High-Fat Diet-Induced Mild Obesity Alters the Activation of T Cells and Maintains Intestinal Homeostasis in Food Allergy Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Feeding
2.2. Experimental BLG-Induced Food Allergy Animal Model
2.3. Serum Lipids Detection
2.4. Serum Biomarker Quantification
2.5. Cell Stimulation and Cytokines Detection
2.6. T Cell Flow Cytometry Analyses
2.7. Tissue Staining and Observation
2.8. RNA Extraction and Analysis
2.9. Statistical Analyses
3. Results
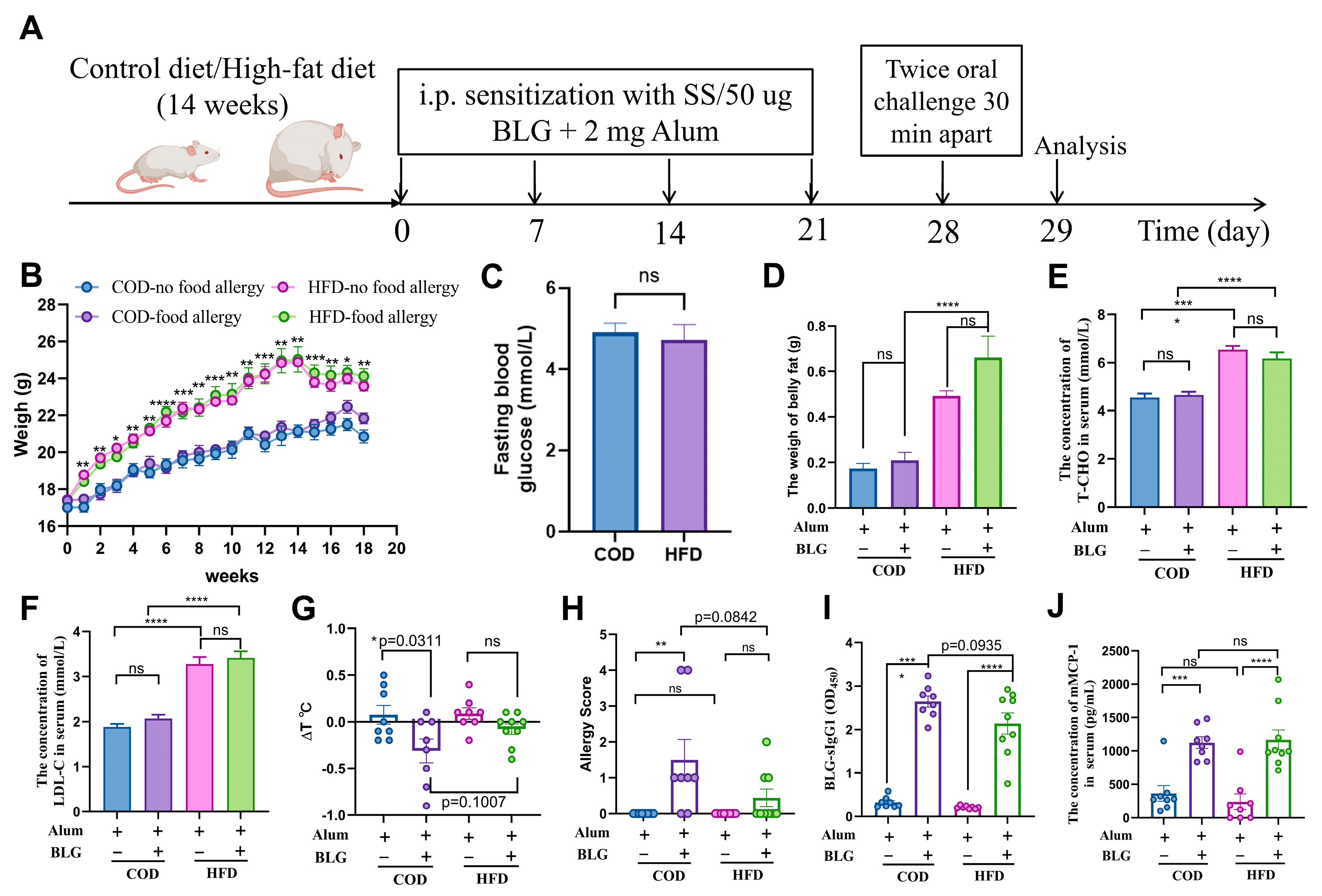
