Carotenoid Yeasts and Their Application Potential
Abstract
:1. Introduction
2. Characteristics of Carotenoids
3. Carotenogenic Microorganisms
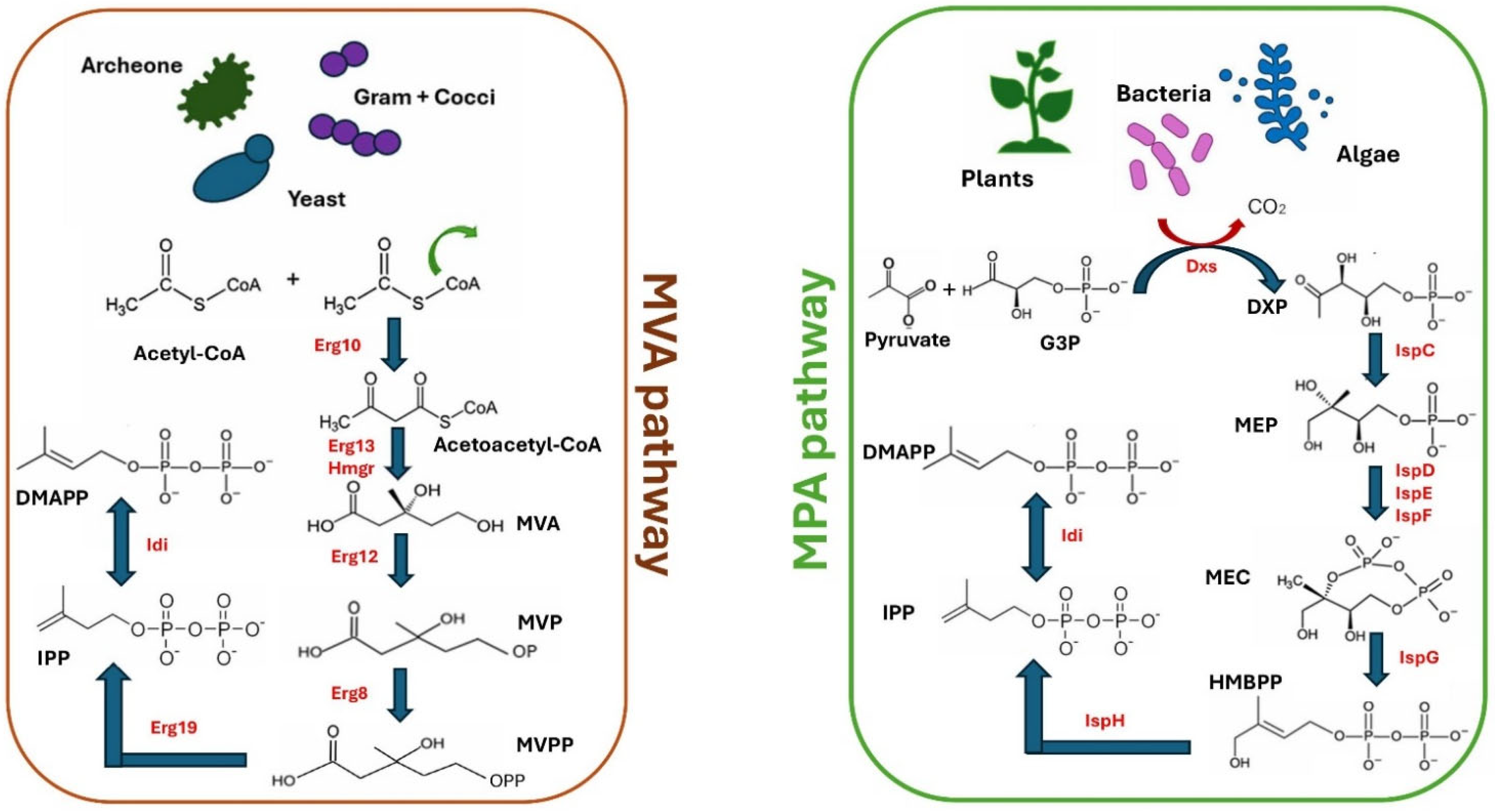
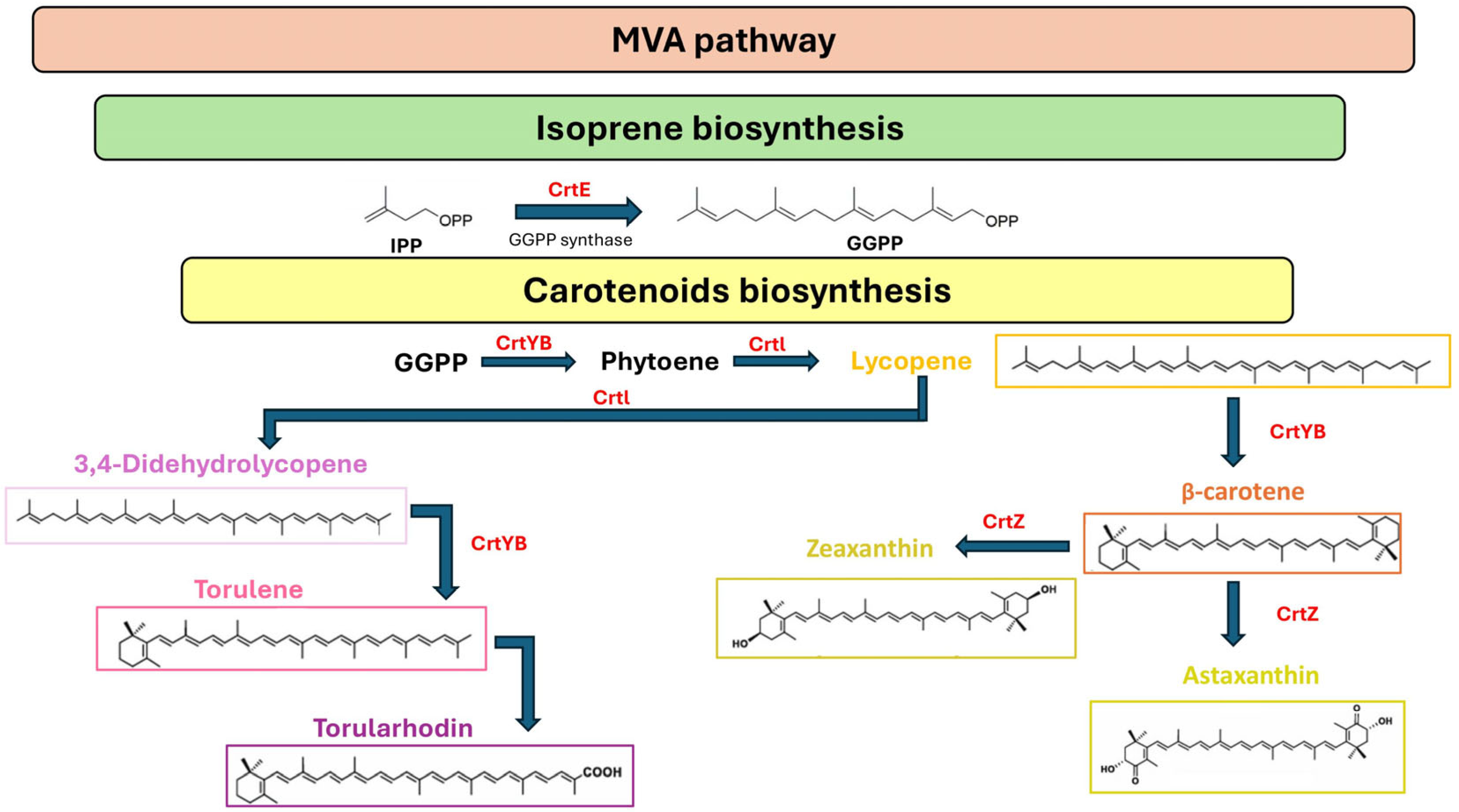
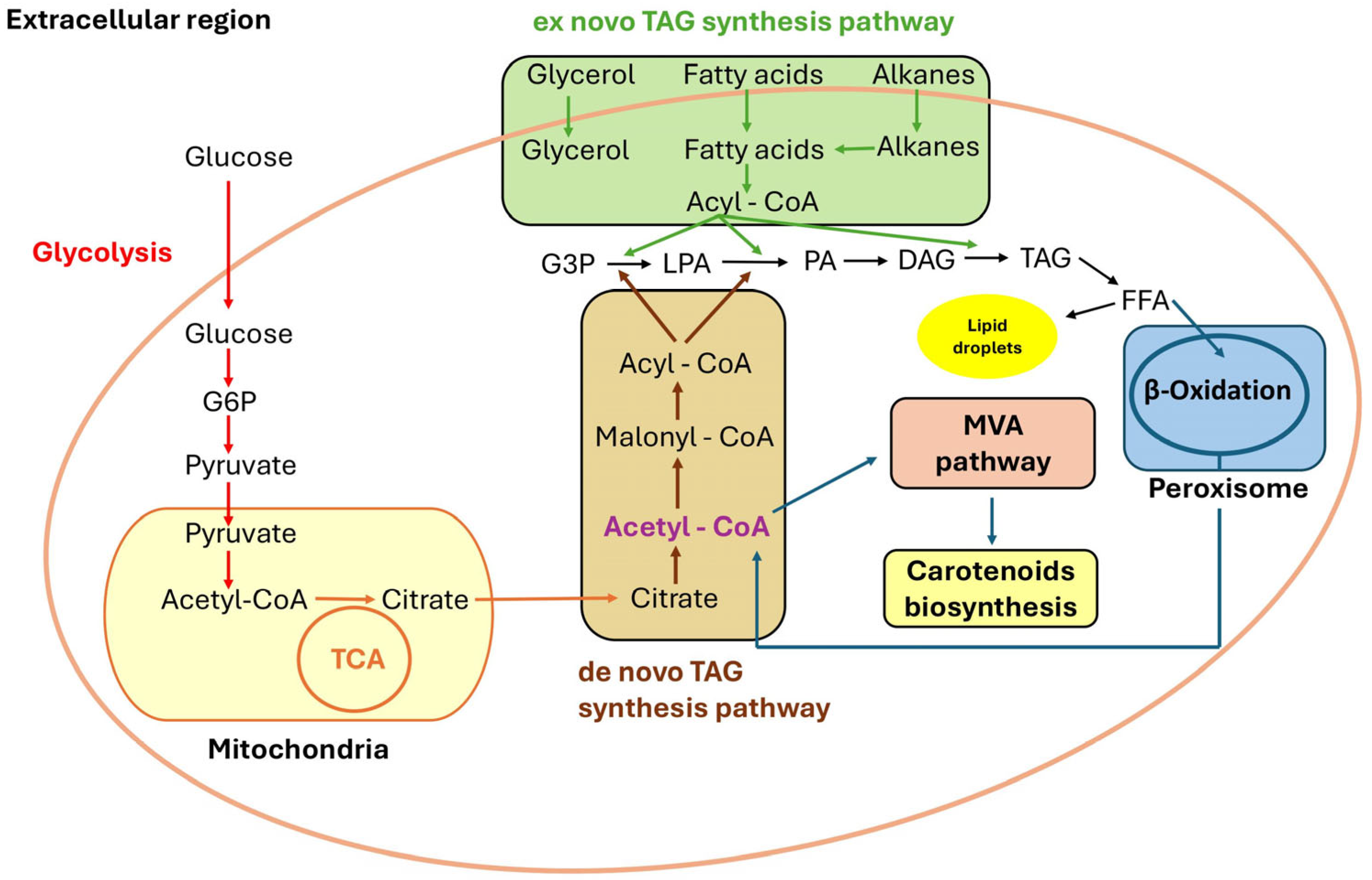
Synthesis of Carotenoid Yeast Metabolites
4. The Application of Carotenoid Yeast
4.1. Colouring Properties
4.2. Antioxidant Properties
4.3. Antimicrobial Properties
4.4. Antimycotic Properties
4.5. Dietary Properties
4.6. Immunostimulatory Properties
4.7. Supporting the Vision Process
5. Carotenoid Bioavailability as a Key Element in the Application of Their Bioactive Properties
6. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogacz-Radomska, L.; Harasym, J.; Piwowar, A. Commercialization aspects of carotenoids. In Carotenoids: Properties, Processing and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 327–357. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- European, C. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union 2008, 354, 16–33. [Google Scholar]
- Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council Text with EEA relevance. Off. J. Eur. Union 2012, 55, L 83.
