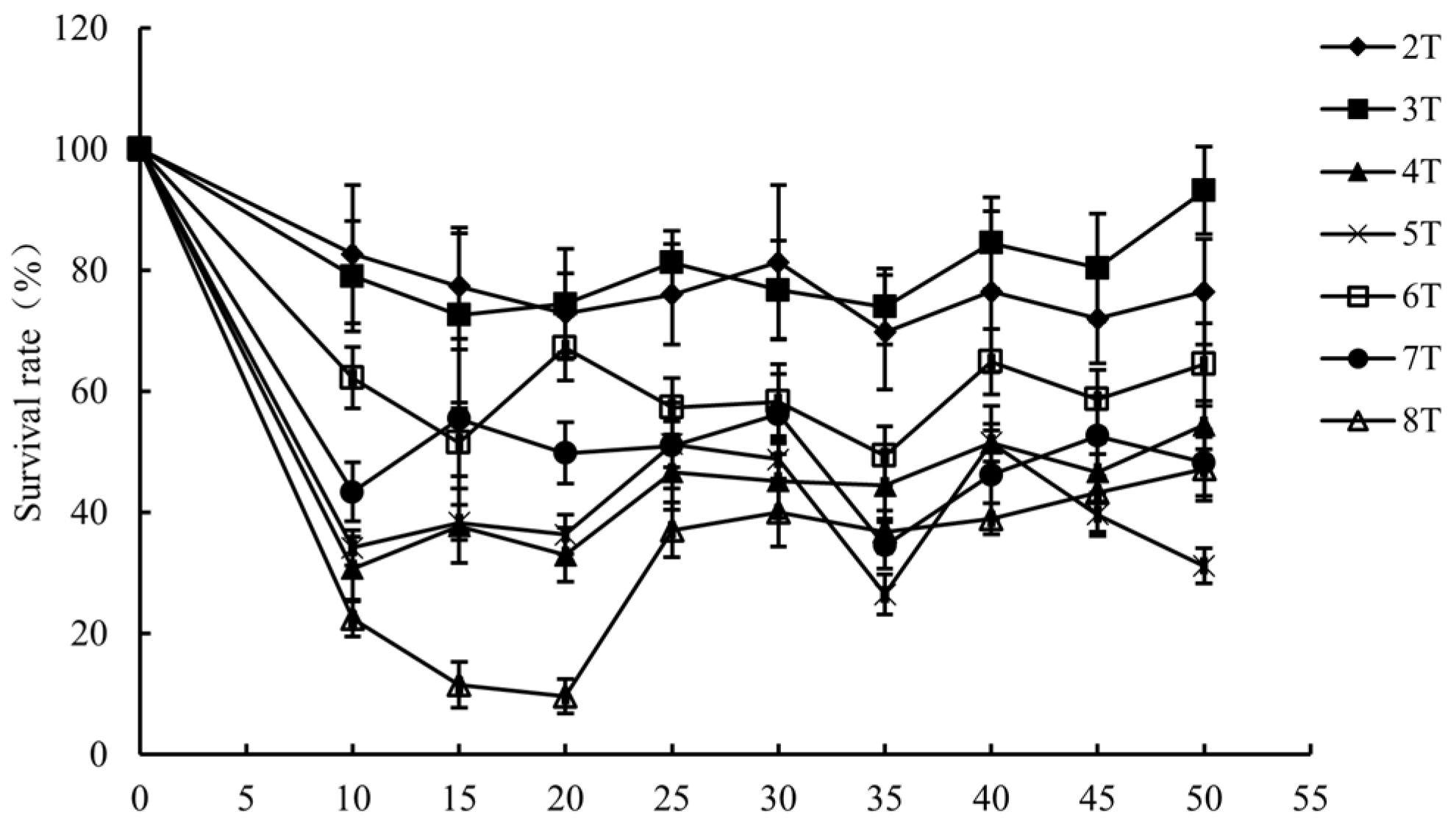
3.1. Inactivation of L. monocytogenes by PMF
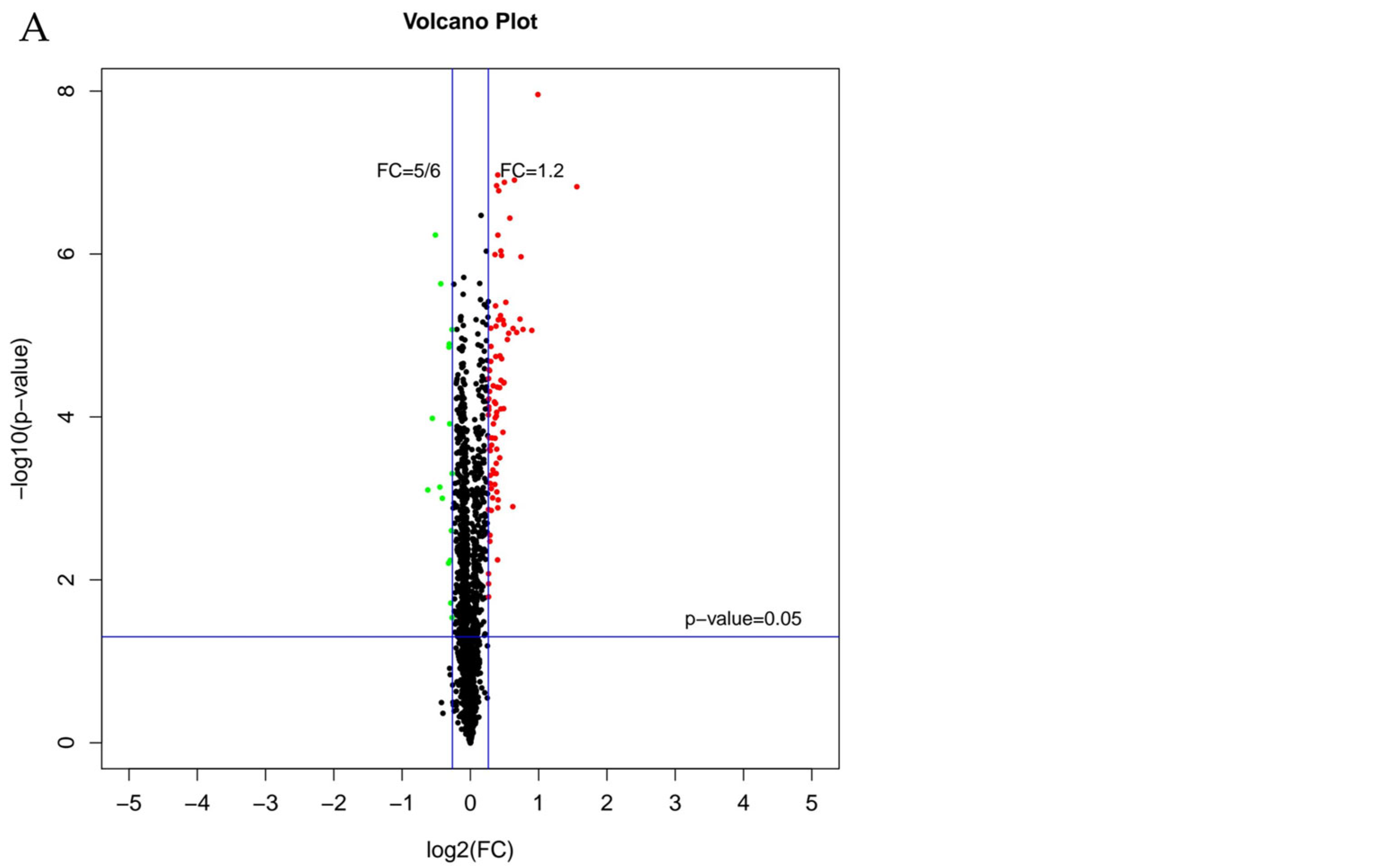
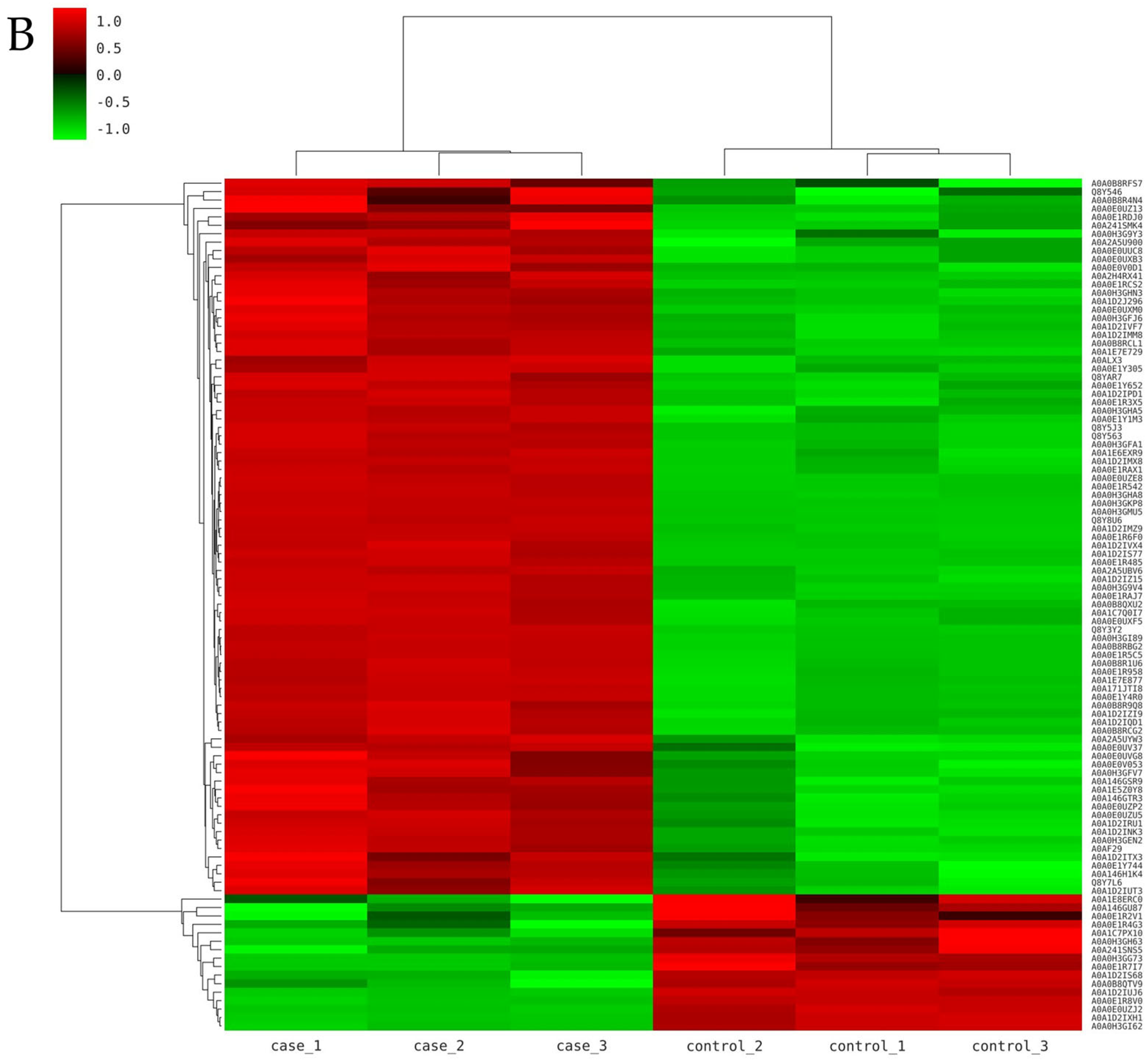
3.2. Identification of Differentially Expressed Proteins
3.3. Analysis of Differentially Expressed Proteins
3.3.1. GO Analysis of DEPs
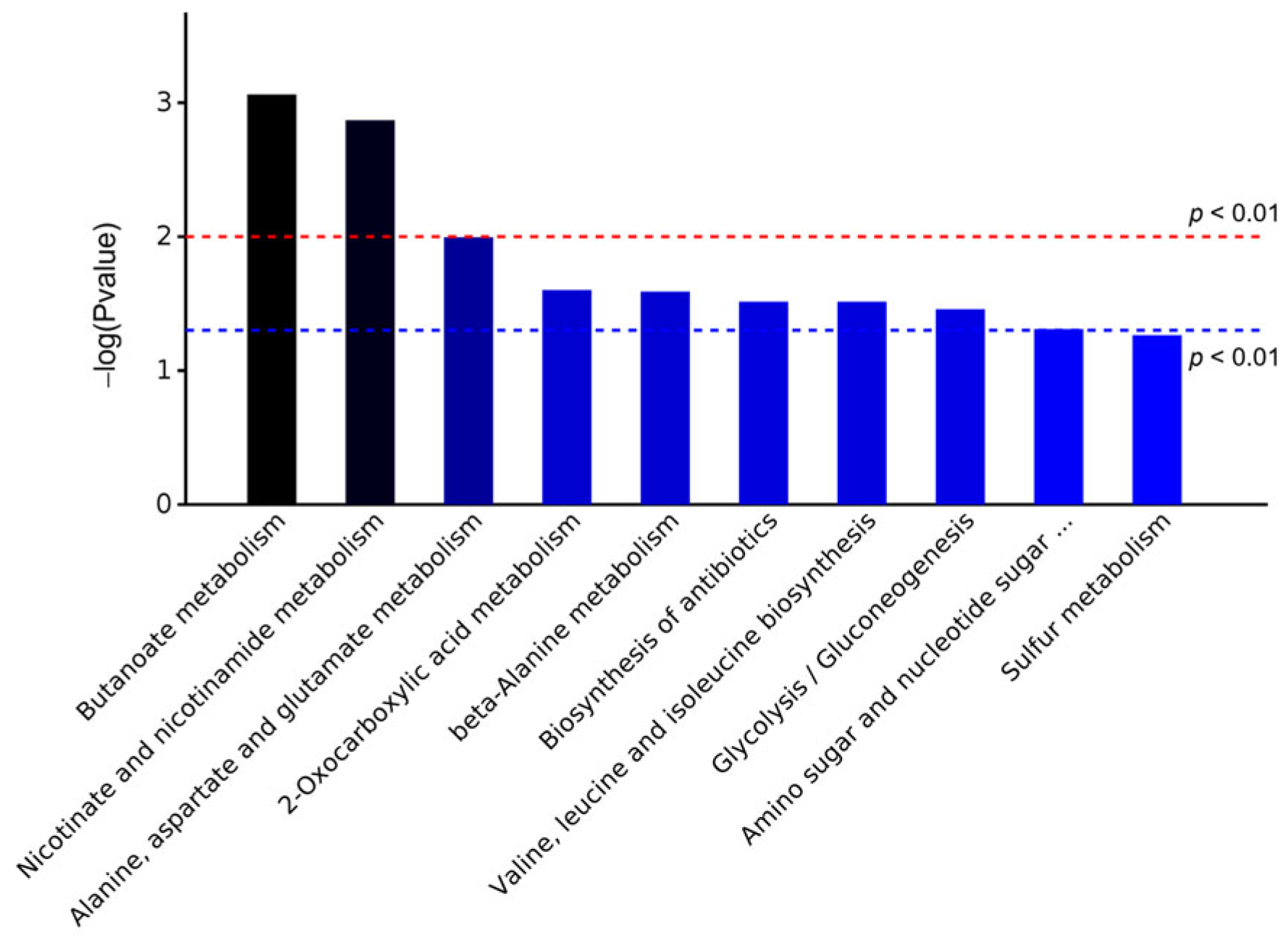
3.3.2. KEGG Analysis of DEPs
3.3.3. DEPs Involved in Transportation
3.3.4. DEPs Involved in Transcription and Translation
3.3.5. DEPs Involved in Carbohydrate Metabolism
3.3.6. DEPs Involved in Amino Acid Metabolism
3.3.7. DEPs Involved in Nicotinate and Nicotinamide Metabolism
3.3.8. DEPs Involved in Other Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Term |
| PMF | Pulsed magnetic field |
| L. monocytogenes | Listeria monocytogenes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| HPP | High-Pressure Processing |
| PEF | Pulsed electric field |
| TMT | Tandem Mass Tag |
| BHI | Brain heart infusion broth |
| CFU | Colony-forming unit |
| IAA | Iodoacetamide |
| TEAB | Triethylammonium bicarbonate |
| MS | Mass spectrometry |
| FA | Formic acid |
| AGC | Automatic gain control |
| HCD | Higher-energy collisional dissociation |
| FDR | False discovery rate |
| DEPs | Differentially expressed proteins |
| FC | Fold change |
| CCR | Carbon catabolite repression |
| HVST | High-voltage short-time ohmic |
| LVLT | Low-voltage long-time ohmic |
| ADHE | Aldehyde–alcohol dehydrogenases |
| T | Tesla |
| HHP | High hydrostatic pressure |
References
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Tech. 2018, 54, 1–13. [Google Scholar] [CrossRef]
- Sevenich, R.; Mathys, A. Continuous Versus Discontinuous Ultra-High-Pressure Systems for Food Sterilization with Focus on Ultra-High-Pressure Homogenization and High-Pressure Thermal Sterilization: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.-J.; Sun, J.; Pan, J.-N.; Zheng, X.; Zhong, J.-J.; Zhou, W.-W. Ultrasound-synergized gas in ensuring the sterilization and physicochemical quality of fruit and vegetables: A review. Postharvest Biol. Technol. 2024, 209, 112705. [Google Scholar] [CrossRef]
- Li, L.; Yang, R.; Zhao, W. The Effect of Pulsed Electric Fields (PEF) Combined with Temperature and Natural Preservatives on the Quality and Microbiological Shelf-Life of Cantaloupe Juice. Foods 2021, 10, 2606. [Google Scholar] [CrossRef]
- Qian, J.; Yan, G.; Huo, S.; Dai, C.; Ma, H.; Kan, J. Effects of pulsed magnetic field on microbial and enzymic inactivation and quality attributes of orange juice. J. Food Process. Preserv. 2021, 45, 1553. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; He, R.; Cui, H. Action mechanism of pulsed magnetic field against E. coli O157:H7 and its application in vegetable juice. Food Control 2019, 95, 150–156. [Google Scholar] [CrossRef]
- Wu, P.; Qu, W.; Abdualrahman, M.A.Y.; Guo, Y.; Xu, K.; Ma, H. Study on inactivation mechanisms of Listeria grayi affected by pulse magnetic field via morphological structure, Ca2+ transmembrane transport and proteomic analysis. Int. J. Food Sci. Technol. 2017, 52, 2049–2057. [Google Scholar] [CrossRef]
- Kp, R.; Renganayaki, P.R.; Sundareswaran, S.; Kumar, S.M.; Kamalakannan, A. Effect of pulsed magnetic field on seed borne pathogen and germination of tomato. Int. J. Chem. Stud. 2021, 9, 1141–1144. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, M.; Dai, C.; Huo, S.; Ma, H. Transcriptomic analysis of Listeria monocytogenes under pulsed magnetic field treatment. Food Res. Int. 2020, 133, 109195. [Google Scholar] [CrossRef]
