Prediction of Key Quality Parameters in Hot Air-Dried Jujubes Based on Hyperspectral Imaging
Abstract
1. Introduction
- (1)
- To evaluate the distribution of jujube quality parameters (L*, a*, b*, SSC, hardness, and moisture) under varying drying temperature conditions;
- (2)
- To acquire the reflectance spectral data of jujubes at different drying temperatures and perform spectral transformations to obtain absorbance spectra and Kubelka–Munk (K-M) spectra;
- (3)
- To apply preprocessing methods, such as standard normal variate (SNV), baseline correction (baseline), and Savitzky–Golay first derivative (SG1st), in combination with support vector regression (SVR), to determine the optimal modeling results and preprocessing methods for different quality parameters;
- (4)
- To apply the best preprocessing method to various deep learning models (BiLSTM, BiGRU, and CNN-BiGRU), train these models and compare their performance with that of SVR to identify the optimal predictive model, thereby enabling the prediction of jujube quality parameters.
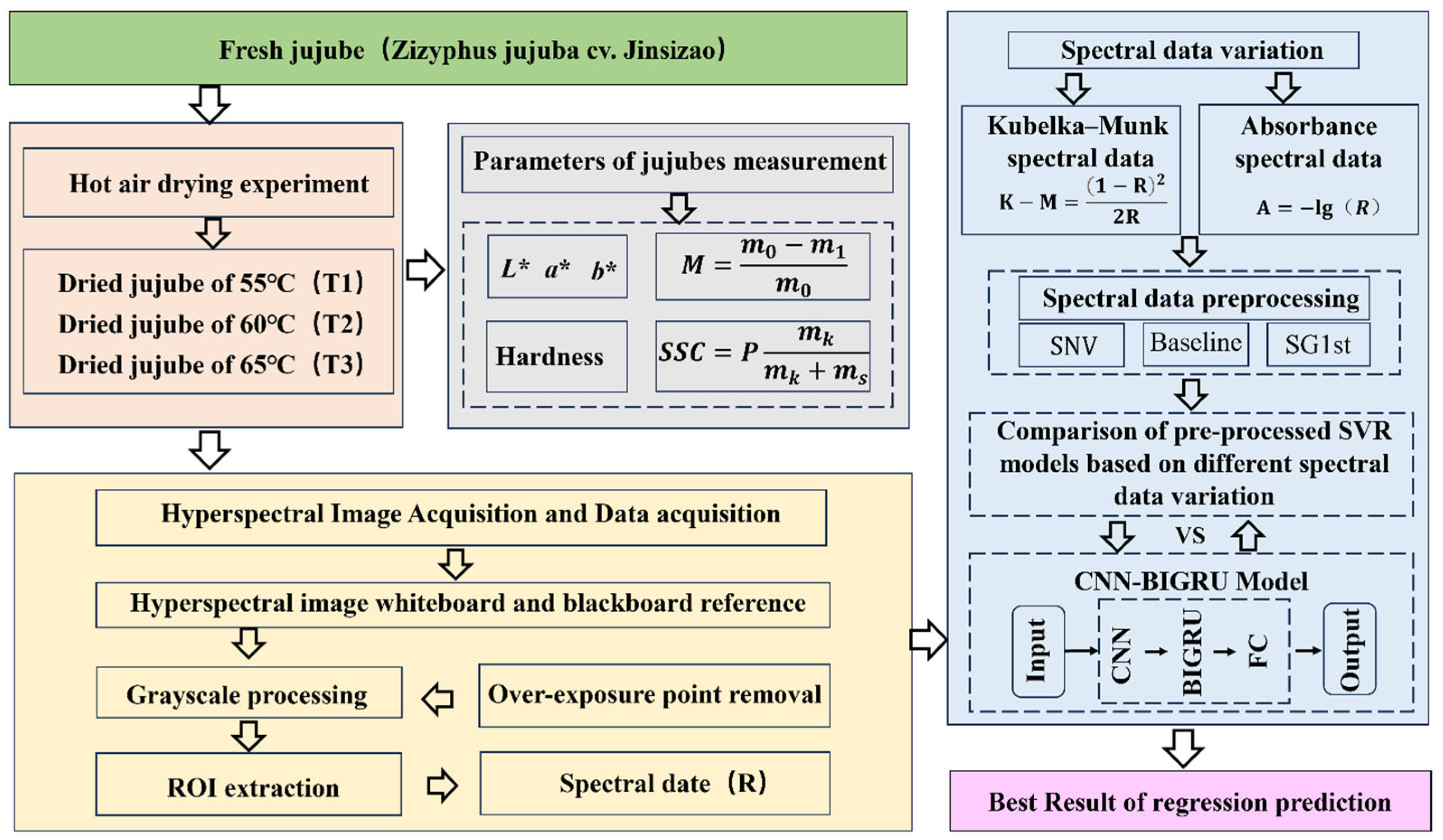
2. Materials and Methods
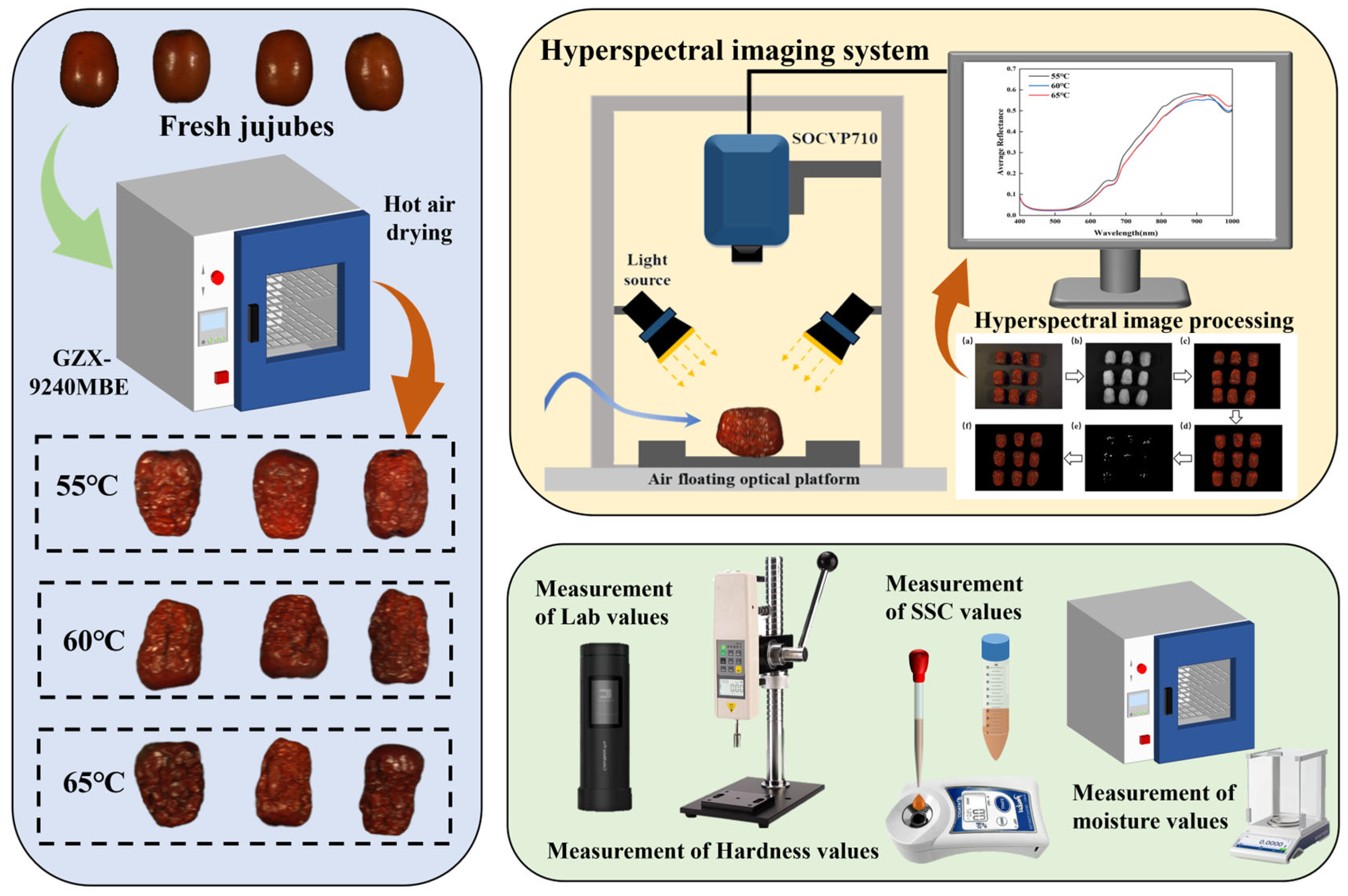
2.1. Jujube Samples and Drying Experiment
2.2. Quality Parameters of Jujubes Measurement
2.3. Hyperspectral Imaging System and Date Acquisition
2.3.1. Hyperspectral Imaging System
2.3.2. Hyperspectral Date Acquisition and Extraction
2.4. Spectral Preprocessing Methods
2.5. Module Establishment and Evaluation
2.5.1. Support Vector Regression Module
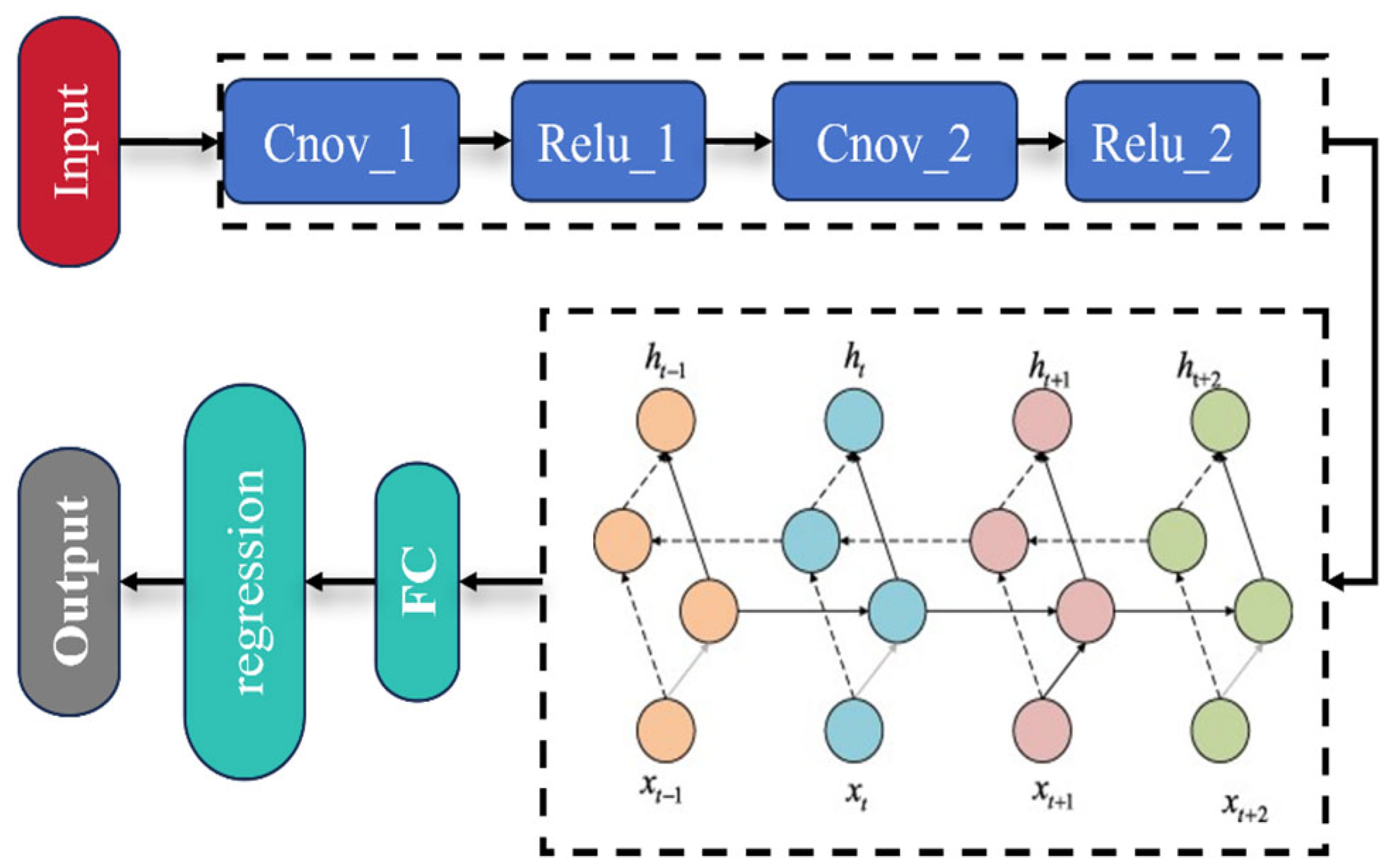
2.5.2. Convolutional Neural Network—Bidirectional Gated Recurrent Unit Module
2.5.3. Dataset Partitioning and Model Evaluation
3. Results
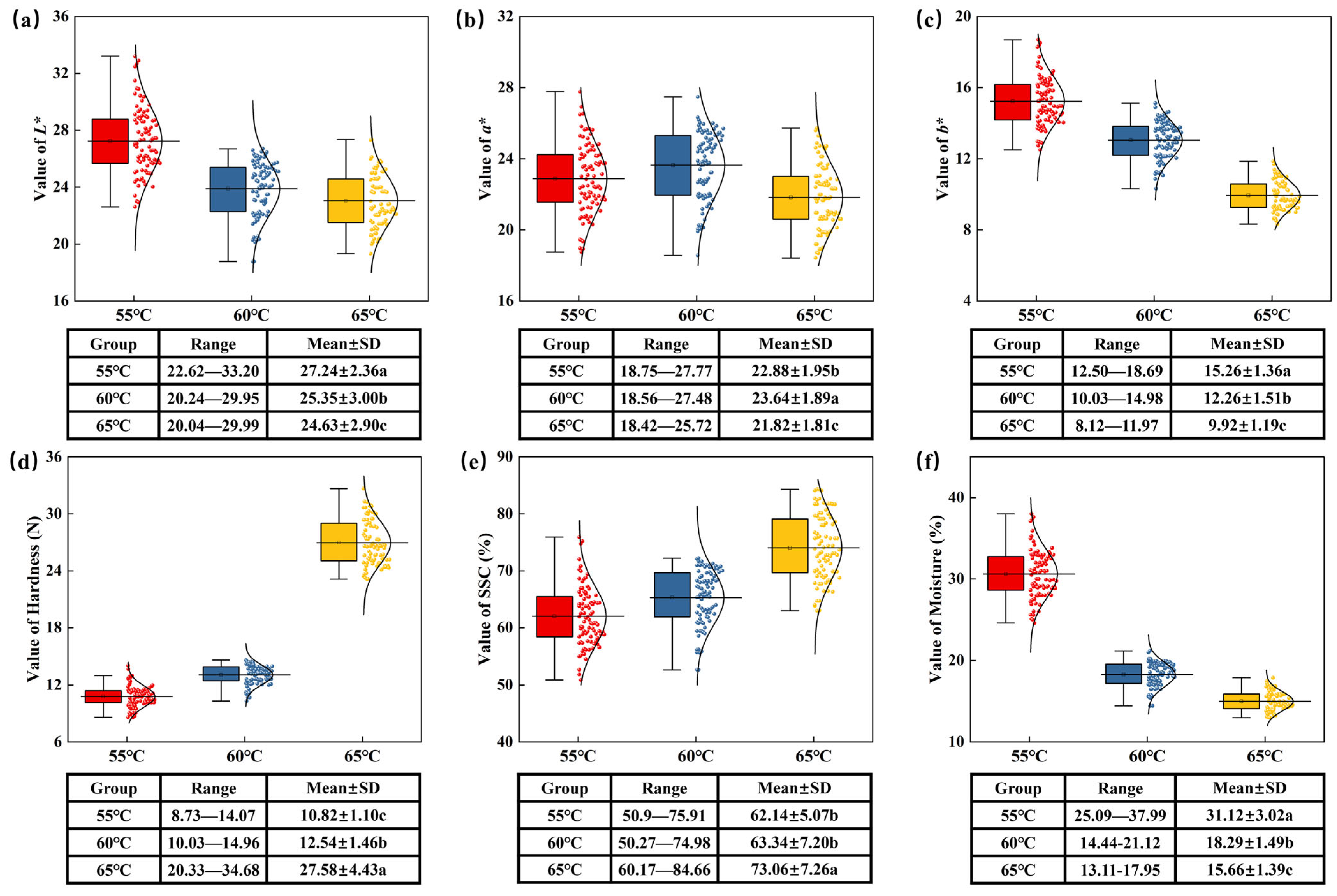
3.1. Statistics of Dired Jujube Quality Parameters
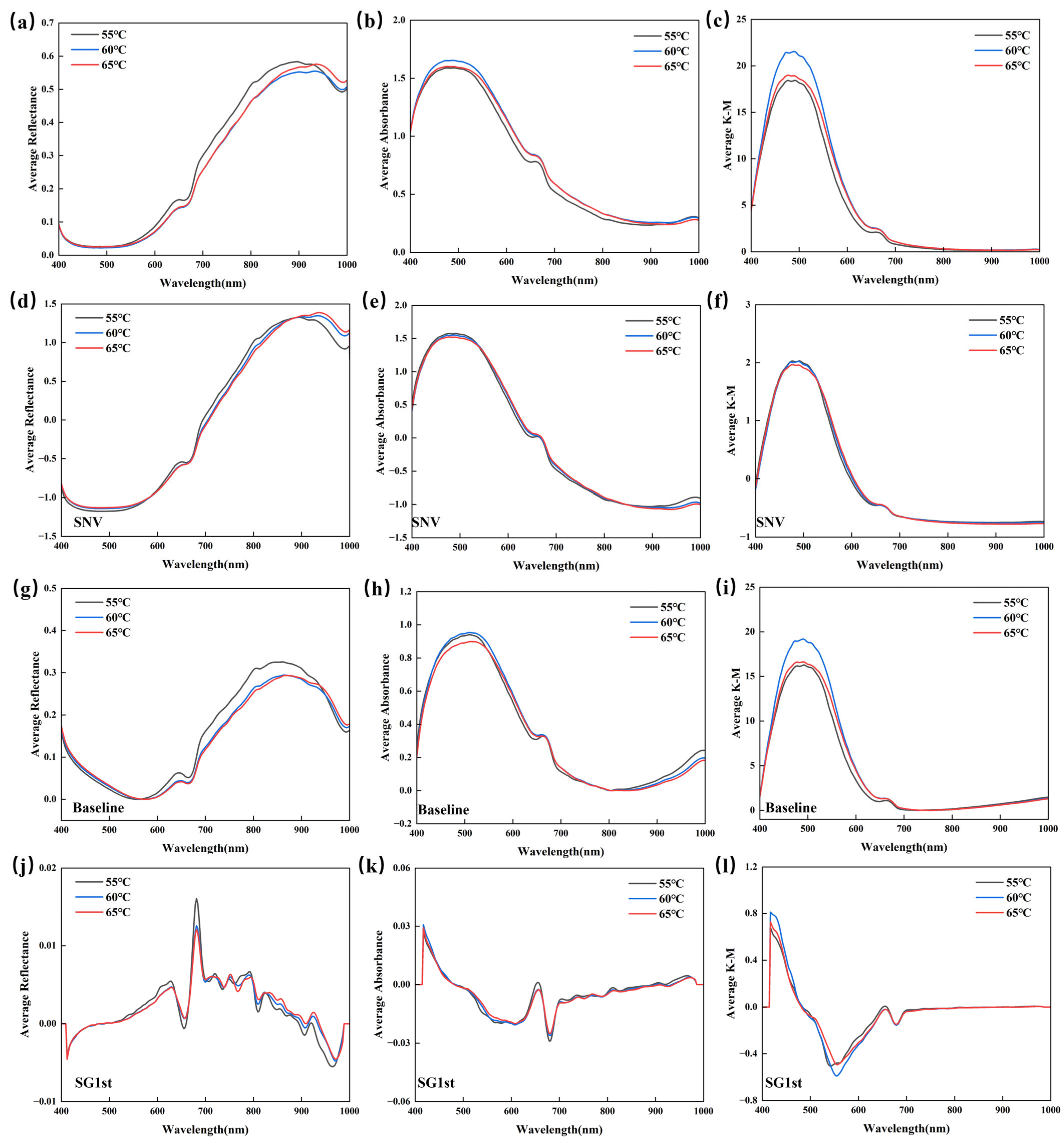
3.2. Spectral Characteristics
3.3. SVR Modeling Results of Different Preprocessing for R, A, and K-M Spectra
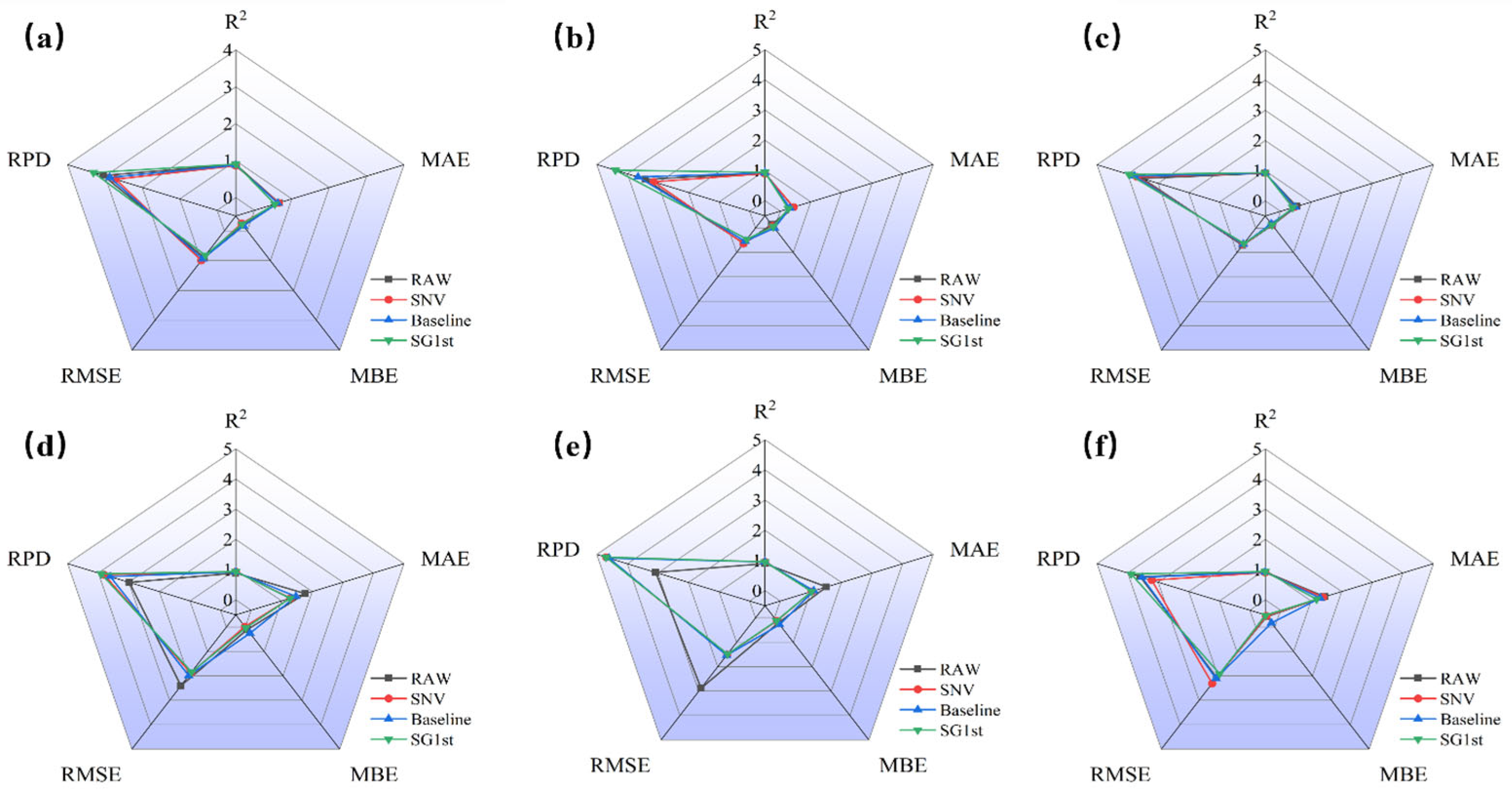