- Kiokias, S.; Proestos, C.; Varzakas, T. A review of the structure, biosynthesis, absorption of carotenoids-analysis and properties of their common natural extracts. Curr. Res. Nutr. Food Sci. J. 2016, 4, 25–37. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Namitha, K.K.; Negi, P.S. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the potential beneficial effects of carotenoids on consumer health and well-being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Li, C.; Swofford, C.A.; Sinskey, A.J. Modular engineering for microbial production of carotenoids. Metab. Eng. Commun. 2020, 10, e00118. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Paredes-Lopez, O. Natural Colorants for Food and Nutraceutical Uses; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Kirti, K.; Amita, S.; Priti, S.; Mukesh Kumar, A.; Jyoti, S. Colorful world of microbes: Carotenoids and their applications. Adv. Biol. 2014, 2014, 837891. [Google Scholar] [CrossRef]
- Naz, T.; Ullah, S.; Nazir, Y.; Li, S.; Iqbal, B.; Liu, Q.; Mohamed, H.; Song, Y. Industrially important fungal carotenoids: Advancements in biotechnological production and extraction. J. Fungi 2023, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Toti, E.; Chen, C.Y.O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-provitamin A and provitamin A carotenoids as immunomodulators: Recommended dietary allowance, therapeutic index, or personalized nutrition? Oxidative Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef]
- Paul, D.; Kumari, P.K.; Siddiqui, N. Yeast carotenoids: Cost-effective fermentation strategies for health care applications. Fermentation 2023, 9, 147. [Google Scholar] [CrossRef]
- da Costa Cardoso, L.A.; Kanno, K.Y.F.; Karp, S.G. Microbial production of carotenoids A review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Ranga, R.; Sarada, A.R.; Baskaran, V.; Ravishankar, G.A. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J. Microbiol. Biotechnol. 2009, 19, 1333–1341. [Google Scholar]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef]
- Kleinegris, D.M.M.; Janssen, M.; Brandenburg, W.A.; Wijffels, R.H. Continuous production of carotenoids from Dunaliella salina. Enzym. Microb. Technol. 2011, 48, 253–259. [Google Scholar] [CrossRef]
- Jin, E.S.; Melis, A. Microalgal biotechnology: Carotenoid production by the green algae Dunaliella salina. Biotechnol. Bioprocess Eng. 2003, 8, 331–337. [Google Scholar] [CrossRef]
- Hegazy, A.A.; Abu-Hussien, S.H.; Elsenosy, N.K.; El-Sayed, S.M.; Abo El-Naga, M.Y. Optimization, characterization and biosafety of carotenoids produced from whey using Micrococcus luteus. BMC Biotechnol. 2024, 24, 74. [Google Scholar] [CrossRef]
- Shahin, Y.H.; Elwakil, B.H.; Ghareeb, D.A.; Olama, Z.A. Micrococcus lylae MW407006 pigment: Production, optimization, nano-pigment synthesis, and biological activities. Biology 2022, 11, 1171. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Surekha, P.; Dhanya, P. Micrococcus luteus strain BAA2, a novel isolate produces carotenoid pigment. Electron. J Biol 2016, 12, 83–89. [Google Scholar]
- Sun, X.-Y.; Dong, H.; Zhang, Y.; Gao, J.-W.; Zhou, P.; Sun, C.; Xu, L. Isolation and cultivation of carotenoid-producing strains from tidal flat sediment and proposal of Croceibacterium aestuarii sp. nov., a novel carotenoid-producing species in the family Erythrobacteraceae. J. Mar. Sci. Eng. 2024, 12, 99. [Google Scholar] [CrossRef]
- Elamin, O.; Marwa, C.; Koraichi, S.I.; Iraqui, H.M. Lycopene production in Mycobacterium smegmatis by expression of crt genes from Mycobacterium aurum and protective effect of lycopene in vivo and in vitro against UV radiation. Int. J. Pharm. Pharm. Sci 2018, 10, 49–53. [Google Scholar] [CrossRef]
- Kerr, S.; Calé, C.; Cabral, J.M.S.; Van Keulen, F. Factors enhancing lycopene production by a new Mycobacterium aurum mutant. Biotechnol. Lett. 2004, 26, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Innocenti, M.; Turchetti, B.; Libkind, D.; van Broock, M.; Mulinacci, N. Carotenoid profiles of yeasts belonging to the genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Can. J. Microbiol. 2007, 53, 1024–1031. [Google Scholar] [CrossRef]
- Han, M.; He, Q.; Zhang, W.-G. Carotenoids production in different culture conditions by Sporidiobolus pararoseus. Prep. Biochem. Biotechnol. 2012, 42, 293–303. [Google Scholar] [CrossRef]