- Li, M.M.H.; MacDonald, M.R.; Rice, C.M. To translate, or not to translate: Viral and host mRNA regulation by interferon-stimulated genes. Trends Cell Biol. 2015, 25, 320–329. [Google Scholar] [CrossRef]
- Jackson, K.A.; Gould, L.H.; Hunter, J.C.; Kucerova, Z.; Jackson, B. Listeriosis Outbreaks Associated with Soft Cheeses, United States, 1998–2014. Emerg. Infect. Dis. 2018, 24, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.N.; Aras, S.; George, J.; Wadood, S.; Chowdhury, S.; Fouladkhah, A.C. High-pressure and thermal-assisted pasteurization of habituated, wild-type, and pressure-stressed Listeria monocytogenes, Listeria innocua, and Staphylococcus aureus. LWT 2021, 137, 110445. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; Zeng, Q.-H.; Liu, Y.; Liu, H.; Zhao, Y.; Wang, J.J. Application of the curcumin-mediated photodynamic inactivation for preserving the storage quality of salmon contaminated with L. monocytogenes. Food Chem. 2021, 359, 129974. [Google Scholar] [CrossRef] [PubMed]
- Serra-Castelló, C.; Jofré, A.; Belletti, N.; Garriga, M.; Bover-Cid, S. Modelling the piezo-protection effect exerted by lactate on the high pressure resistance of Listeria monocytogenes in cooked ham. Food Res. Int. 2021, 140, 110003. [Google Scholar] [CrossRef]
- Nangul, A.; Bozkurt, H.; Gupta, S.; Woolf, A.; Phan-thien, K.-y.; McConchie, R.; Fletcher, G.C. Decline of Listeria monocytogenes on fresh apples during long-term, low-temperature simulated international sea-freight transport. Int. J. Food Microbiol. 2021, 341, 109069. [Google Scholar] [CrossRef]
- Kyere, E.O.; Foong, G.; Palmer, J.; Wargent, J.J.; Fletcher, G.C.; Flint, S. Rapid attachment of Listeria monocytogenes to hydroponic and soil grown lettuce leaves. Food Control 2019, 101, 77–80. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Ali, S.; Haruna, S.A.; He, P.; Li, H.; Ouyang, Q.; Chen, Q. Development of a fluorescence aptasensor for rapid and sensitive detection of Listeria monocytogenes in food. Food Control 2021, 122, 107808. [Google Scholar] [CrossRef]
- Evert-Arriagada, K.; Trujillo, A.J.; Amador-Espejo, G.G.; Hernández-Herrero, M.M. High pressure processing effect on different Listeria spp. in a commercial starter-free fresh cheese. Food Microbiol. 2018, 76, 481–486. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Shen, X.; Zhang, S.; Chen, X. Lethal and sublethal injury and kinetics of Escherichia coli, Listeria monocytogenes and Staphylococcus aureus in milk by pulsed electric fields. Food Control 2013, 32, 6–12. [Google Scholar] [CrossRef]
- Iorio, M.C.; Bevilacqua, A.; Corbo, M.R.; Campaniello, D.; Sinigaglia, M.; Altieri, C. A case study on the use of ultrasound for the inhibition of Escherichia coli O157:H7 and Listeria monocytogenes in almond milk. Ultrason. Sonochem. 2019, 52, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-Y.; Kuo, S.-H.; Chen, S.-T.; Hwang, D.-F. Differential proteomics to explore the inhibitory effects of acidic, slightly acidic electrolysed water and sodium hypochlorite solution on Vibrio parahaemolyticus. Food Chem. 2016, 194, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, Y.; An, H.; Hao, Y.; Hu, X.; Liao, X.; Doores, S. New Insights into the Formation of Viable but Nonculturable Escherichia coli O157:H7 Induced by High-Pressure CO2. mBio 2016, 7, e00961-16. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, C.; Xia, X.; Garba, B.; Shang, L.; Wang, Y. Proteomic analysis of the inhibitory effect of chitosan on Penicillium expansum. Food Sci. Technol. 2020, 40, 250–257. [Google Scholar] [CrossRef]