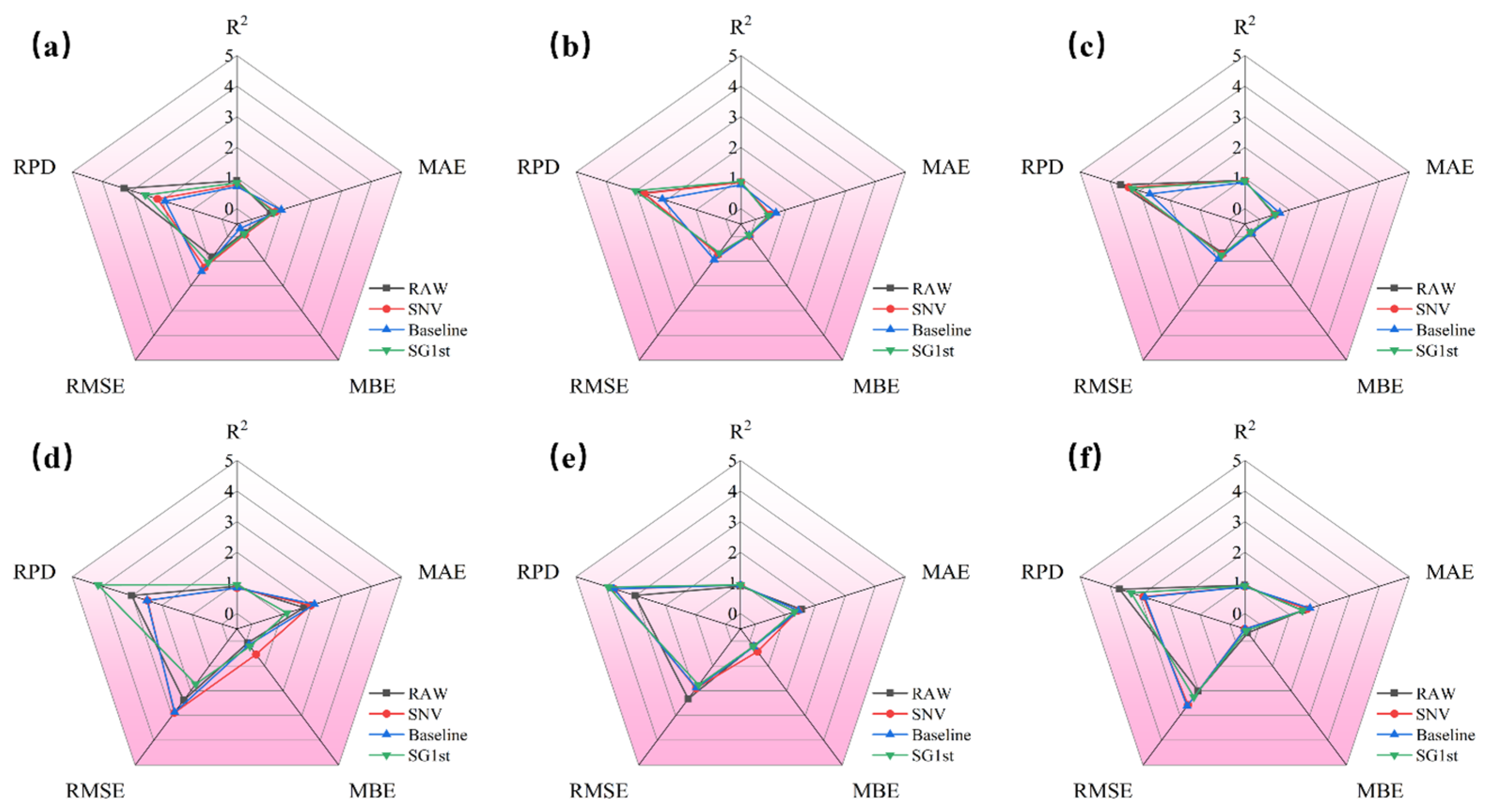
3.3.1. SVR Modeling Results of Different Preprocessing for R Spectra
3.3.2. SVR Modeling Results of Different Preprocessing for A Spectra
3.3.3. SVR Modeling Results of Different Preprocessing for K-M Spectra
3.3.4. Best SVR Modeling Results of Different Preprocessing
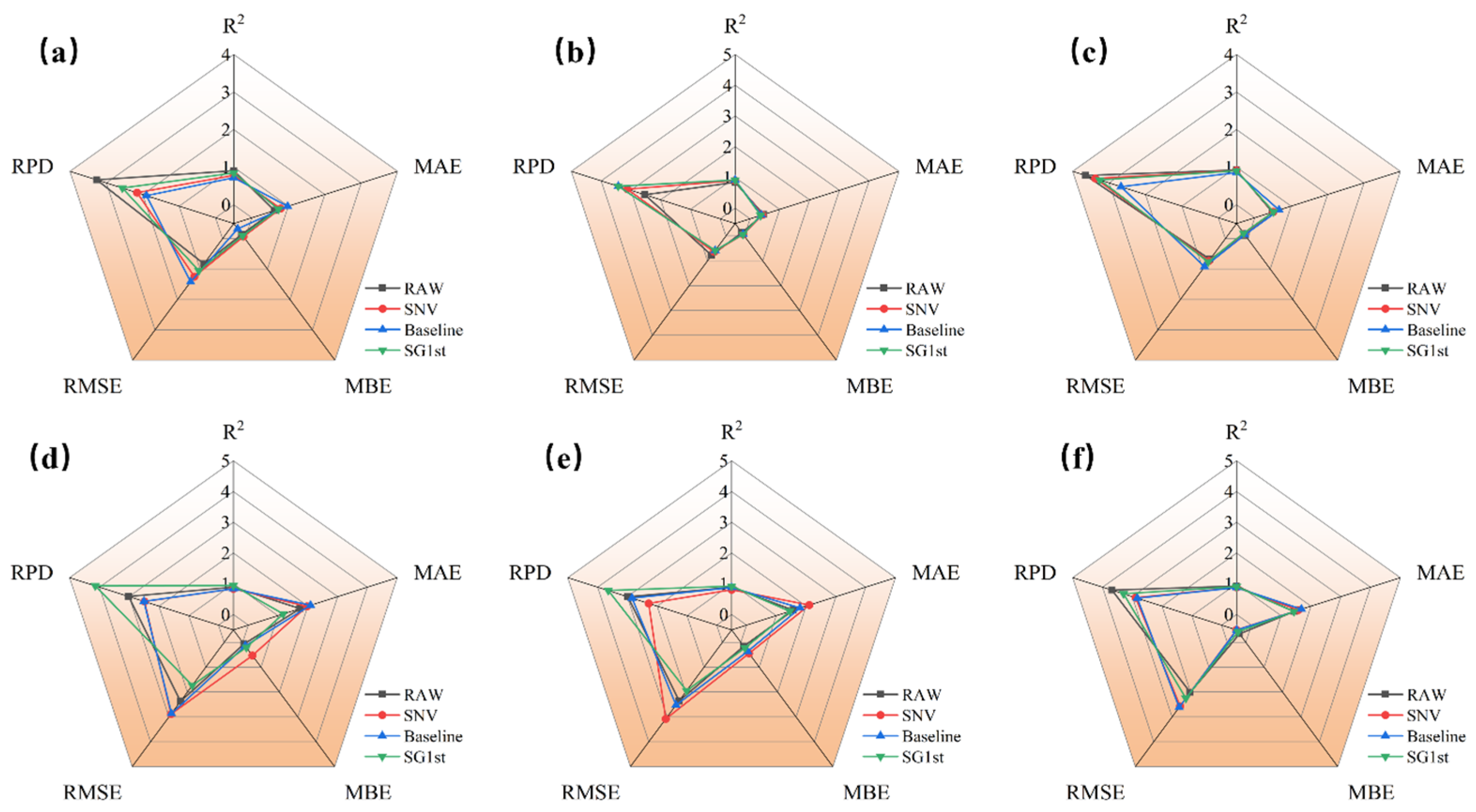
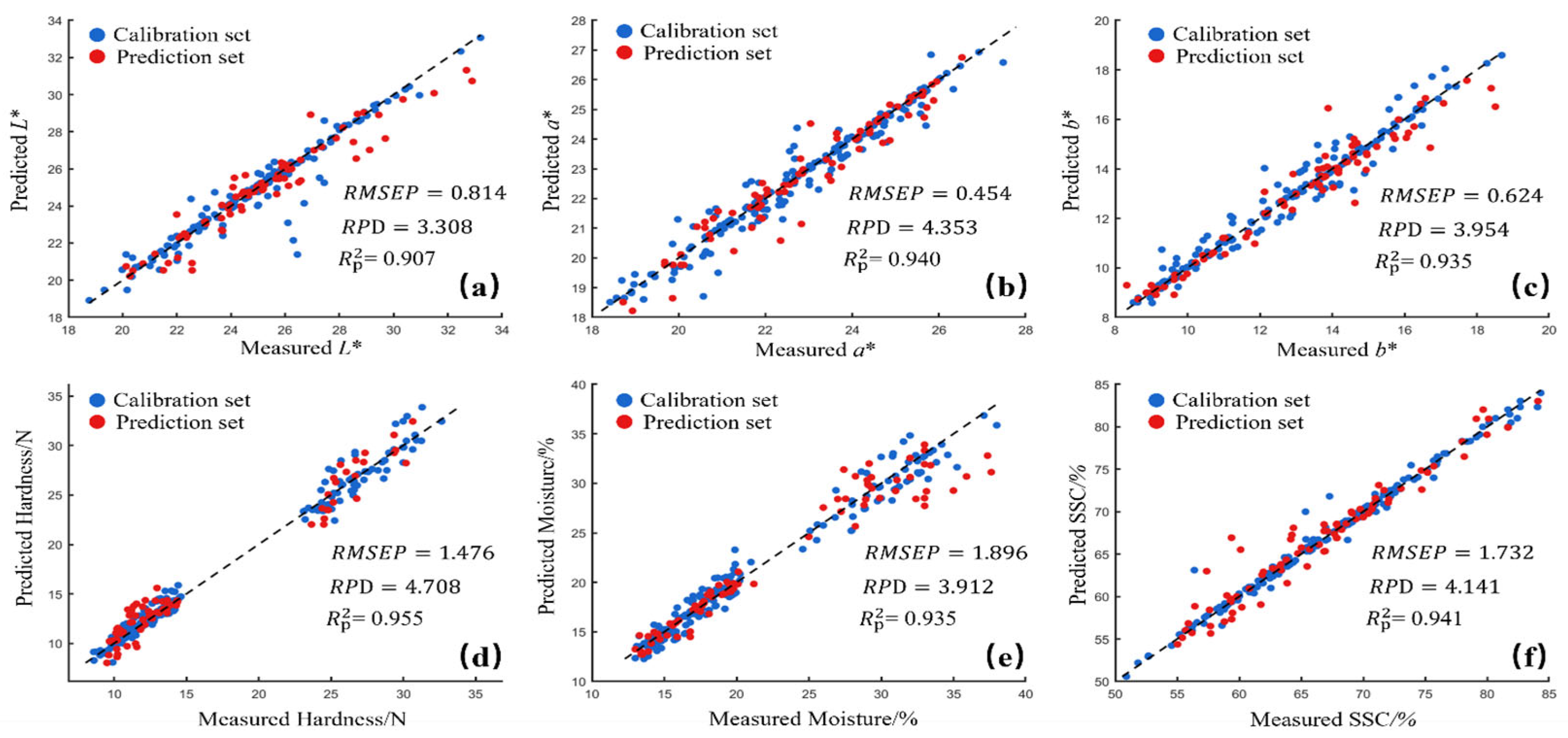
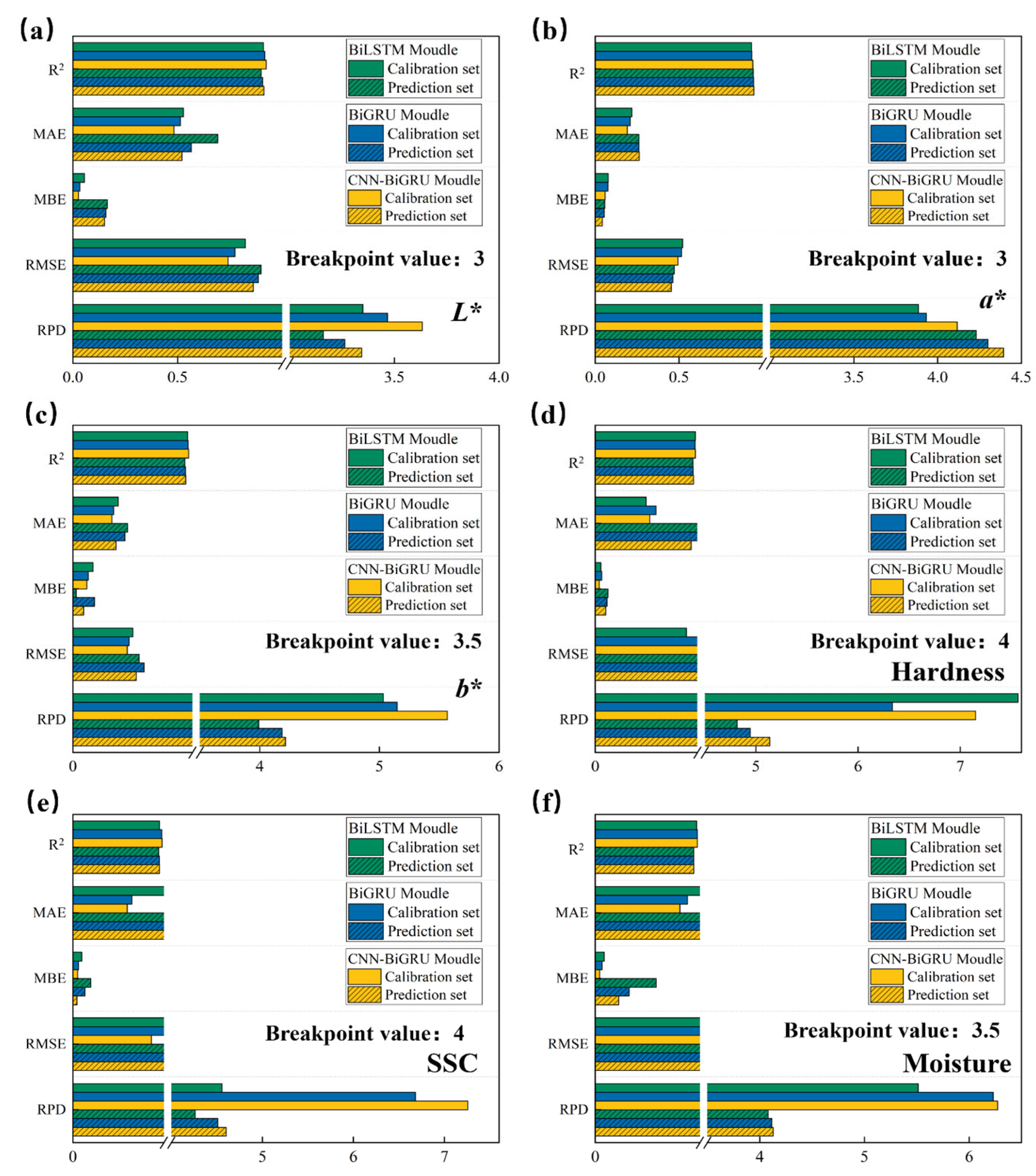
3.4. Result of Optimal Preprocessing on Quality Parameter Modeling in Deep Learning
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Z.; Wu, X.; Jayan, H.; Yin, L.; Xue, S.; El-Seedi, H.R.; Zou, X. Recent developments and applications of surface enhanced Raman scattering spectroscopy in safety detection of fruits and vegetables. Food Chem. 2024, 434, 137469. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liang, C.; Bao, F.; Zhang, T.; Cheng, Y.; Zhang, W.; Lu, Y. Chemometric analysis illuminates the relationship among browning, polyphenol degradation, Maillard reaction and flavor variation of 5 jujube fruits during air-impingement jet drying. Food Chem. X 2024, 22, 101425. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, A.K.; Karim, N.; Shishir, M.R.I.; Bao, T.; Lu, Y.; Chen, W. Jujube fruit: A potential nutritious fruit for the development of functional food products. J. Funct. Foods 2020, 75, 104205. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Fan, S.; Jiang, X.; Chen, Y.; Wang, F.; Cao, X.; Yan, L. Development of a predictive model for assessing quality of winter jujube during storage utilizing hyperspectral imaging technology. J. Food Process Eng. 2024, 47, e14688. [Google Scholar] [CrossRef]
- Song, J.; Jin, X.; Han, Y.; Zhai, S.; Zhang, K.; Jia, W.; Chen, J. Infrared drying effects on the quality of jujube and process optimization using response surface methodology. LWT 2024, 214, 117089. [Google Scholar] [CrossRef]