- Wei, C.; Wu, T.; Ao, H.; Qian, X.; Wang, Z.; Sun, J. Increased torulene production by the red yeast, Sporidiobolus pararoseus, using citrus juice. Prep. Biochem. Biotechnol. 2020, 50, 66–73. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Silva, P.G.P.; Amantino, C.F.; Burkert, J.F.M.; Primo, F.L.; Pessoa Jr, A.; Santos-Ebinuma, V.C. Production of natural astaxanthin by Phaffia rhodozyma and its potential application in textile dyeing. Biochem. Eng. J. 2022, 187, 108658. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, J.I.; Lee, N.K.; Hahm, Y.T.; Baik, M.Y.; Kim, B.Y. Extraction of β-carotene produced from yeast Rhodosporidium sp. and its heat stability. Food Sci. Biotechnol. 2010, 19, 263–266. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Montanez, J.C.; Aguilar-Gonzalez, M.A.; Martínez-Ávila, C.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Biosci. 2014, 5, 64–72. [Google Scholar] [CrossRef]
- Kulczyk-Małysa, E.; Bogusławska-Wąs, E. Optymalizacja warunków biosyntezy wybranych karotenoidów przez drożdże z rodzaju Rhodotorula. ŻYWNOŚĆ. Nauka. Technologia. Jakość. 2024, 31, 45–62. [Google Scholar] [CrossRef]
- Durakli Velioglu, S.; Tirpanci Sivri, G. Optimizing β-carotene production by Blakeslea trispora using bug damaged wheat. Pigment Resin Technol. 2018, 47, 189–195. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Tairis, N.; Polycratis, A. Production of β-carotene by Sclerotinia sclerotiorum and its role in sclerotium differentiation. Mycol. Res. 2001, 105, 1110–1115. [Google Scholar] [CrossRef]
- Gmoser, R.; Ferreira, J.A.; Lundin, M.; Taherzadeh, M.J.; Lennartsson, P.R. Pigment production by the edible filamentous fungus Neurospora intermedia. Fermentation 2018, 4, 11. [Google Scholar] [CrossRef]
- Harding, R.W.; Philip, D.Q.; Drozdowicz, B.Z.; Williams, N.P. A Neurospora crassa mutant which over accumulates carotenoid pigments. Fungal Genet. Rep. 1984, 31, 23. [Google Scholar] [CrossRef]
- Parra-Rivero, O.; Paes de Barros, M.; Prado, M.d.M.; Gil, J.-V.; Hornero-Méndez, D.; Zacarías, L.; Rodrigo, M.J.; Limón, M.C.; Avalos, J. Neurosporaxanthin overproduction by Fusarium fujikuroi and evaluation of its antioxidant properties. Antioxidants 2020, 9, 528. [Google Scholar] [CrossRef]
- Naz, T.; Saeed, T.; Ullah, S.; Nazir, Y.; Assefa, M.; Liu, Q.; Fan, Z.; Mohamed, H.; Song, Y. Metabolic engineering of Mucor circinelloides to improve astaxanthin production. World J. Microbiol. Biotechnol. 2024, 40, 1–16. [Google Scholar] [CrossRef]
- Kanzy, H.M.; Nasr, N.F.; El-Shazly, H.A.M.; Barakat, O.S. Optimization of carotenoids production by yeast strains of Rhodotorula using salted cheese whey. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 456–469. [Google Scholar]
- Marova, I.; Haronikova, A.; Petrik, S.; Dvorakova, T.; Breierova, E. Production of enriched biomass by red yeasts of Sporobolomyces sp. grown on waste substrates. J. Microbiol. Biotechnol. Food Sci. 2012, 1, 534–551. [Google Scholar]
- Han, M.; Xu, J.-Z.; Liu, Z.-M.; Qian, H.; Zhang, W.-G. Co-production of microbial oil and exopolysaccharide by the oleaginous yeast Sporidiobolus pararoseus grown in fed-batch culture. Rsc Adv. 2018, 8, 3348–3356. [Google Scholar] [CrossRef]
- Cabral, M.M.S.; Cence, K.; Zeni, J.; Tsai, S.M.; Durrer, A.; Foltran, L.L.; Toniazzo, G.; Valduga, E.; Treichel, H. Carotenoids production from a newly isolated Sporidiobolus pararoseus strain by submerged fermentation. Eur. Food Res. Technol. 2011, 233, 159–166. [Google Scholar] [CrossRef]
- Li, C.; Xie, Z.; Zhao, D.; Li, B.; Wang, D.; Chang, L.; Feng, F.; Zheng, L.; Wang, X.; Shao, M. Multi-omics analysis provides insights into the enhancement of β-carotene and torularhodin production in oleaginous red yeast Sporobolomyces pararoseus under H2O2-induced oxidative stress. LWT 2024, 197, 115947. [Google Scholar] [CrossRef]
- Johnson, E.A. Phaffia rhodozyma: Colorful odyssey. Int. Microbiol. 2003, 6, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Wu, J.Y. Optimization of cell growth and carotenoid production of Xanthophyllomyces dendrorhous through statistical experiment design. Biochem. Eng. J. 2007, 36, 182–189. [Google Scholar] [CrossRef]
- Freitas, C.; Parreira, T.M.; Roseiro, J.; Reis, A.; da Silva, T.L. Selecting low-cost carbon sources for carotenoid and lipid production by the pink yeast Rhodosporidium toruloides NCYC 921 using flow cytometry. Bioresour. Technol. 2014, 158, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.Y.; Keasling, J.D. Production of isoprenoid pharmaceuticals by engineered microbes. Nat. Chem. Biol. 2006, 2, 674–681. [Google Scholar] [CrossRef]
