Oleuropein Regulates Bile Acid Metabolism via Modulating the Gut Microbiota, Thereby Alleviating DSS-Induced Ulcerative Colitis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experiments
2.2. Disease Activity Index (DAI) Analysis
2.3. Measurement of Pro-Inflammatory Cytokine Levels
2.4. Measurement of Oxidative Stress Levels
2.5. Tissue Section Staining
2.6. Western Blot Analysis
2.7. 16S rRNA Analysis of Mouse Fecal Microbiota
2.8. Gut Metabolite Assay
2.9. Statistical Analysis
3. Results
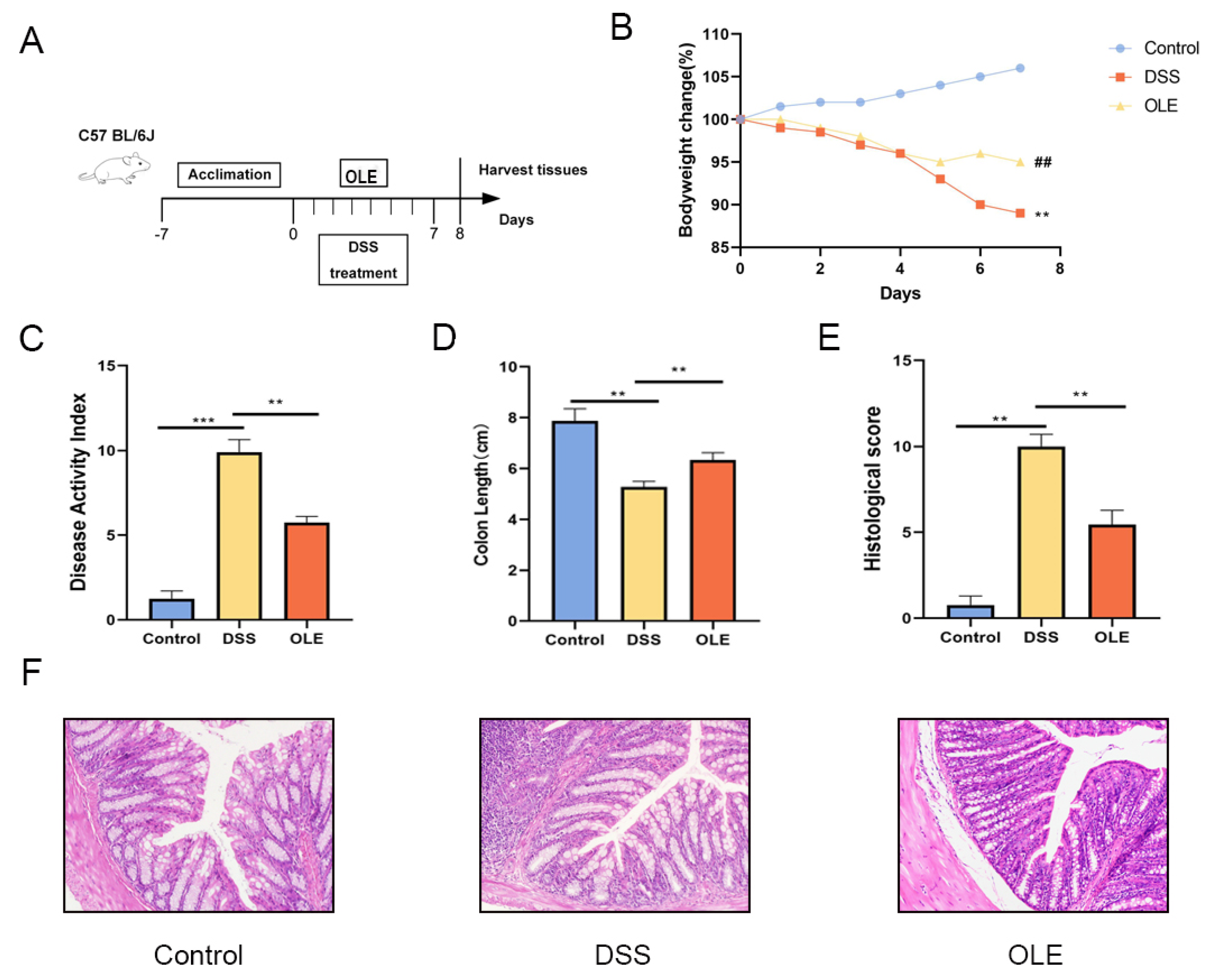
3.1. OLE Alleviated DSS-Induced Colitis in Mice
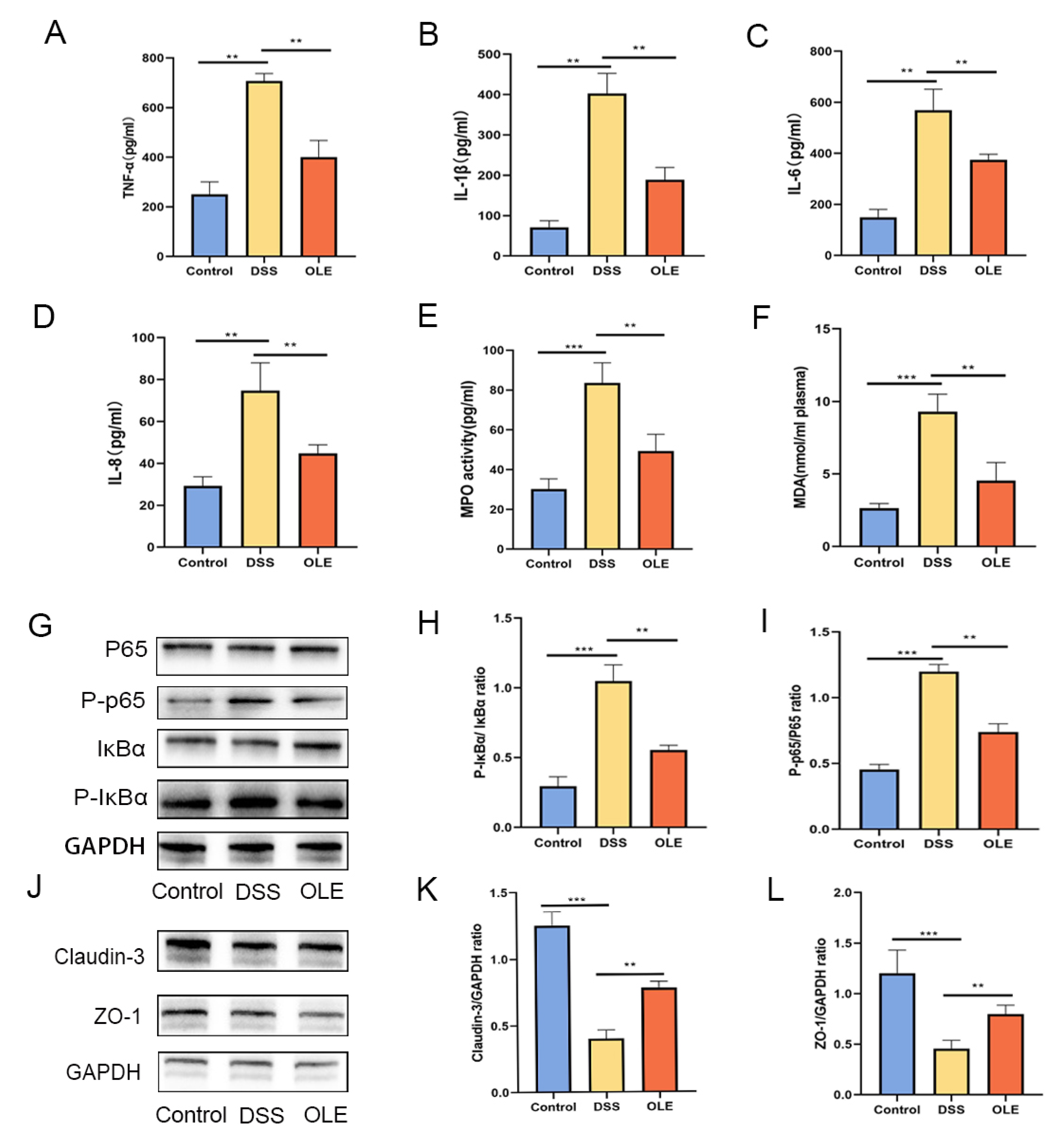
3.2. OLE Protected the Intestinal Mucosal Barrier in UC Mice and Inhibited the Expression of NF-κB Signaling
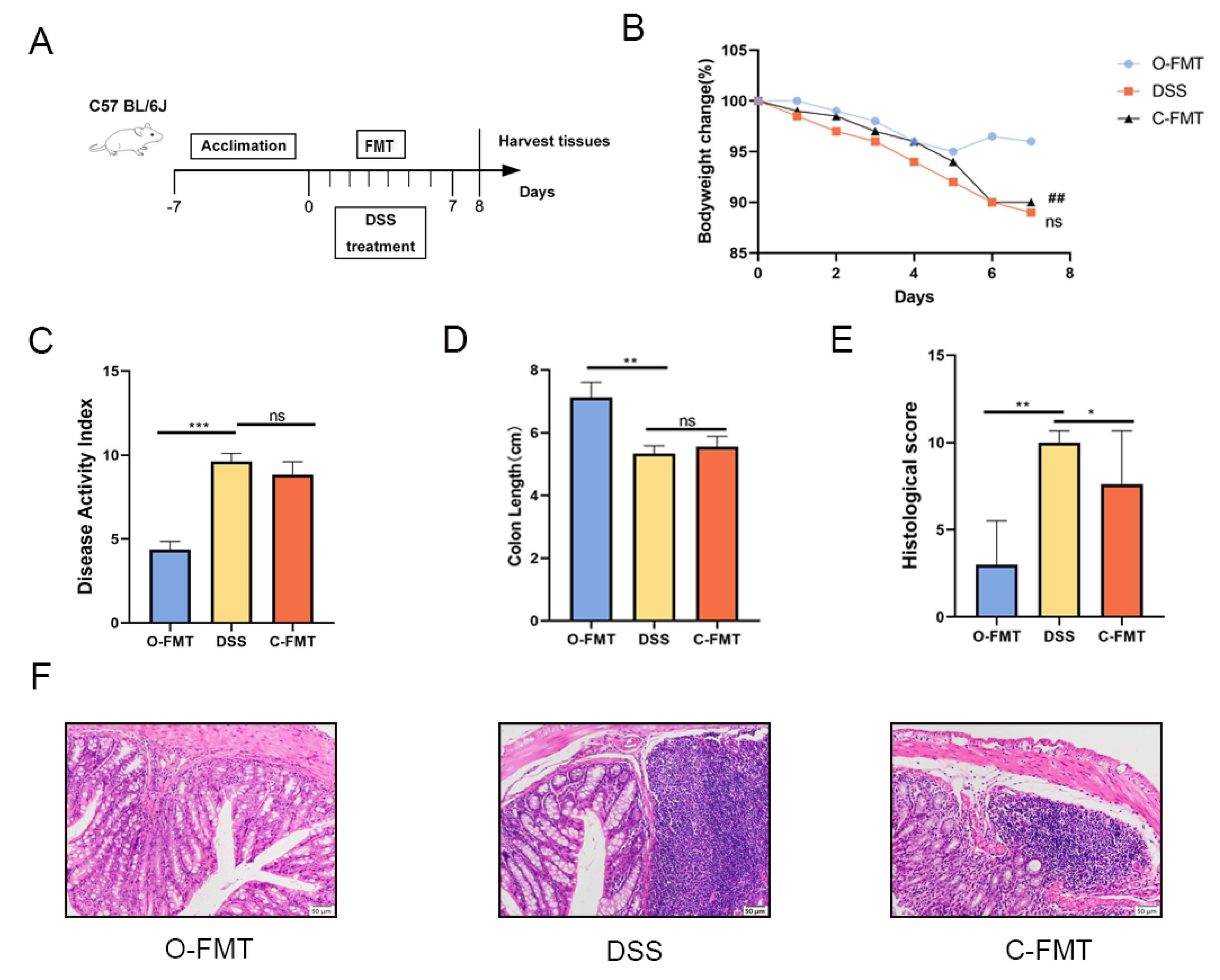
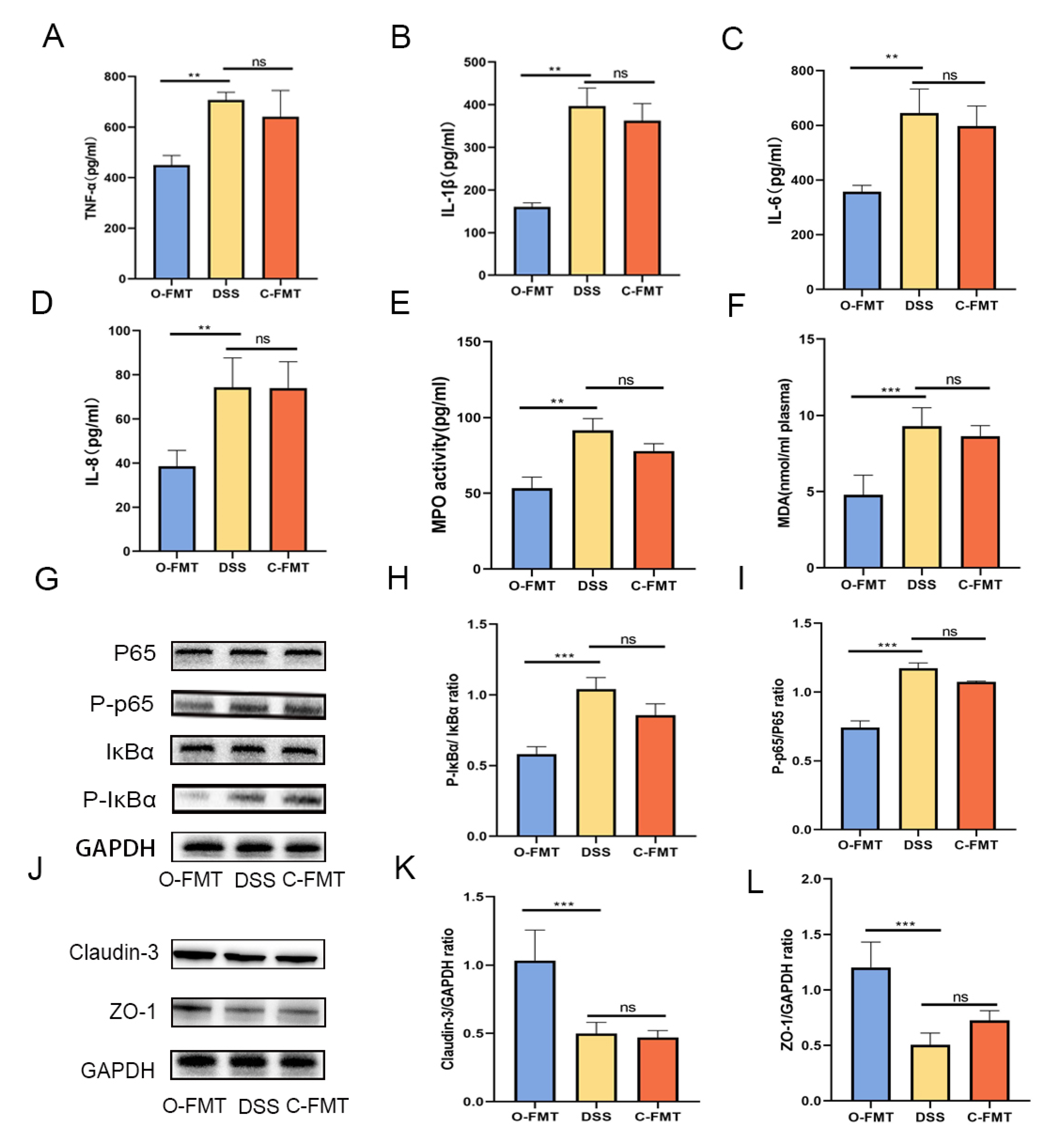
3.3. The FMT of OLE Alleviated DSS-Induced Colitis in Mice
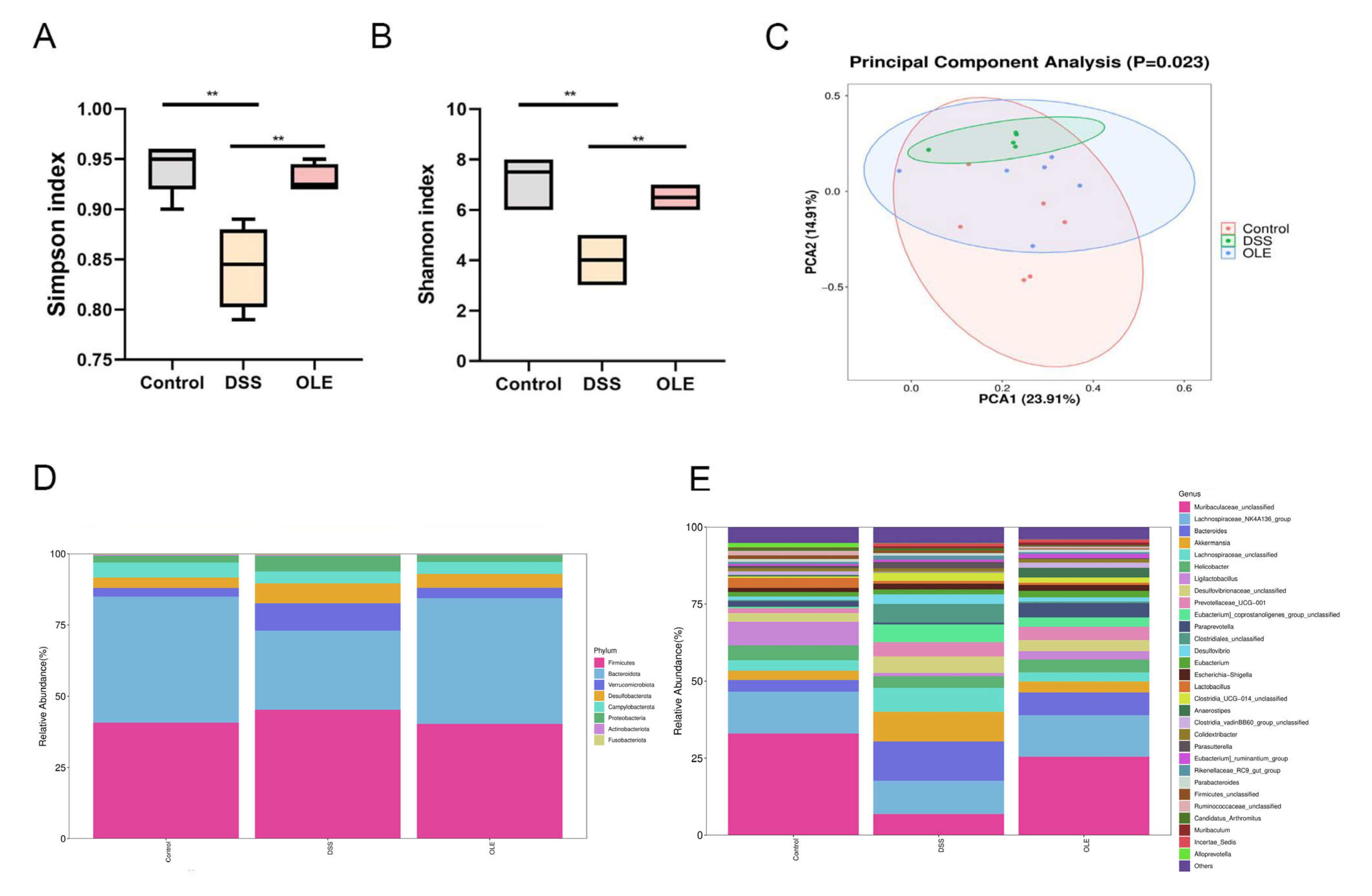
3.4. OLE Altered the Gut Microbiota of Mice with UC
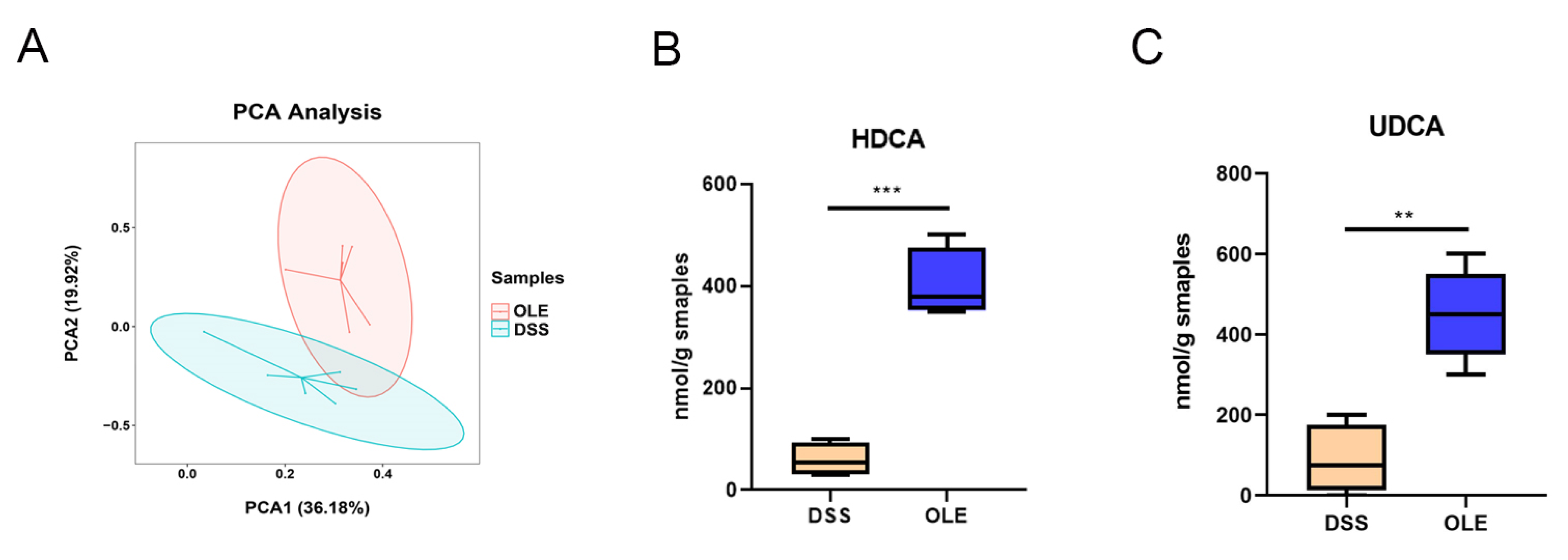
3.5. OLE Alleviated UC in Mice by Modulating the Level of Bile Acids in the Gut Microbiota
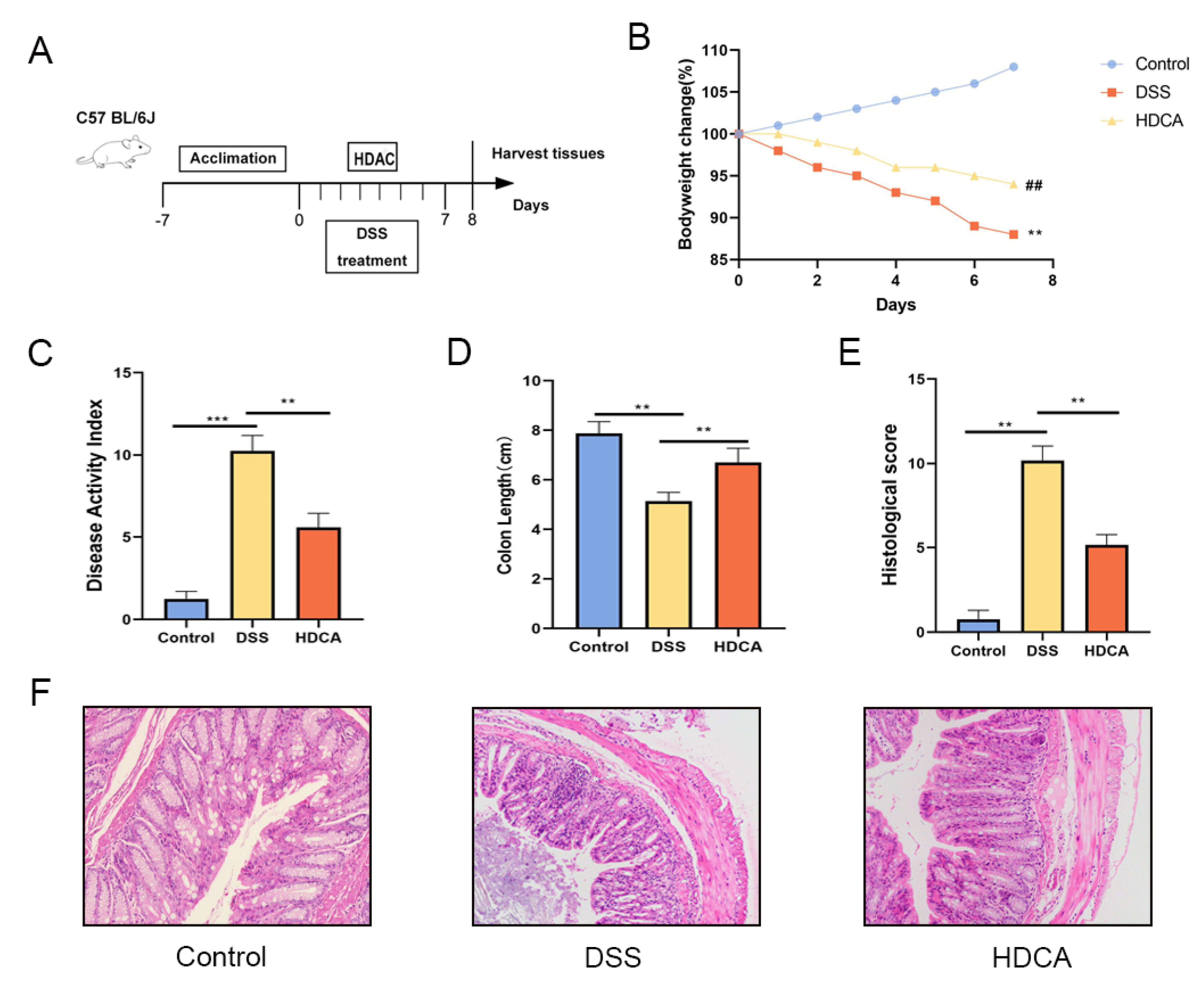
3.6. HDCA Exerted Anti-Inflammatory and Barrier-Protective Effects in Mice with UC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Ding, C.B.; Liu, X.L.; Zhao, Y.C.; Li, S.S.; Ding, Q.T.; Zhao, T.; Ma, S.; Li, W.; Liu, W.C. New resource food-arabinogalactan improves DSS-induced acute colitis through intestinal flora and NLRP3 signaling pathway. Int. J. Biol. Macromol. 