- Mao, J.; Sun, H.; Cao, L.; Deng, K.; Xu, J.; Fu, J.; Chen, Y.; Xie, H. Comparative analysis of the quality characteristics of high-moisture jujube with different degrees of dehydration. LWT 2024, 198, 116026. [Google Scholar] [CrossRef]
- Asghar, Z.; Amin, M.; Ullah, S.; Hassan, H.; Habiba, U.; Razzaq, K.; Rajwana, I.A.; Akhtar, G.; Faried, H.N.; Aiyub, M.; et al. Antioxidative enzymes, phytochemicals and proximate components in jujube fruit (Ziziphus mauritiana L.) with respect to genotypes and harvest maturity. Food Biosci. 2024, 60, 104504. [Google Scholar] [CrossRef]
- Song, J.; Han, J.; Fu, L.; Shang, H.; Yang, L. Assessment of characteristics aroma of heat pump drying (HPD) jujube based on HS-SPME/GC–MS and e-nose. J. Food Compos. Anal. 2022, 110, 104402. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Legua, P.; Lech, K.; Carbonell-Barrachina, Á.A.; Hernández, F. Chemical composition, antioxidant capacity, and sensory quality of dried jujube fruits as affected by cultivar and drying method. Food Chem. 2016, 207, 170–179. [Google Scholar] [CrossRef]
- Oladejo, A.O.; Gruber, S.; Foerst, P. Applications of non-invasive measuring techniques of internal changes during drying of food products. J. Food Eng. 2025, 396, 112558. [Google Scholar] [CrossRef]
- Ding, H.; Yang, C.; Cao, S.; Gu, J.; Li, Y.; Zang, Y.; Yao, X.; Zhu, R.; Wang, Q.; Dong, W.; et al. Prediction models of texture attributes for radiofrequency assisted hot air drying of jujube slices based on morphological parameters. LWT 2023, 190, 115527. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Wang, C.; Liu, X.; El-Seedi, H.R.; Gómez, P.L.; Alzamora, S.M.; Zou, X.; Guo, Z. Enhanced composite Co-MOF-derived sodium carboxymethyl cellulose visual films for real-time and in situ monitoring fresh-cut apple freshness. Food Hydrocoll. 2024, 157, 110475. [Google Scholar] [CrossRef]