- Han, J.; Gao, P.; Zhao, S.; Bie, X.; Lu, Z.; Zhang, C.; Lv, F. iTRAQ-based proteomic analysis of LI-F type peptides produced by Paenibacillus polymyxa JSa-9 mode of action against Bacillus cereus. J. Proteom. 2017, 150, 130–140. [Google Scholar] [CrossRef]
- Ning, Y.; Fu, Y.; Hou, L.; Ma, M.; Wang, Z.; Li, X.; Jia, Y. iTRAQ-based quantitative proteomic analysis of synergistic antibacterial mechanism of phenyllactic acid and lactic acid against Bacillus cereus. Food Res. Int. 2021, 139, 109562. [Google Scholar] [CrossRef]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-M.; Xu, D.; Ren, H.; Geng, J.; Xu, K. Metagenomic insights into the “window” effect of static magnetic field on nitrous oxide emission from biological nitrogen removal process at low temperature. J. Environ. Manag. 2021, 298, 113377. [Google Scholar] [CrossRef]
- Su, D.B.; Zhao, Z.X.; Yin, D.C.; Ye, Y.J. Promising application of pulsed electromagnetic fields on tissue repair and regeneration. Prog. Biophys. Mol. Bio. 2024, 187, 36–50. [Google Scholar] [CrossRef]
- Guo, L.; Azam, S.M.R.; Guo, Y.; Liu, D.; Ma, H. Germicidal efficacy of the pulsed magnetic field against pathogens and spoilage microorganisms in food processing: An overview. Food Control 2022, 136, 108496. [Google Scholar] [CrossRef]
- Naskar, S.; Chandan; Baskaran, D.; Roy Choudhury, A.N.; Chatterjee, S.; Karunakaran, S.; Murthy, B.V.S.; Basu, B. Dosimetry of pulsed magnetic field towards attaining bacteriostatic effect on Enterococcus faecalis: Implications for endodontic therapy. Int. Endod. J. 2021, 54, 1878–1891. [Google Scholar] [CrossRef]
- Dao, T.T.M.; Truong, D.D.; Duong, L.N.H.; Nguyen, N.N.Y.; Nguyen, H.D. Preparation of Bacillus subtilis Cell Samples and Generation of an SDS-PAGE. BioTechniques 2023, 74, 123–129. [Google Scholar] [CrossRef]
- Bassey, A.P.; Zhang, Y.; Zhu, Y.; Cui, X.; Zhang, X.; Corradini, M.G.; Singh, M.; Liu, X.; Zhang, H. Tandem mass tag-based quantitative proteomics elucidates the inactivation mechanisms of high-power pulsed microwave treatment on Pseudomonas aeruginosa PAO1. Innov. Food Sci. Emerg. 2024, 91, 103532. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, J.; Wang, S.; Xu, F. Proteomic Analysis Reveals Differentially Expressed Proteins in Cordyceps militaris Cultured with Different Media. Curr. Microbiol. 2024, 82, 29. [Google Scholar] [CrossRef]
- Liu, X.; Suo, R.; Wang, H.; Wang, W.; Sun, J.; Wang, J. TMT proteomics establishes correlations between solar drying and quality modifications in Penaeus vannamei. Food Chem. 2024, 441, 138330. [Google Scholar] [CrossRef]
- Hudek, L.; Premachandra, D.; Webster, W.A.J.; Bräu, L.; Liu, S.J. Role of Phosphate Transport System Component PstB1 in Phosphate Internalization by Nostoc punctiforme. Appl. Environ. Microbiol. 2016, 82, 6344–6356. [Google Scholar] [CrossRef]