2024, 258 Pt 2, 129118. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Khan, A.; Chen, W.T.; Chai, W.Q.; Wang, C.F. Advancements in Genetic Biomarkers and Exogenous Antioxidant administration for Safeguarding Mammalian Cells against Heat-Induced Oxidative Stress and Apoptosis. Antioxidants 2024, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.K.; Lee, J.W.; Woo, J.H.; Kim, H.J.; Kim, D.H.; Choi, J.H. Regulation of colonic neuropeptide Y expression by the gut microbiome in patients with ulcerative colitis and its association with anxiety- and depression-like behavior in mice. Gut Microbes. 2024, 16, 2319844. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Z.; Hu, X.Y.; Ren, H.L.; Wang, X.X.; Shi, R.R.; Guo, J.; Chang, J.; Zhou, X.S.; Jin, Y.Y.; Li, Y.S.; et al. Protective effect of synbiotic combination of and olive oil extract tyrosol in a murine model of ulcerative colitis. J. Transl. Med. 2024, 22, 308. [Google Scholar] [CrossRef]
- Motawea, M.H.; Abd Elmaksoud, H.A.; Elharrif, M.G.; Desoky, A.A.E.; Ibrahimi, A. Evaluation of Anti-inflammatory and Antioxidant Profile of Oleuropein in Experimentally Induced Ulcerative Colitis. Int. J. Mol. Cell Med. 2020, 9, 224–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larussa, T.; Oliverio, M.; Suraci, E.; Greco, M.; Placida, R.; Gervasi, S.; Marasco, R.; Imeneo, M.; Paolino, D.; Tucci, L.; et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients 2017, 9, 391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, Z.C.; Lu, Y.; Liu, B.; Ran, C.W.; Lei, X.; Wang, M.F.; Wu, S.T.; Yang, Y.F.; Wu, H.Z. Glycolysis, a new mechanism of oleuropein against liver tumor. Phytomedicine 2023, 114, 154770. [Google Scholar] [CrossRef]
- Singh, R.; Zahra, W.; Singh, S.S.; Birla, H.; Rathore, A.S.; Keshri, P.K.; Dilnashin, H.; Singh, S.; Singh, S.P. Oleuropein confers neuroprotection against rotenone-induced model of Parkinson’s disease via BDNF/CREB/Akt pathway. Sci. Rep. 2023, 13, 2452. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Frost, B.; Liu, J. Oleuropein, unexpected benefits! Oncotarget 2017, 8, 17409. [Google Scholar] [CrossRef] [PubMed]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. BioFactors 2017, 43, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, K.I.A.; Zamil, S.J. Effect of injection of epigallocatechin-3 gallate and oleuropein on hatching, productive and physiological aspects of broiler chicks exposed to short heat stress. J. Indones. Trop. Anim. 2024, 49, 1–15. [Google Scholar] [CrossRef]
- Nsairat, H.; Jaber, A.M.; Faddah, H.; Ahmad, S. Oleuropein impact on colorectal cancer. Future Sci. OA 2024, 10, FSO. [Google Scholar] [CrossRef]
- Blanco, E.; Silva-Pilipich, N.; Bocanegra, A.; Chocarro, L.; Procopio, A.; Ausin, K.; Fernandez-Irigoyen, J.; Fernandez, L.; Razquin, N.; Igea, A.; et al. Oleuropein-driven reprogramming of the myeloid cell compartment to sensitise tumours to PD-1/PD-L1 blockade strategies. Br. J. Cancer 2024, 130, 869–879. [Google Scholar] [CrossRef]