- Dou, J.-F.; Kou, X.-H.; Wu, C.-E.; Fan, G.-J.; Li, T.-T.; Li, X.-J.; Zhou, D.-D.; Yan, Z.-C.; Zhu, J.-P. Recent advances and development of postharvest management research for fresh jujube fruit: A review. Sci. Hortic. 2023, 310, 111769. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Mohanty, P.; Sahu, J.K.; Sahu, J.N. A critical review on drying of food materials: Recent progress and key challenges. Int. Commun. Heat Mass Transf. 2024, 158, 107863. [Google Scholar] [CrossRef]
- Liu, L.; Zareef, M.; Wang, Z.; Li, H.; Chen, Q.; Ouyang, Q. Monitoring chlorophyll changes during Tencha processing using portable near-infrared spectroscopy. Food Chem. 2023, 412, 135505. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, G.; He, J.; Wan, G.; Fan, N.; Li, Y.; Sun, Y. Classification of Lingwu long jujube internal bruise over time based on visible near-infrared hyperspectral imaging combined with partial least squares-discriminant analysis. Comput. Electron. Agric. 2021, 182, 106043. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Zhu, S.; Li, Y.; He, Y.; Liu, F. Shape induced reflectance correction for non-destructive determination and visualization of soluble solids content in winter jujubes using hyperspectral imaging in two different spectral ranges. Postharvest Biol. Technol. 2020, 161, 111080. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Q.; Yan, L.; Cao, X.; Chen, Y.; Wei, Y.; Wang, F.; Xing, H. Hyperspectral imaging combined with spectral-imagery feature fusion convolutional neural network to discriminate different geographical origins of wolfberries. J. Food Compos. Anal. 2024, 132, 106259. [Google Scholar] [CrossRef]
- Tan, F.; Mo, X.; Ruan, S.; Yan, T.; Xing, P.; Gao, P.; Xu, W.; Ye, W.; Li, Y.; Gao, X.; et al. Combining Vis-NIR and NIR Spectral Imaging Techniques with Data Fusion for Rapid and Nondestructive Multi-Quality Detection of Cherry Tomatoes. Foods 2023, 12, 3621. [Google Scholar] [CrossRef]