- Galinier, A.; Deutscher, J. Sophisticated Regulation of Transcriptional Factors by the Bacterial Phosphoenolpyruvate: Sugar Phosphotransferase System. J. Mol. Biol. 2017, 429, 773–789. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Shi, C.; Alharbi, M.; Cui, H.; Lin, L. Phosphoproteomics analysis reveals the anti-bacterial and anti-virulence mechanism of eugenol against Staphylococcus aureus and its application in meat products. Int. J. Food Microbiol. 2024, 414, 110621. [Google Scholar] [CrossRef] [PubMed]
- Deutscher, J. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2008, 11, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Kim, Y.; Kim, D.; Yoon, S.H. Machine learning identifies key metabolic reactions in bacterial growth on different carbon sources. Mol. Syst. Biol. 2024, 20, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, V.M.; Fonseca, G.G. Effects of the carbon source and the interaction between carbon sources on the physiology of the industrial Saccharomyces cerevisiae CAT-1. Prep. Biochem. Biotechnol. 2019, 50, 349–356. [Google Scholar] [CrossRef]
- Qian, J.Y.; Fall, A.N.; Zhang, M.; Huo, S.H.; Ma, H.L. Increase of intracellular Ca2+ concentration in Listeria monocytogenes under pulsed magnetic field. J. Magn. Magn. Mater. 2022, 553, 7. [Google Scholar] [CrossRef]
- Liu, J.; Huang, C.; Shin, D.-H.; Yokota, H.; Jancarik, J.; Kim, J.-S.; Adams, P.D.; Kim, R.; Kim, S.-H. Crystal Structure of a Heat-inducible Transcriptional Repressor HrcA from Thermotoga maritima: Structural Insight into DNA Binding and Dimerization. J. Mol. Biol. 2005, 350, 987–996. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, Y.; Lin, Z.; Wang, G.; Jiang, S.; Sun, X.; Tu, H.; Yu, Z.; Qu, D. ClpP participates in stress tolerance, biofilm formation, antimicrobial tolerance, and virulence of Enterococcus faecalis. BMC Microbiol. 2020, 20, 30. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Pang, X.; Wu, Y.; Wu, Y.; Shu, Q.; Chen, Q.; Zhang, X. Synergistic antibacterial effect and mechanism of high hydrostatic pressure and mannosylerythritol Lipid-A on Listeria monocytogenes. Food Control 2022, 135, 108797. [Google Scholar] [CrossRef]
- Tian, X.; Yu, Q.; Wu, W.; Li, X.; Dai, R. Comparative proteomic analysis of Escherichia coli O157:H7 following ohmic and water bath heating by capillary-HPLC-MS/MS. Int. J. Food Microbiol. 2018, 285, 42–49. [Google Scholar] [CrossRef]
- Roth, P.; Jeckelmann, J.-M.; Fender, I.; Ucurum, Z.; Lemmin, T.; Fotiadis, D. Structure and mechanism of a phosphotransferase system glucose transporter. Nat. Commun. 2024, 15, 1878–1891. [Google Scholar] [CrossRef]
- Wei, D.; Wang, M.; Jiang, B.; Shi, J.; Hao, J. Role of dihydroxyacetone kinases I and II in the dha regulon of Klebsiella pneumoniae. J. Biotechnol. 2014, 177, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, N.; Hirata, K.; Matsutani, M.; Ano, Y.; Nguyen, T.M.; Adachi, O.; Matsushita, K.; Yakushi, T. Three ATP-dependent phosphorylating enzymes in the first committed step of dihydroxyacetone metabolism in Gluconobacter thailandicus NBRC3255. Appl. Microbiol. Biotechnol. 2021, 105, 1227–1236. [Google Scholar] [CrossRef]
- Ethayathulla, A.S.; Yousef, M.S.; Amin, A.; Leblanc, G.; Kaback, H.R.; Guan, L. Structure-based mechanism for Na+/melibiose symport by MelB. Nat. Commun. 2014, 5, 7992. [Google Scholar] [CrossRef]