- Na, K.; Wei, J.N.; Zhang, L.; Fang, Y.; Li, X.Y.; Lu, S.; Guo, X.H. Effects of chitosan oligosaccharides (COS) and FMT from COS-dosed mice on intestinal barrier function and cell apoptosis. Carbohyd. Polym. 2022, 297, 120043. [Google Scholar] [CrossRef]
- Tao, Y.L.; Wang, L.; Ye, X.F.; Qian, X.; Pan, D.Y.; Dong, X.Y.; Jiang, Q.; Hu, P. Huang Qin decoction increases SLC6A4 expression and blocks the NFκB-mediated NLRP3/Caspase1/GSDMD pathway to disrupt colitis-associated carcinogenesis. Funct. Integr. Genom. 2024, 24, 55. [Google Scholar] [CrossRef]
- Yue, Y.S.; Shi, M.X.; Song, X.Y.; Ma, C.; Li, D.T.; Hu, X.S.; Chen, F. Lycopene Ameliorated DSS-Induced Colitis by Improving Epithelial Barrier Functions and Inhibiting the Escherichia coli Adhesion in Mice. J. Agric. Food Chem. 2024, 72, 5784–5796. [Google Scholar] [CrossRef]
- Wei, S.X.; Wang, L.F.; Chen, X.D.; Wang, Y.; Tong, L.L.; Wang, L.L.; Han, Q.Y.; Guo, D.S.; Ren, B. Polysaccharide from Boletus aereus ameliorates DSS-induced colitis in mice by regulating the MANF/MUC2 signaling and gut microbiota. Int. J. Biol. Macromol. 2024, 266 Pt 1, 131232. [Google Scholar] [CrossRef]
- Chen, Y.H.; Huang, C.; Du, F.; Xiao, Z.L.; Qian, W.; Bai, T.; Song, J.; Song, Y.H.; Hou, X.H.; Zhang, L. EphB2 promotes enteric nitrergic hyperinnervation and neurogenic inflammation in DSS-induced chronic colitis in mice. Int. Immunopharmacol. 2024, 129, 111591. [Google Scholar] [CrossRef]