- Wei, Y.; Yuan, M.; Hu, H.; Xu, H.; Mao, X. Estimation for soluble solid content in Hetian jujube using hyperspectral imaging with fused spectral and textural Features. J. Food Compos. Anal. 2024, 128, 106079. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, X.; Wang, F.; Zhu, B.; Yan, L.; Wei, Y.; Chen, Y. Detection of dried jujube from fresh jujube with different variety and maturity after hot air drying based on hyperspectral imaging technology. J. Food Compos. Anal. 2024, 133, 106378. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.; Yang, H.; Jiang, H.; Li, L.; Yang, G. Non-destructive and in-site estimation of apple quality and maturity by hyperspectral imaging. Comput. Electron. Agric. 2022, 195, 106843. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, J.; Huang, H.; Hu, X.; Han, L.; Huang, D.; Luo, H. Nondestructive visualization and quantification of total acid and reducing sugar contents in fermented grains by combining spectral and color data through hyperspectral imaging. Food Chem. 2022, 386, 132779. [Google Scholar] [CrossRef]
- Günaydın, S.; Çetin, N.; Sağlam, C.; Sacilik, K.; Jahanbakhshi, A. Comparative analysis of visible and near-infrared (Vis-NIR) spectroscopy and prediction of moisture ratio using machine learning algorithms for jujube dried under different conditions. Appl. Food Res. 2025, 5, 100699. [Google Scholar] [CrossRef]
- Yang, C.; Guo, Z.; Fernandes Barbin, D.; Dai, Z.; Watson, N.; Povey, M.; Zou, X. Hyperspectral Imaging and Deep Learning for Quality and Safety Inspection of Fruits and Vegetables: A Review. J. Agric. Food Chem. 2025, 73, 10019–10035. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, X.; Liu, Q.; Wang, F.; Fan, S.; Yan, L.; Wei, Y.; Chen, Y.; Yang, G.; Xu, B.; et al. Prediction of multi-task physicochemical indices based on hyperspectral imaging and analysis of the relationship between physicochemical composition and sensory quality of tea. Food Res. Int. 2025, 211, 116455. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, X.; Wang, F.; Fan, S.; Zhu, B.; Yan, L.; Chen, Y.; Wei, Y.; Chen, W. Evaluation and process monitoring of jujube hot air drying using hyperspectral imaging technology and deep learning for quality parameters. Food Chem. 2025, 467, 141999. [Google Scholar] [CrossRef]