- Fraser, H.I.; Kvaratskhelia, M.; White, M.F. The two analogous phosphoglycerate mutases of Escherichia coli. FEBS Lett. 1999, 455, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Pohl, S.; Tu, W.Y.; Aldridge, P.D.; Gillespie, C.; Hahne, H.; Mäder, U.; Read, T.D.; Harwood, C.R. Combined proteomic and transcriptomic analysis of the response of Bacillus anthracis to oxidative stress. Proteomics 2011, 11, 3036–3055. [Google Scholar] [CrossRef]
- Cui, Y.; Qu, X. Genetic mechanisms of prebiotic carbohydrate metabolism in Lactic acid bacteria: Emphasis on Lacticaseibacillus casei and Lacticaseibacillus paracasei as flexible, diverse and outstanding prebiotic carbohydrate starters. Trends Food Sci. Technol. 2021, 115, 486–499. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Hao, L.; Cao, J.; Jiang, L.; Yi, H. Metabolomic Approaches to Study the Potential Inhibitory Effects of Plantaricin Q7 against Listeria monocytogenes Biofilm. Foods 2024, 13, 2573. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Wu, N.; Yu, Y.; Li, T.; Ji, X.; Jiang, L.; Zong, J.; Huang, H. Investigating the Influence of MoS2 Nanosheets on E. coli from Metabolomics Level. PLoS ONE 2016, 11, e0167245. [Google Scholar] [CrossRef]
- Yarabbi, H.; Mortazavi, S.A.; Yavarmanesh, M.; Javadmanesh, A. Molecular cloning, gene overexpression and characterization of glutamate decarboxylase from Enterococcus faecium DO. LWT 2021, 148, 111699. [Google Scholar] [CrossRef]
- Azcarate-Peril, M.A.; Altermann, E.; Hoover-Fitzula, R.L.; Cano, R.J.; Klaenhammer, T.R. Identification and Inactivation of Genetic Loci Involved with Lactobacillus acidophilus Acid Tolerance. Appl. Environ. Microbiol. 2004, 70, 5315–5322. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; O’Byrne, C. Metabolic reprogramming in the food-borne pathogen Listeria monocytogenes as a critical defence against acid stress. FEMS Microbiol. Lett. 2024, 371, fnae060. [Google Scholar] [CrossRef]
- Boura, M.; Brensone, D.; Karatzas, K.A.G. A novel role for the glutamate decarboxylase system in Listeria monocytogenes; protection against oxidative stress. Food Microbiol. 2020, 85, 103284. [Google Scholar] [CrossRef]
- Singh, N.; Chauhan, A.; Kumar, R.; Singh, S.K. Biochemical and functional characterization of Mycobacterium tuberculosis ketol-acid reductoisomerase. Microbiology 2021, 167, 1087. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chauhan, A.; Kumar, R.; Singh, S.K. Mycobacterium tuberculosis ketol-acid reductoisomerase down-regulation affects its ability to persist, and its survival in macrophages and in mice. Microb. Infect. 2022, 24, 105000. [Google Scholar] [CrossRef]
- Jaworski, A.F.; Aitken, S.M. Exploration of the six tryptophan residues of Escherichia coli cystathionine β-lyase as probes of enzyme conformational change. Arch. Biochem. Biophys. 2013, 538, 138–144. [Google Scholar] [CrossRef]
- Ji, L.; Chen, C.; Zhu, J.; Hong, X.; Liu, X.; Wei, C.; Zhu, X.; Li, W. Integrated time-series biochemical, transcriptomic, and metabolomic analyses reveal key metabolites and signaling pathways in the liver of the Chinese soft-shelled turtle (Pelodiscus sinensis) against Aeromonas hydrophila infection. Front. Immunol. 2024, 15, 1376860. [Google Scholar] [CrossRef] [PubMed]
- Shats, I.; Williams, J.G.; Liu, J.; Makarov, M.V.; Wu, X.; Lih, F.B.; Deterding, L.J.; Lim, C.; Xu, X.; Randall, T.A.; et al. Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab. 2020, 31, 564–579. [Google Scholar] [CrossRef]