- Di, Y.; Song, Y.; Xu, K.J.; Wang, Q.X.; Zhang, L.; Liu, Q.; Zhang, M.; Liu, X.B.; Wang, Y.T. Chicoric Acid Alleviates Colitis via Targeting the Gut Microbiota Accompanied by Maintaining Intestinal Barrier Integrity and Inhibiting Inflammatory Responses. J. Agric. Food Chem. 2024, 72, 6276–6288. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.G.; Zhang, T.; Zhang, T.S.; Park, S. The impact of gut microbiome enterotypes on ulcerative colitis: Identifying key bacterial species and revealing species co-occurrence networks using machine learning. Gut. Microbes 2024, 16, 2292254. [Google Scholar] [CrossRef]
- Li, A.; Liu, A.J.; Wang, J.; Song, H.A.; Luo, P.F.; Zhan, M.; Zhou, X.L.; Chen, L.H.; Zhang, L. The prophylaxis functions of GLF-217 and FLP-215 on ulcerative colitis via modulating gut microbiota of mice. J. Sci. Food Agric. 2024, 104, 5816–5825. [Google Scholar] [CrossRef]
- Shu, Y.F.; Huang, Y.J.; Dong, W.; Fan, X.; Sun, Y.; Chen, G.J.; Zeng, X.X.; Ye, H. The polysaccharides from alleviate non-alcoholic fatty liver diseasemodulating gut microbiota and bile acids metabolism. Int. J. Biol. Macromol. 2023, 246, 125662. [Google Scholar] [CrossRef]
- Petito-da-Silva, T.I.; Villardi Júnior, F.M.; Penna-de-Carvalho, A.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V.; Barbosa-da-Silva, S. An Intestinal FXR Agonist Mitigates Dysbiosis, Intestinal Tight Junctions, and Inflammation in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2024, 68, e2300148. [Google Scholar] [CrossRef]