- Li, X.; Zhou, S.; Wang, F. A CNN-BiGRU sea level height prediction model combined with bayesian optimization algorithm. Ocean Eng. 2025, 315, 119849. [Google Scholar] [CrossRef]
- Ma, H.; Peng, T.; Zhang, C.; Ji, C.; Li, Y.; Nazir, M.S. Developing an evolutionary deep learning framework with random forest feature selection and improved flow direction algorithm for NOx concentration prediction. Eng. Appl. Artif. Intell. 2023, 123, 106367. [Google Scholar] [CrossRef]
- Aldhanhani, A.R.H.; Ahmed, Z.F.R.; Tzortzakis, N.; Singh, Z. Maturity stage at harvest influences antioxidant phytochemicals and antibacterial activity of jujube fruit (Ziziphus mauritiana Lamk. and Ziziphus spina-christi L.). Ann. Agric. Sci. 2022, 67, 196–203. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Li, Y.; Ma, B.; Li, C.; Yu, G. Accurate prediction of soluble solid content in dried Hami jujube using SWIR hyperspectral imaging with comparative analysis of models. Comput. Electron. Agric. 2022, 193, 106655. [Google Scholar] [CrossRef]
- Yuan, R.; Guo, M.; Li, C.; Chen, S.; Liu, G.; He, J.; Wan, G.; Fan, N. Detection of early bruises in jujubes based on reflectance, absorbance and Kubelka-Munk spectral data. Postharvest Biol. Technol. 2022, 185, 111810. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, H.; Chen, M.; Zhang, L.; Cheng, Y.; Geng, W.; Guan, J. Detection of Pear Quality Using Hyperspectral Imaging Technology and Machine Learning Analysis. Foods 2024, 13, 3956. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, Y.; Huang, Y.; Zheng, C.; Liao, S.; Xiao, L.; Zhao, J. Prediction of the Quality of Anxi Tieguanyin Based on Hyperspectral Detection Technology. Foods 2024, 13, 4126. [Google Scholar] [CrossRef]