- Diner, B.A.; Fan, J.; Scotcher, M.C.; Wells, D.H.; Whited, G.M. Synthesis of heterologous mevalonic acid pathway enzymes in Clostridium ljungdahlii for the conversion of fructose and of syngas to mevalonate and isoprene. Appl. Environ. Microbiol. 2018, 84, e01723-17. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.-W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; León, P.; Boronat, A.; Rodríguez-Concepción, M. The plastidial MEP pathway: Unified nomenclature and resources. Trends Plant Sci. 2008, 13, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Abdallah, I.I.; de Haan, I.E.M.; Sibbald, M.J.J.B.; Quax, W.J. Enhanced C 30 carotenoid production in Bacillus subtilis by systematic overexpression of MEP pathway genes. Appl. Microbiol. Biotechnol. 2015, 99, 5907–5915. [Google Scholar] [CrossRef]
- Feng, Y.; Morgan, R.M.L.; Fraser, P.D.; Hellgardt, K.; Nixon, P.J. Crystal structure of geranylgeranyl pyrophosphate synthase (CrtE) involved in cyanobacterial terpenoid biosynthesis. Front. Plant Sci. 2020, 11, 589. [Google Scholar] [CrossRef]
- Xie, Z.-T.; Mi, B.-Q.; Lu, Y.-J.; Chen, M.-T.; Ye, Z.-W. Research progress on carotenoid production by Rhodosporidium toruloides. Appl. Microbiol. Biotechnol. 2024, 108, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Peng, H.; Yang, C.; Guo, W.; Wang, M.; Li, G.; Liu, D. Metabolic engineering of model microorganisms for the production of xanthophyll. Microorganisms 2023, 11, 1252. [Google Scholar] [CrossRef]
- Qi, F.; Shen, P.; Hu, R.; Xue, T.; Jiang, X.; Qin, L.; Chen, Y.; Huang, J. Carotenoids and lipid production from Rhodosporidium toruloides cultured in tea waste hydrolysate. Biotechnol. Biofuels 2020, 13, 74. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef]
- Perveen, I.; Abbas, N.; Bukhari, B.; Saleem, Y.; Mazhar, S.; Nawaz, S.; Syed, Q.; Abidi, S.H.I.; Riaz, S.; Akram, F. Bioengineering of the Optimized Biosynthesis of Commercially Vital Carotenoids-Techno-Advanced Applications: Biosynthesis of Commercially Vital Carotenoids. Pak. Biomed. J. 2023, 6, 19–31. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa—Alternative sources of natural carotenoids, lipids, and enzymes for industrial use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef]
- Misawa, N. Carotenoids: Biosynthetic and Biofunctional Approaches; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2021, 62, 6932–6946. [Google Scholar] [CrossRef] [PubMed]
- Galafassi, S.; Cucchetti, D.; Pizza, F.; Franzosi, G.; Bianchi, D.; Compagno, C. Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour. Technol. 2012, 111, 398–403. [Google Scholar] [CrossRef] [PubMed]
- El Kantar, S.; Khelfa, A.; Vorobiev, E.; Koubaa, M. Strategies for increasing lipid accumulation and recovery from Y. lipolytica: A review. OCL 2021, 28, 51. [Google Scholar] [CrossRef]
- Brink, D.P.; Mierke, F.; Norbeck, J.; Siewers, V.; Andlid, T. Expanding the genetic toolbox of Rhodotorula toruloides by identification and validation of six novel promoters induced or repressed under nitrogen starvation. Microb. Cell Factories 2023, 22, 160. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, N.; Lazar, Z.; Chatzivasileiou, A.; Ward, V.; Chen, J.; Zhou, J.; Stephanopoulos, G. Enhancing isoprenoid synthesis in Yarrowia lipolytica by expressing the isopentenol utilization pathway and modulating intracellular hydrophobicity. Metab. Eng. 2020, 61, 344–351. [Google Scholar] [CrossRef]
- Tkáčová, J.; Klempová, T.; Čertík, M. Kinetic study of growth, lipid and carotenoid formation in β-carotene producing Rhodotorula glutinis. Chem. Pap. 2018, 72, 1193–1203. [Google Scholar] [CrossRef]
- Tkáčová, J.; Čaplová, J.; Klempová, T.; Čertík, M. Correlation between lipid and carotenoid synthesis in torularhodin-producing Rhodotorula glutinis. Ann. Microbiol. 2017, 67, 541–551. [Google Scholar] [CrossRef]
- Simova, E.D.; Frengova, G.I.; Beshkova, D.M. Exopolysaccharides produced by mixed culture of yeast Rhodotorula rubra GED10 and yogurt bacteria (Streptococcus thermophilus 13a+ Lactobacillus bulgaricus 2-11). J. Appl. Microbiol. 2004, 97, 512–519. [Google Scholar] [CrossRef]
- Simova, E.D.; Frengova, G.I.; Beshkova, D.M. Synthesis of mannose-rich exopolysaccharide by Rhodotorula glutinis 16P co-cultured with yeast or bacteria. Z. Für Naturforschung C 2000, 55, 540–545. [Google Scholar] [CrossRef]