- Beau, A.; Benoit, B.; Le Barz, M.; Meugnier, E.; Penhoat, A.; Calzada, C.; Pinteur, C.; Loizon, E.; Chanon, S.; Vieille-Marchiset, A.; et al. Inhibition of intestinal FXR activity as a possible mechanism for the beneficial effects of a probiotic mix administration on lipid metabolism alterations and weight gain in mice fed a high fat diet. Gut Microbes 2023, 15, 2281015. [Google Scholar] [CrossRef]
- Rao, Y.F.; Wen, Q.; Liu, R.H.; He, M.Z.; Jiang, Z.H.; Qian, K.; Zhou, C.Q.; Li, J.M.; Du, H.; Ouyang, H.; et al. PL-S2, a homogeneous polysaccharide from, attenuates hyperlipidemia via farnesoid X receptor (FXR) pathway-modulated bile acid metabolism. Int. J. Biol. Macromol. 2020, 165, 1694–1705. [Google Scholar] [CrossRef]
- Pan, D.B.; Wang, J.; Ye, H.L.; Qin, Y.; Xu, S.Q.; Ye, G.X.; Shen, H.J. Tauroursodeoxycholic acid suppresses biliary epithelial cell apoptosis and endoplasmic reticulum stress by miR-107/NCK1 axis in a FXR-dependent manner. Drug Chem. Toxicol. 2024, 47, 839–847. [Google Scholar] [CrossRef]
- Han, B.; Lv, X.D.; Liu, G.F.; Li, S.Q.; Fan, J.H.; Chen, L.; Huang, Z.X.; Lin, G.F.; Xu, X.F.; Huang, Z.Q.; et al. Gut microbiota-related bile acid metabolism-FXR/TGR5 axis impacts the response to anti-α4β7-integrin therapy in humanized mice with colitis. Gut Microbes 2023, 15, 2232143. [Google Scholar] [CrossRef]