- Martin, J.P.; Rasor, B.J.; DeBonis, J.; Karim, A.S.; Jewett, M.C.; Tyo, K.E.J.; Broadbelt, L.J. A dynamic kinetic model captures cell-free metabolism for improved butanol production. Metab. Eng. 2023, 76, 133–145. [Google Scholar] [CrossRef]
- van Lis, R.; Popek, M.; Couté, Y.; Kosta, A.; Drapier, D.; Nitschke, W.; Atteia, A. Concerted Up-regulation of Aldehyde/Alcohol Dehydrogenase (ADHE) and Starch in Chlamydomonas reinhardtii Increases Survival under Dark Anoxia. J. Biol. Chem. 2017, 292, 2395–2410. [Google Scholar] [CrossRef]
- Uroz, M.; Wistorf, S.; Serra-Picamal, X.; Conte, V.; Sales-Pardo, M.; Roca-Cusachs, P.; Guimerà, R.; Trepat, X. Regulation of cell cycle progression by cell–cell and cell–matrix forces. Nat. Cell Biol. 2018, 20, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Pis Diez, C.M.; Juncos, M.J.; Villarruel Dujovne, M.; Capdevila, D.A. Bacterial Transcriptional Regulators: A Road Map for Functional, Structural, and Biophysical Characterization. Int. J. Mol. Sci. 2022, 23, 2179. [Google Scholar] [CrossRef]

| Spot No. | Gene/ORF Name | Description | Accession No. | pI/MW (kDa) | Scores | Seq Cov (%) | Fold Change |
|---|---|---|---|---|---|---|---|
| 1 | ylbF * | Regulatory protein ylbF | A0A0E1R7I7 | 5.68/18.7 | 5.81 | 6 | −1.54 |
| 2 | LMRG_00836 | UPF0176 protein LMRG_00836 | A0A0H3GG73 | 4.98/36.3 | 219.75 | 59 | −1.47 |
| 3 | rpmI | 50S ribosomal protein L35 | A0A0E1R8V0 | 12.56/7.7 | 116.31 | 35 | −1.43 |
| 4 | hemA * | Glutamyl-tRNA reductase | A0A0H3GH63 | 5.33/49.2 | 21.34 | 5 | −1.37 |
| 5 | CDR86_15435 | tRNA-dihydrouridine synthase | A0A1D2IUJ6 | 6.44/36.8 | 326.28 | 64 | −1.35 |
| 6 | A410_1127 * | UPF0358 protein A410_1127 | A0A241SNS5 | 8.63/12.6 | 2.5 | 10 | −1.33 |
| 7 | SAMD00023519_01241 | Dipicolinate synthase | A0A146GU87 | 8.66/20.8 | 17.66 | 13 | −1.25 |
| 8 | lysP * | Lysine-specific permease | A0A0E1R4G3 | 9.41/53.1 | 4.52 | 2 | −1.23 |
| 9 | CDR86_13105 | Carbonic anhydrase | A0A1D2IXH1 | 4.97/27.2 | 405.4 | 49 | −1.23 |
| 10 | BB718_05970 | DNA-binding response regulator | A0A1D2IS68 | 6.13/28.5 | 61.22 | 22 | −1.23 |
| 11 | secE * | Protein translocase subunit SecE | A0A0E1R2V1 | 9.54/6.9 | 2.24 | 14 | −1.20 |
| 12 | LmNIHS28_00558 * | Sodium-dependent phosphate transporter | A0A0B8QTV9 | 5.45/59.7 | 5.77 | 1 | −1.20 |
| 13 | rpsN | 30S ribosomal protein S14 type Z | A0A0E0UZJ2 | 10.49/7.1 | 21.41 | 34 | −1.20 |
| 14 | CDR86_09705 | Rhodanese-like domain-containing protein | A0A1C7PX10 | 4.84/10.8 | 52.88 | 58 | −1.20 |
| 15 | LMRG_01479 | Glutamate decarboxylase | A0A0H3GMU5 | 5.22/53.5 | 305.63 | 38 | 2.95 |
| 16 | LMRG_01332 | Aldehyde–alcohol dehydrogenase | A0A0H3GKP8 | 6.93/94.6 | 2625.08 | 57 | 1.99 |
| 17 | LMRG_01480 * | Glutamate/gamma-aminobutyrate antiporter | A0A0H3GFA1 | 9.09/55.1 | 21.75 | 4 | 1.87 |
| 18 | lmo2067 | Lmo2067 protein | Q8Y5J3 | 5.15/36.8 | 38.58 | 19 | 1.71 |
| 19 | inlB * | Internalin B | A0A0E1R485 | 9.41/71.2 | 20.55 | 5 | 1.67 |
| 20 | CDR86_05685 | GNAT family N-acetyltransferase | A0A1D2IS77 | 4.94/10.2 | 59.52 | 23 | 1.65 |
| 21 | LMRG_00327 | Cadmium-translocating P-type ATPase | A0A0H3G9V4 | 5.72/67.6 | 238.61 | 23 | 1.60 |
| 22 | spsB | Signal peptidase I | A0A0E1R6F0 | 5.19/21.1 | 50.79 | 28 | 1.56 |