- Ouyang, Q.; Rong, Y.; Wu, J.; Wang, Z.; Lin, H.; Chen, Q. Application of colorimetric sensor array combined with visible near-infrared spectroscopy for the matcha classification. Food Chem. 2023, 420, 136078. [Google Scholar] [CrossRef]
- Amoriello, T.; Ciorba, R.; Ruggiero, G.; Masciola, F.; Scutaru, D.; Ciccoritti, R. Vis/NIR Spectroscopy and Vis/NIR Hyperspectral Imaging for Non-Destructive Monitoring of Apricot Fruit Internal Quality with Machine Learning. Foods 2025, 14, 196. [Google Scholar] [CrossRef]
- Tamburini, E.; Vincenzi, F.; Costa, S.; Mantovi, P.; Pedrini, P.; Castaldelli, G. Effects of Moisture and Particle Size on Quantitative Determination of Total Organic Carbon (TOC) in Soils Using Near-Infrared Spectroscopy. Sensors 2017, 17, 2366. [Google Scholar] [CrossRef] [PubMed]
- Duckena, L.; Alksnis, R.; Erdberga, I.; Alsina, I.; Dubova, L.; Duma, M. Non-Destructive Quality Evaluation of 80 Tomato Varieties Using Vis-NIR Spectroscopy. Foods 2023, 12, 1990. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Bai, T.; Wu, C.; He, Y.; Huang, J.; Li, X.; Shi, Z.; Hou, K. Variety classification and identification of jujube based on near-infrared spectroscopy and 1D-CNN. Comput. Electron. Agric. 2024, 223, 109122. [Google Scholar] [CrossRef]
- Ram, B.G.; Oduor, P.; Igathinathane, C.; Howatt, K.; Sun, X. A systematic review of hyperspectral imaging in precision agriculture: Analysis of its current state and future prospects. Comput. Electron. Agric. 2024, 222, 109037. [Google Scholar] [CrossRef]
- Zozio, S.; Servent, A.; Cazal, G.; Mbéguié-A-Mbéguié, D.; Ravion, S.; Pallet, D.; Abel, H. Changes in antioxidant activity during the ripening of jujube (Ziziphus mauritiana Lamk). Food Chem. 2014, 150, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, X.; Liu, Q.; Wei, Y.; Wang, F.; Yan, L.; Zhao, J.; Cao, X.; Xing, H. A hyperspectral imaging technique for rapid non-destructive detection of soluble solid content and firmness of wolfberry. Food Meas. 2024, 18, 7927–7941. [Google Scholar] [CrossRef]