- Wang, C.; Guo, H.; Bai, J.Y.; Yu, L.L.; Tian, F.W.; Zhao, J.X.; Zhang, H.; Chen, W.; Zhai, Q.X. The roles of different strains in alleviating DSS-induced ulcerative colitis and related functional genes. Food Funct. 2024, 15, 3327–3339. [Google Scholar] [CrossRef]
- Zhao, R.Q.; Ji, Y.; Chen, X.; Ma, G.X.; Yao, H.L.; Li, J.; Hu, Q.H.; Zhao, L.Y. Flammulina velutipes polysaccharides regulate lipid metabolism disorders in HFD-fed mice via bile acids metabolism. Int. J. Biol. Macromol. 2023, 253, 127308. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.; Mountifield, R. Active inflammatory bowel disease is associated with reduced sleep efficiency and sleep architecture changes: Preliminary results of IBD-SLEEP. J. Gastroenterol. Hepatol. 2023, 38, 134–135. [Google Scholar]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X receptor (FXR): Structures and ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef]
- Panzitt, K.; Zollner, G.; Marschall, H.U.; Wagner, M. Recent advances on FXR-targeting therapeutics. Mol. Cell Endocrinol. 2022, 552, 111678. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Lu, Z.; Wang, R.; Xiang, Y.; Li, H.; Lu, J.; Zhang, L.; Chen, H.; Li, W.; Luan, X.; et al. FXR agonists for colorectal and liver cancers, as a stand-alone or in combination therapy. Biochem. Pharmacol. 2023, 212, 115570. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core altered microorganisms in colitis mouse model: A comprehensive time-point and fecal microbiota transplantation analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef]