- Shim, S.-M.; Seo, S.H.; Lee, Y.; Moon, G.-I.; Kim, M.-S.; Park, J.-H. Consumers’ knowledge and safety perceptions of food additives: Evaluation on the effectiveness of transmitting information on preservatives. Food Control 2011, 22, 1054–1060. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Characterization and cytotoxic activity of pigment extracted from Rhodotorula mucilaginosa to assess its potential as bio-functional additive in confectionary products. J. Food Sci. Technol. 2021, 58, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Grigore, D.-M.; Ungureanu-Iuga, M.; Pogurschi, E.N.; Băbeanu, N.E. Transforming Rhodotorula sp. biomass to active biologic compounds for poultry nutrition. Agriculture 2023, 13, 1159. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, L.; Ge, J.; Wu, X.; Peng, Y.; Zhang, T.; Jiang, M. Astaxanthin supplementation enriches productive performance, physiological and immunological responses in laying hens. Anim. Biosci. 2020, 34, 443. [Google Scholar] [CrossRef]
- Johnson, E.A.; Villa, T.G.; Lewis, M.J. Phaffia rhodozyma as an astaxanthin source in salmonid diets. Aquaculture 1980, 20, 123–134. [Google Scholar] [CrossRef]
- Ueno, R.; Hamada-Sato, N.; Ishida, M.; Urano, N. Potential of carotenoids in aquatic yeasts as a phylogenetically reliable marker and natural colorant for aquaculture. Biosci. Biotechnol. Biochem. 2011, 75, 1654–1661. [Google Scholar] [CrossRef]
- An, G.H.; Song, J.Y.; Chang, K.S.; Lee, B.D.; Chae, H.S.; Jang, B.G. Pigmentation and delayed oxidation of broiler chickens by the red carotenoid, astaxanthin, from chemical synthesis and the yeast, Xanthophyllomyces dendrorhous. Asian-Australas. J. Anim. Sci. 2004, 17, 1309–1314. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Tang, Z.; Zhang, X.; Chen, J.; Sun, Z. Effects of Rhodotorula mucilaginosa fermentation product on the laying performance, egg quality, jejunal mucosal morphology and intestinal microbiota of hens. J. Appl. Microbiol. 2020, 128, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Abbas, C.A. Production of antioxidants, aromas, colours, flavours, and vitamins by yeasts. In Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006; pp. 285–334. [Google Scholar]
- Manimala, M.R.A.; Murugesan, R. In vitro antioxidant and antimicrobial activity of carotenoid pigment extracted from Sporobolomyces sp. isolated from natural source. J. Appl. Nat. Sci. 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Stachowiak, B. The antioxidant potential of carotenoid extract from Phaffia rhodozyma. Acta Sci. Pol. Technol. Aliment. 2010, 9, 171–188. [Google Scholar]
- García-Béjar, B.; Sánchez-Carabias, D.; Alarcon, M.; Arévalo-Villena, M.; Briones, A. Autochthonous yeast from pork and game meat fermented sausages for application in meat protection and aroma developing. Animals 2020, 10, 2340. [Google Scholar] [CrossRef]
- Terao, J.; Minami, Y.; Bando, N. Singlet molecular oxygen-quenching activity of carotenoids: Relevance to protection of the skin from photoaging. J. Clin. Biochem. Nutr. 2010, 48, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef]
- Sriphuttha, C.; Limkul, S.; Pongsetkul, J.; Phiwthong, T.; Massu, A.; Sumniangyen, N.; Boontawan, P.; Ketudat-Cairns, M.; Boontawan, A.; Boonchuen, P. Effect of fed dietary yeast (Rhodotorula paludigena CM33) on shrimp growth, gene expression, intestinal microbial, disease resistance, and meat composition of Litopenaeus vannamei. Dev. Comp. Immunol. 2023, 147, 104896. [Google Scholar] [CrossRef]
- Wang, J.-h.; Zhao, L.-q.; Liu, J.-f.; Wang, H.; Xiao, S. Effect of potential probiotic Rhodotorula benthica D30 on the growth performance, digestive enzyme activity and immunity in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2015, 43, 330–336. [Google Scholar] [CrossRef]
- Elwan, H.A.M.; Elnesr, S.S.; Abdallah, Y.; Hamdy, A.; El-Bogdady, A.H. Red yeast (Phaffia rhodozyma) as a source of astaxanthin and its impacts on productive performance and physiological responses of poultry. World’s Poult. Sci. J. 2019, 75, 273–284. [Google Scholar] [CrossRef]
- Naisi, S.; Bayat, M.; Salehi, T.Z.; Zarif, B.R.; Yahyaraeyat, R. Antimicrobial and anti-biofilm effects of carotenoid pigment extracted from Rhodotorula glutinis strain on food-borne bacteria. Iran. J. Microbiol. 2023, 15, 79. [Google Scholar] [CrossRef]