- Cozzolino, D.; Williams, P.J.; Hoffman, L.C. An overview of pre-processing methods available for hyperspectral imaging applications. Microchem. J. 2023, 193, 109129. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Wang, J.; Liu, Y.; Jayan, H.; El-Seedi, H.R.; Alzamora, S.M.; Gómez, P.L.; Zou, X. Detection model transfer of apple soluble solids content based on NIR spectroscopy and deep learning. Comput. Electron. Agric. 2023, 212, 108127. [Google Scholar] [CrossRef]
- Ouyang, Q.; Fan, Z.; Chang, H.; Shoaib, M.; Chen, Q. Analyzing TVB-N in snakehead by Bayesian-optimized 1D-CNN using molecular vibrational spectroscopic techniques: Near-infrared and Raman spectroscopy. Food Chem. 2025, 464, 141701. [Google Scholar] [CrossRef]

| Parameters | Range | Parameters | Range |
|---|---|---|---|
| Wavelength range | 400–1000 nm | Variables | 228 |
| Spectral resolution | 4.69 nm | Dynamic range | 12 bit |
| Speed | 23.2 s/cube | Exposure time | 10 -> 103 ms |
| Pixels per line | 520 | Lines per cube | 696 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yu, C.; Ma, Y.; Zhang, H.; Yan, L.; Fan, S. Prediction of Key Quality Parameters in Hot Air-Dried Jujubes Based on Hyperspectral Imaging. Foods 2025, 14, 1855. https://doi.org/10.3390/foods14111855
Liu Q, Yu C, Ma Y, Zhang H, Yan L, Fan S. Prediction of Key Quality Parameters in Hot Air-Dried Jujubes Based on Hyperspectral Imaging. Foods. 2025; 14(11):1855. https://doi.org/10.3390/foods14111855
Chicago/Turabian StyleLiu, Quancheng, Chunzhan Yu, Yuxuan Ma, Hongwei Zhang, Lei Yan, and Shuxiang Fan. 2025. "Prediction of Key Quality Parameters in Hot Air-Dried Jujubes Based on Hyperspectral Imaging" Foods 14, no. 11: 1855. https://doi.org/10.3390/foods14111855
APA StyleLiu, Q., Yu, C., Ma, Y., Zhang, H., Yan, L., & Fan, S. (2025). Prediction of Key Quality Parameters in Hot Air-Dried Jujubes Based on Hyperspectral Imaging. Foods, 14(11), 1855. https://doi.org/10.3390/foods14111855