- Fu, T.; Li, Y.; Oh, T.G.; Cayabyab, F.; He, N.; Tang, Q.; Coulter, S.; Truitt, M.; Medina, P.; He, M.; et al. FXR mediates ILC-intrinsic responses to intestinal inflammation. Proc. Natl. Acad. Sci. USA 2022, 119, e2213041119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, R.; Zhou, R.; Li, Y.; Liu, Z.; Wu, H.; Lu, L.; Xu, H. Oleuropein Regulates Bile Acid Metabolism via Modulating the Gut Microbiota, Thereby Alleviating DSS-Induced Ulcerative Colitis in Mice. Foods 2025, 14, 1863. https://doi.org/10.3390/foods14111863
Zang R, Zhou R, Li Y, Liu Z, Wu H, Lu L, Xu H. Oleuropein Regulates Bile Acid Metabolism via Modulating the Gut Microbiota, Thereby Alleviating DSS-Induced Ulcerative Colitis in Mice. Foods. 2025; 14(11):1863. https://doi.org/10.3390/foods14111863
Chicago/Turabian StyleZang, Rongxin, Rui Zhou, Yaodong Li, Zhouliang Liu, Huihao Wu, Liping Lu, and Hongwei Xu. 2025. "Oleuropein Regulates Bile Acid Metabolism via Modulating the Gut Microbiota, Thereby Alleviating DSS-Induced Ulcerative Colitis in Mice" Foods 14, no. 11: 1863. https://doi.org/10.3390/foods14111863
APA StyleZang, R., Zhou, R., Li, Y., Liu, Z., Wu, H., Lu, L., & Xu, H. (2025). Oleuropein Regulates Bile Acid Metabolism via Modulating the Gut Microbiota, Thereby Alleviating DSS-Induced Ulcerative Colitis in Mice. Foods, 14(11), 1863. https://doi.org/10.3390/foods14111863