- Ungureanu, C.; Popescu, S.; Purcel, G.; Tofan, V.; Popescu, M.; Sălăgeanu, A.; Pîrvu, C. Improved antibacterial behavior of titanium surface with torularhodin–polypyrrole film. Mater. Sci. Eng. C 2014, 42, 726–733. [Google Scholar] [CrossRef]
- Sinha, S.; Das, S.; Saha, B.; Paul, D.; Basu, B. Anti-microbial, anti-oxidant, and anti-breast cancer properties unraveled in yeast carotenoids produced via cost-effective fermentation technique utilizing waste hydrolysate. Front. Microbiol. 2023, 13, 1088477. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Chakrabarti, A.; Singh, G.; Kumar, K.K.; Gaur, N.A.; Arora, A.; Singh, K.N.; Singh, S.; Paul, D. Isolation and identification of carotenoid-producing yeast and evaluation of antimalarial activity of the extracted carotenoid (s) against P. falciparum. Biol. Futur. 2021, 72, 325–337. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Chen, J.; Wang, T.; Huang, X.; Chen, G. The extraction of β-carotene from microalgae for testing their health benefits. Foods 2022, 11, 502. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Adelusi, O.A.; Gbashi, S.; Adebiyi, J.A.; Makhuvele, R.; Aasa, A.O.; Oladeji, O.M.; Khoza, M.; Okoth, S.; Njobeh, P.B. Seasonal Diversity and Occurrence of Filamentous Fungi in Smallholder Dairy Cattle Feeds and Feedstuffs in South Africa. J. Fungi 2022, 8, 1192. [Google Scholar] [CrossRef] [PubMed]
- Sukmawati, D.; Saidah, N.; Handayani, K.T.; Rahayu, S. The characteristics of fungi contaminating chicken feed in Tegal, Bogor, West Java. Asian J. Agric. Biol. 2018, 6, 472–480. [Google Scholar]
- Kulczyk-Małysa, E.; Bogusławska-Wąs, E. Effect of storage conditions on the micrbiological quality of feed. Acta Sci. Pol. Zootech. 2024, 22, 21–32. [Google Scholar]
- Lawniczek-Walczyk, A.; Gorny, R.L.; Golofit-Szymczak, M.; Niesler, A.; Wlazlo, A. Occupational exposure to airborne microorganisms, endotoxins and beta-glucans in poultry houses at different stages of the production cycle. Ann. Agric. Environ. Med. 2013, 20, 259–268. [Google Scholar]
- Srinual, O.; Moonmanee, T.; Lumsangkul, C.; Doan, H.V.; Punyatong, M.; Yachai, M.; Chaiyaso, T.; Kongtong, K.; Tapingkae, W. Can red yeast (Sporidiobolus pararoseus) be used as a novel feed additive for mycotoxin binders in broiler chickens? Toxins 2022, 14, 678. [Google Scholar] [CrossRef]
- Weaver, A.C.; King, W.D.; Verax, M.; Fox, U.; Kudupoje, M.B.; Mathis, G.; Lumpkins, B.; Yiannikouris, A. Impact of chronic levels of naturally multi-contaminated feed with Fusarium mycotoxins on broiler chickens and evaluation of the mitigation properties of different titers of yeast cell wall extract. Toxins 2020, 12, 636. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Coppa, C.F.S.C.; Neeff, D.V.D.; Corassin, C.H.; Oliveira, C.A.F. Mycotoxins in fruits and fruit-based products: Occurrence and methods for decontamination. Toxin Rev. 2019, 38, 263–272. [Google Scholar] [CrossRef]
- Ianiri, G.; Pinedo, C.; Fratianni, A.; Panfili, G.; Castoria, R. Patulin degradation by the biocontrol yeast Sporobolomyces sp. is an inducible process. Toxins 2017, 9, 61. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L. Dairy products and health: Recent insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef]
- Cheng, S.; Li, W.; Wu, S.; Ge, Y.; Wang, C.; Xie, S.; Wu, J.; Chen, X.; Cheong, L.-Z. Functional butter for reduction of consumption risk and improvement of nutrition. Grain Oil Sci. Technol. 2023, 6, 172–184. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, I.H. Effect of astaxanthin produced by Phaffia rhodozyma on growth performance, meat quality, and fecal noxious gas emission in broilers. Poult. Sci. 2014, 93, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-K.; Li, Y.-X.; Liu, D.; Yan, K.-C.; Zhang, C.; Li, W.; Cao, Z.-J.; Lu, Z.-M. Effects of dietary supplementation of Phaffia rhodozyma on fetal days, serum antioxidant and immune indexes of perinatal dairy cows. Chin. J. Anim. Nutr. 2023, 35, 3761–3770. [Google Scholar]
- Takahashi, K.; Takimoto, T.; Sato, K.; Akiba, Y. Effect of dietary supplementation of astaxanthin from Phaffia rhodozyma on lipopolysaccharide-induced early inflammatory responses in male broiler chickens (Gallus gallus) fed a corn-enriched diet. Anim. Sci. J. 2011, 82, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Woollard, G. Retinol, retinoic acid, carotenes and carotenoids: Vitamin A structure and terminology. In Vitamin A and Carotenoids: Chemistry, Analysis, Function and Effects; RSC Publishing: London, UK, 2012; pp. 3–22. [Google Scholar]