| 23 | LmNIHS28_02228 | Fumarate reductase | A0A0B8RBG2 | 5.94/54.5 | 754.17 | 57 | 1.49 |
| 24 | dhaK_2 | PTS-dependent dihydroxyacetone kinase, dihydroxyacetone-binding subunit dhaK | A0A0E1R958 | 4.93/34.9 | 123.73 | 19 | 1.48 |
| 25 | gadG | Glutamate decarboxylase | A0A0E1RAJ7 | 5.22/54.8 | 76.2 | 15 | 1.43 |
| 26 | lmo0796 | Lmo0796 protein | Q8Y8U6 | 4.84/19.3 | 404.6 | 88 | 1.41 |
| 27 | LMM7_2086 * | Fructosamine deglycase | A0A0E0UXM0 | 5.54/38 | 21.25 | 9 | 1.41 |
| 28 | NT04LM_1576 | Lipoprotein, putative (Fragment) | A0A0E1Y652 | 4.77/8.4 | 23.34 | 19 | 1.40 |
| 29 | CDR86_01030 | PTS mannose/fructose/sorbose transporter subunit IIB | A0A1C7Q0I7 | 6.34/17.2 | 21.69 | 17 | 1.40 |
| 30 | CXL08_12865 | NAD-dependent dehydratase | A0A1D2IMX8 | 6.38/22.7 | 76.14 | 35 | 1.39 |
| 31 | LMM7_2092 * | Putative transcription regulator, GntR family | A0A0E0UXB3 | 5.45/28.0 | 2.09 | 3 | 1.39 |
| 32 | dhaL | Dihydroxyacetone kinase, C-terminal domain protein | A0A0E0UZE8 | 5.33/21.5 | 228.46 | 52 | 1.37 |
| 33 | CDR86_02755 | Carbohydrate kinase | A0A1D2IMM8 | 4.96/40.6 | 35.57 | 10 | 1.37 |
| 34 | lwe2587 | lipoprotein | A0ALX3 | 5.91/32.9 | 1369.32 | 53 | 1.36 |
| 35 | gpmA | 2,3-Bisphosphoglycerate-dependent phosphoglycerate mutase | A0A0H3GI89 | 5.69/26.4 | 285.71 | 39 | 1.36 |
| 36 | manZ_3 * | Mannose permease IID component | A0A0E1RAX1 | 8.34/31.8 | 35.5 | 7 | 1.36 |
| 37 | CDR86_08010 | Anaerobic ribonucleoside-triphosphate reductase | A0A1D2IZ15 | 5.94/82.1 | 131.03 | 24 | 1.35 |
| 38 | BN389_17220 | Epimerase family protein SE_0553 | A0A0E1RDJ0 | 7.68/34.6 | 20.27 | 17 | 1.35 |
| 39 | NT04LM_1565 | DNA protection during starvation protein 2 | A0A0E1Y4R0 | 4.93/18.0 | 915.53 | 79 | 1.33 |
| 40 | LMRG_00977 | Short chain dehydrogenase | A0A0H3GHA8 | 6.19/20.9 | 91.47 | 38 | 1.33 |
| 41 | LMRG_00977 | Lmo1261 protein | Q8Y7L6 | 9.41/42.3 | 122.24 | 18 | 1.33 |
| 42 | manX_2 | PTS system mannose-specific EIIAB component | A0A0E1R542 | 9.33/19.7 | 262.23 | 46 | 1.32 |
| 43 | lmo2697 | Lmo2697 protein | Q8Y3Y2 | 4.7/13.4 | 115.1 | 54 | 1.32 |
| 44 | Fief * | Putative Zn/Cd/Fe cation exporter | A0A0E0UZ13 | 5.78/31.8 | 10.55 | 6 | 1.32 |
| 45 | CLN77_13670 | FMN-binding protein | A0A2H4RX41 | 6.65/32.7 | 1370.82 | 50 | 1.31 |
| 46 | psuG | Pseudouridine-5’-phosphate glycosidase | A0A1D2IMZ9 | 4.84/32.5 | 385.23 | 61 | 1.31 |
| 47 | SAMD00023519_01958 * | Putative activator of (R)-hydroxyglutaryl-CoA | A0A146GTR3 | 6.34/164.1 | 61 | 7 | 1.31 |
| 48 | A4P56_12940 * | Cell surface protein | A0A2A5UYW3 | 5.15/34.6 | 14.81 | 16 | 1.31 |
| 49 | AF973_12935 * | Amino acid ABC transporter permease | A0A1E6EXR9 | 9.66/23.9 | 9.96 | 6 | 1.31 |
| 50 | CDR86_13550 | Pyruvate oxidase | A0A1D2INK3 | 5.02/62.8 | 266.31 | 35 | 1.30 |
| 51 | lmo2213 | Lmo2213 protein | Q8Y563 | 7.21/19.5 | 101.16 | 35 | 1.30 |
| 52 | SAMD00023519_01961 * | PTS mannose transporter subunit IIC | A0A146GSR9 | 5.54/32.2 | 24.98 | 4 | 1.30 |
| 53 | AJL15_04430 | FMN-binding protein | A0A1E7E877 | 6.65/32.7 | 1328.39 | 50 | 1.29 |
| 54 | pdxH | Putative general stress protein 26 putative pyridoxamine/pyridoxine 5’-phosphate oxidase | A0A0E0UZU5 | 4.68/15.7 | 59.03 | 35 | 1.29 |
| 55 | lacD | Tagatose 1,6-diphosphate aldolase | A0A0E1R5C5 | 5.1/37.9 | 599.72 | 68 | 1.28 |