- Somboonchai, T.; Foiklang, S.; Panatuk, J.; Cherdthong, A.; Laorodphan, N.; Wanapat, M.; Yammuen-Art, S.; Kang, S. Replacement of soybean meal by red yeast fermented tofu waste on feed intake, growth performance, carcass characteristics, and meat quality in Thai Brahman crossbred beef cattle. Trop. Anim. Health Prod. 2022, 54, 133. [Google Scholar] [CrossRef]
- Parker, R.S.; Swanson, J.E.; You, C.-S.; Edwards, A.J.; Huang, T. Bioavailability of carotenoids in human subjects. Proc. Nutr. Soc. 1999, 58, 155–162. [Google Scholar] [CrossRef]
- You, C.-S.; Parker, R.S.; Goodman, K.J.; Swanson, J.E.; Corso, T.N. Evidence of cis-trans isomerization of 9-cis-beta-carotene during absorption in humans. Am. J. Clin. Nutr. 1996, 64, 177–183. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Kot, A.M.; Kieliszek, M.; Piwowarek, K.; Błażejak, S.; Mussagy, C.U. Sporobolomyces and Sporidiobolus–non-conventional yeasts for use in industries. Fungal Biol. Rev. 2021, 37, 41–58. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Levy, Y. Bioavailability of a natural isomer mixture compared with synthetic all-trans beta-carotene in human serum. Am. J. Clin. Nutr. 1996, 63, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dai, Z.; Shi, E.; Wan, P.; Chen, G.; Zhang, Z.; Xu, Y.; Gao, R.; Zeng, X.; Li, D. Study on the interaction between β-carotene and gut microflora using an in vitro fermentation model. Food Sci. Hum. Wellness 2023, 12, 1369–1378. [Google Scholar] [CrossRef]
- Roy, U.; Gálvez, E.J.C.; Iljazovic, A.; Lesker, T.R.; Błażejewski, A.J.; Pils, M.C.; Heise, U.; Huber, S.; Flavell, R.A.; Strowig, T. Distinct microbial communities trigger colitis development upon intestinal barrier damage via innate or adaptive immune cells. Cell Rep. 2017, 21, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Drai, J.; Borel, P.; Faure, H.; Galabert, C.; Moël, G.L.; Laromiguière, M.; Fayol, V. Fasting plasma carotenoids concentrations in Crohn’s and pancreatic cancer patients compared to control subjects. Int. J. Vitam. Nutr. Res. 2009, 79, 87–94. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J. Lycopene alleviates DSS-induced colitis and behavioral disorders via mediating microbes-gut–brain axis balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
| Microorganism | Type | Carotenoid Fraction Produced | Production Efficiency | References |
|---|---|---|---|---|
| Algae | Haematococcus | canthaxanthin astaxanthin | 1.8 mg/g DB 22.0 mg/g w/w | [19,20] |
| Chlorella | neoxanthin α-carotene β-carotene | 0.2 μg/g 4.2 mg/g 4.3 mg/g | [21] | |
| Dunaliella | β-carotene zeaxanthin | 8.3–13.5 mg 5.9 mg/g | [22,23] | |
| Bacteria | Micrococcus | lycopene neoxanthin zeaxanthin β-carotene | 1.5 mg/mL 2.6 mg/mL 0.9 mg/mL 0.7 g/L | [24,25,26] |
| Croceibacterium | zeaxanthin α-carotene | 412.4 μg/g 42.6 μg/g | [27] | |
| Mycobacterium | lycopene | 1.4 mg/g DW 7.4 mg/g biomass | [28,29] | |
| Yeast | Sporobolomyces | torulene torularodine | 3.8–33.3 μg/g DW 3.1–22.9 μg/g DW | [30] |
| Sporidiobolus | torulene torularodine β-carotene | 17.0–3 1.7 mg/L 1.5–41.2 μg/g DW 10.4–18.9 mg/L | [30,31,32] | |
| Phaffia | astaxanthin β-carotene | 150.0–503.6 μg/g DW 110.3–22.7 μg/g DW | [33] | |
| Rhodosporidium | β-carotene | 3.3–5.1 μg/mL | [34] | |
| Rhodotorula | torulene torularodine β-carotene | 20.3–95.2 μg/g DW 1.5–4.3 mg/100 g DW 38.7–77.25 μg/g DW | [35,36] | |
| Moulds | Blakeslea | β-carotene | 72.2–250.4 mg/L | [37] |
| Sclerotinia | β-carotene | 0.5–2.1 nmol/g DW | [38] | |
| Neurospora | β-carotene neurosporaxanthin | 0.1–1.2 mg/g DB 0.2–3.7 ug/g DW | [39,40] | |
| Fusarium | neurosporaxanthin | 0.02–8.3 ug/g DW | [41] | |
| Mucor | astaxanthin | 9.1–11.2 g/L | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulczyk-Małysa, E.; Bogusławska-Wąs, E. Carotenoid Yeasts and Their Application Potential. Foods 2025, 14, 1866. https://doi.org/10.3390/foods14111866
Kulczyk-Małysa E, Bogusławska-Wąs E. Carotenoid Yeasts and Their Application Potential. Foods. 2025; 14(11):1866. https://doi.org/10.3390/foods14111866
Chicago/Turabian StyleKulczyk-Małysa, Ewa, and Elżbieta Bogusławska-Wąs. 2025. "Carotenoid Yeasts and Their Application Potential" Foods 14, no. 11: 1866. https://doi.org/10.3390/foods14111866
APA StyleKulczyk-Małysa, E., & Bogusławska-Wąs, E. (2025). Carotenoid Yeasts and Their Application Potential. Foods, 14(11), 1866. https://doi.org/10.3390/foods14111866