| 56 | hrcA | Heat-inducible transcription repressor HrcA | A0A0E1Y305 | 5.58/40.4 | 67.28 | 18 | 1.28 |
| 57 | AJZ74_10515 * | Serine/threonine protein phosphatase | A0A1E5Z0Y8 | 5.08/26.7 | 16 | 14 | 1.28 |
| 58 | SAMD00023520_02065 * | Cyclic nucleotide-binding protein | A0A146H1K4 | 9.22/32.8 | 3.94 | 6 | 1.27 |
| 59 | deoC | Deoxyribose-phosphate aldolase | A0A1D2IZI9 | 5.39/23.5 | 454.21 | 70 | 1.26 |
| 60 | mgtA * | Magnesium-translocating P-type ATPase | A0A1D2IRU1 | 7.99/94.8 | 52.56 | 9 | 1.26 |
| 61 | ilvC | Ketol-acid reductoisomerase (NADP(+)) | A0A0H3GHN3 | 5.36/36.4 | 33.9 | 16 | 1.26 |
| 62 | LMRG_02097 | ATP-dependent Clp protease ATP-binding subunit ClpE | A0A0H3GFJ6 | 5.21/80.2 | 226.18 | 31 | 1.25 |
| 63 | LMM7_0960 | Putative efflux ABC transporter, ATP-binding protein (N-terminal part) | A0A0E0UVG8 | 6.11/25.9 | 41.68 | 30 | 1.25 |
| 64 | LMM7_0961 * | Putative efflux ABC transporter, ATP binding and permease protein | A0A0E0UUC8 | 7.18/41.3 | 15.71 | 5 | 1.24 |
| 65 | pncA * | Putative nicotinamidase | A0A0E0V053 | 4.77/23.5 | 5.27 | 3 | 1.24 |
| 66 | CDR86_04330 | Carnitine transport ATP-binding protein OpuCA | A0A1D2IQD1 | 5.16/45.2 | 289.6 | 52 | 1.23 |
| 67 | gabD | NAD-dependent succinate-semialdehyde dehydrogenase | A0A2A5UBV6 | 6.09/53.1 | 335.88 | 38 | 1.23 |
| 68 | metC * | Cystathionine beta-lyase | A0A0E0UXF5 | 5.41/41.7 | 4.85 | 2 | 1.23 |
| 69 | mntA | Manganese-binding lipoprotein MntA | A0A2A5U900 | 5.52/34.4 | 717.31 | 46 | 1.22 |
| 70 | ctsR | Transcriptional regulator CtsR | A0AF29 | 6.23/17.5 | 90.67 | 37 | 1.22 |
| 71 | LmNIHS28_01175 | UPF0473 protein LmNIHS28_01175 | A0A0B8QXU2 | 3.89/12.1 | 59.49 | 29 | 1.22 |
| 72 | phoU * | Phosphate-specific transport system accessory protein PhoU | A0A1D2ITX3 | 5.05/25.0 | 7.64 | 13 | 1.22 |
| 73 | lmo0047 | Lmo0047 protein | Q8YAR7 | 4.56/22.7 | 126.33 | 35 | 1.21 |
| 74 | BB664_03185 | Lipase | A0A1D2J296 | 4.61/40.4 | 40.15 | 14 | 1.21 |
| 75 | CDR86_03830 | DUF1049 domain-containing protein | A0A1D2IVF7 | 9.33/12.7 | 29.94 | 14 | 1.21 |
| 76 | uspF * | Putative universal stress protein UspA and related nucleotide-binding protein | A0A0E0V0D1 | 8.88/17.2 | 11.51 | 8 | 1.21 |
| 77 | opuCA | Glycine betaine/carnitine/choline transport ATP-binding protein OpuCA | A0A0E1RCS2 | 5.29/36.7 | 66.89 | 34 | 1.20 |
| 78 | lmo2230 * | Lmo2230 protein | Q8Y546 | 4.87/15.9 | 7.32 | 19 | 1.20 |
| 79 | Ung * | Uracil-DNA glycosylase | A0A0B8RFS7 | 7.91/26.4 | 4.41 | 4 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Qian, J.; Huo, S.; Wang, F.; Ma, H.; Liu, S. Proteomic Analysis of Listeria monocytogenes Subjected to Pulsed Magnetic Field. Foods 2025, 14, 1871. https://doi.org/10.3390/foods14111871
Chen D, Qian J, Huo S, Wang F, Ma H, Liu S. Proteomic Analysis of Listeria monocytogenes Subjected to Pulsed Magnetic Field. Foods. 2025; 14(11):1871. https://doi.org/10.3390/foods14111871
Chicago/Turabian StyleChen, Di, Jingya Qian, Shuhao Huo, Feng Wang, Haile Ma, and Shan Liu. 2025. "Proteomic Analysis of Listeria monocytogenes Subjected to Pulsed Magnetic Field" Foods 14, no. 11: 1871. https://doi.org/10.3390/foods14111871
APA StyleChen, D., Qian, J., Huo, S., Wang, F., Ma, H., & Liu, S. (2025). Proteomic Analysis of Listeria monocytogenes Subjected to Pulsed Magnetic Field. Foods, 14(11), 1871. https://doi.org/10.3390/foods14111871