3.1. HFD-Induced Mild Obesity Slightly Alleviates BLG-Induced Allergic Response in Female BALB/c Mice
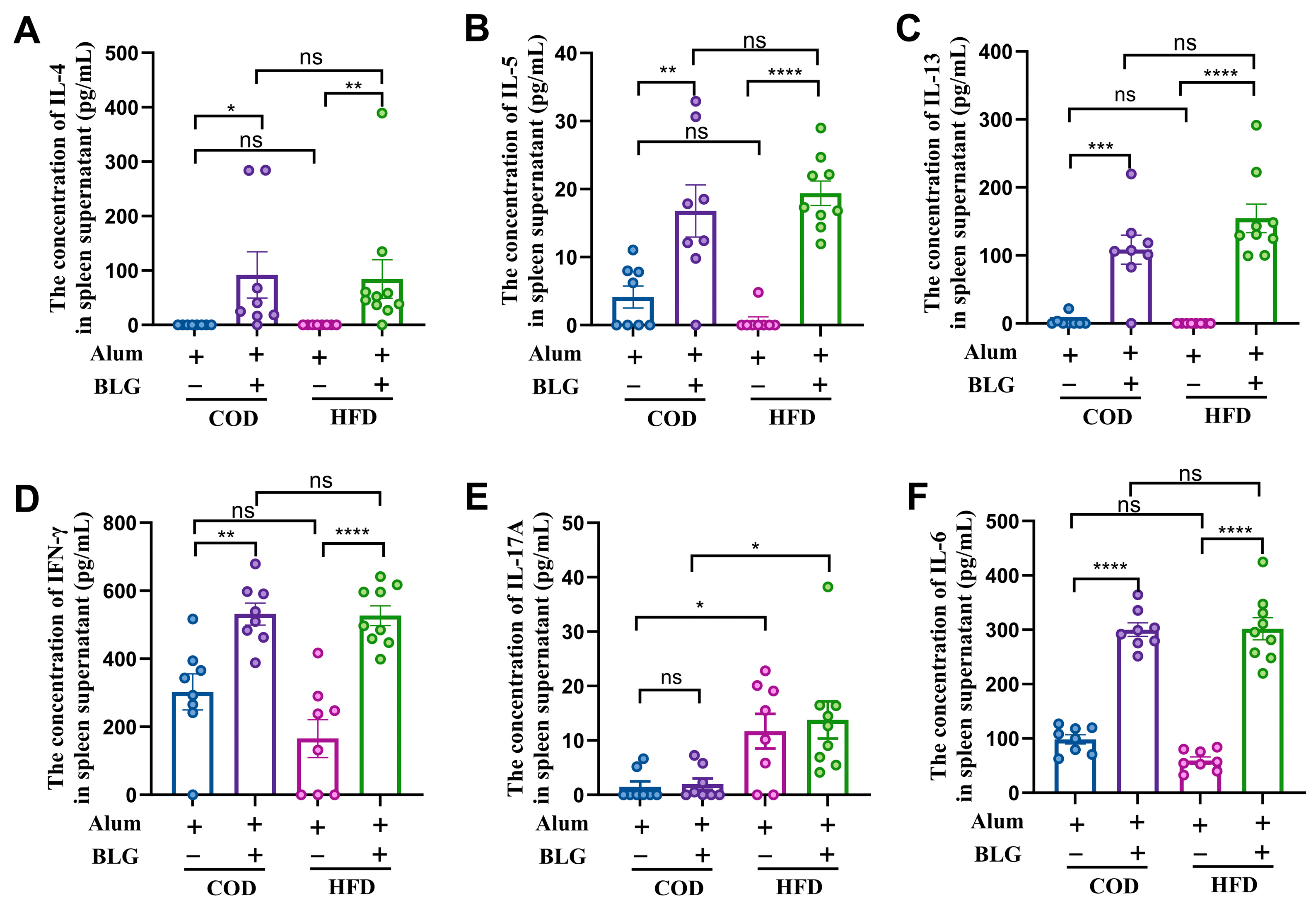
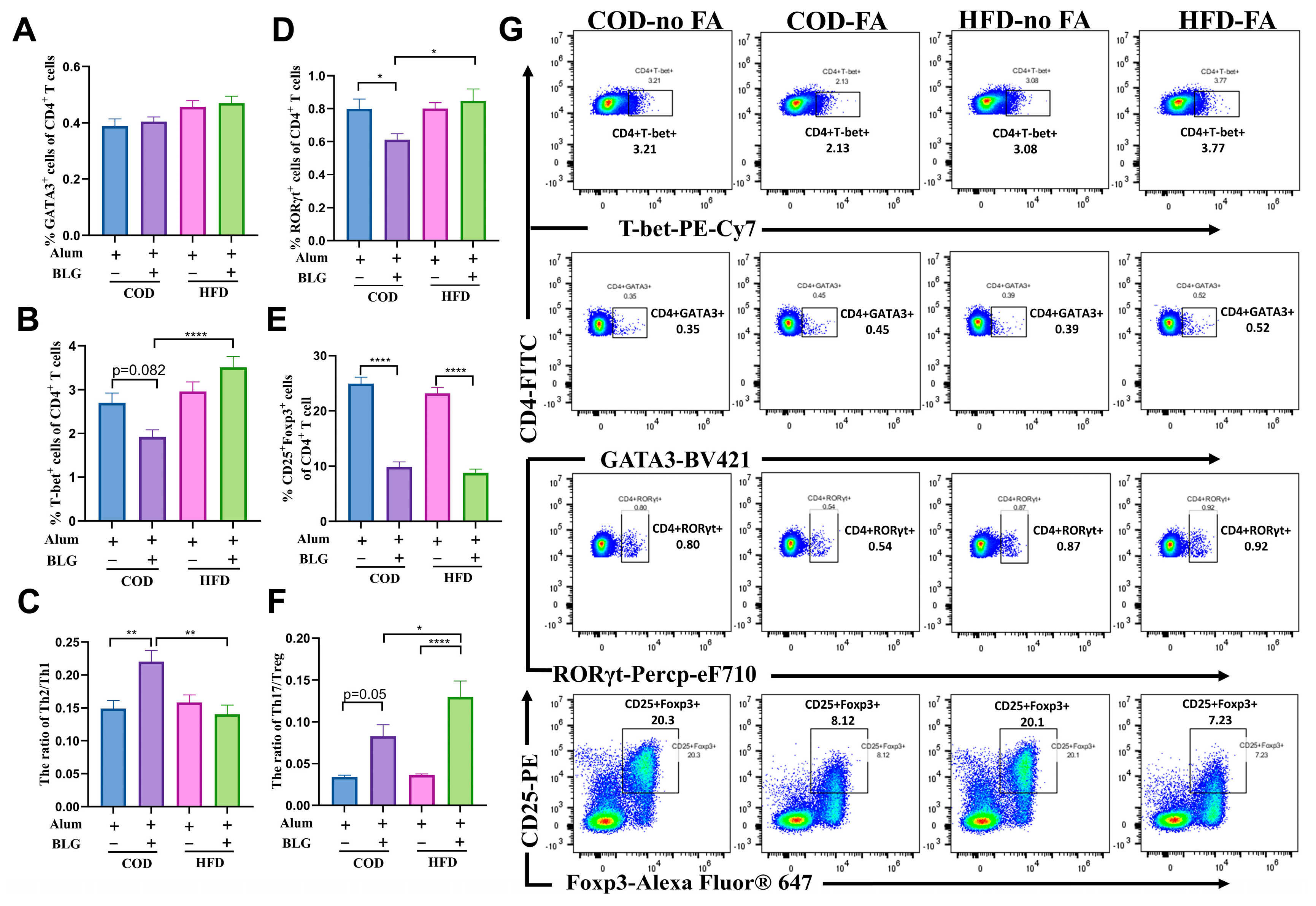
3.2. HFD Maintains Th2/Th1 Balance and Activates Th17 Cells in the Spleen
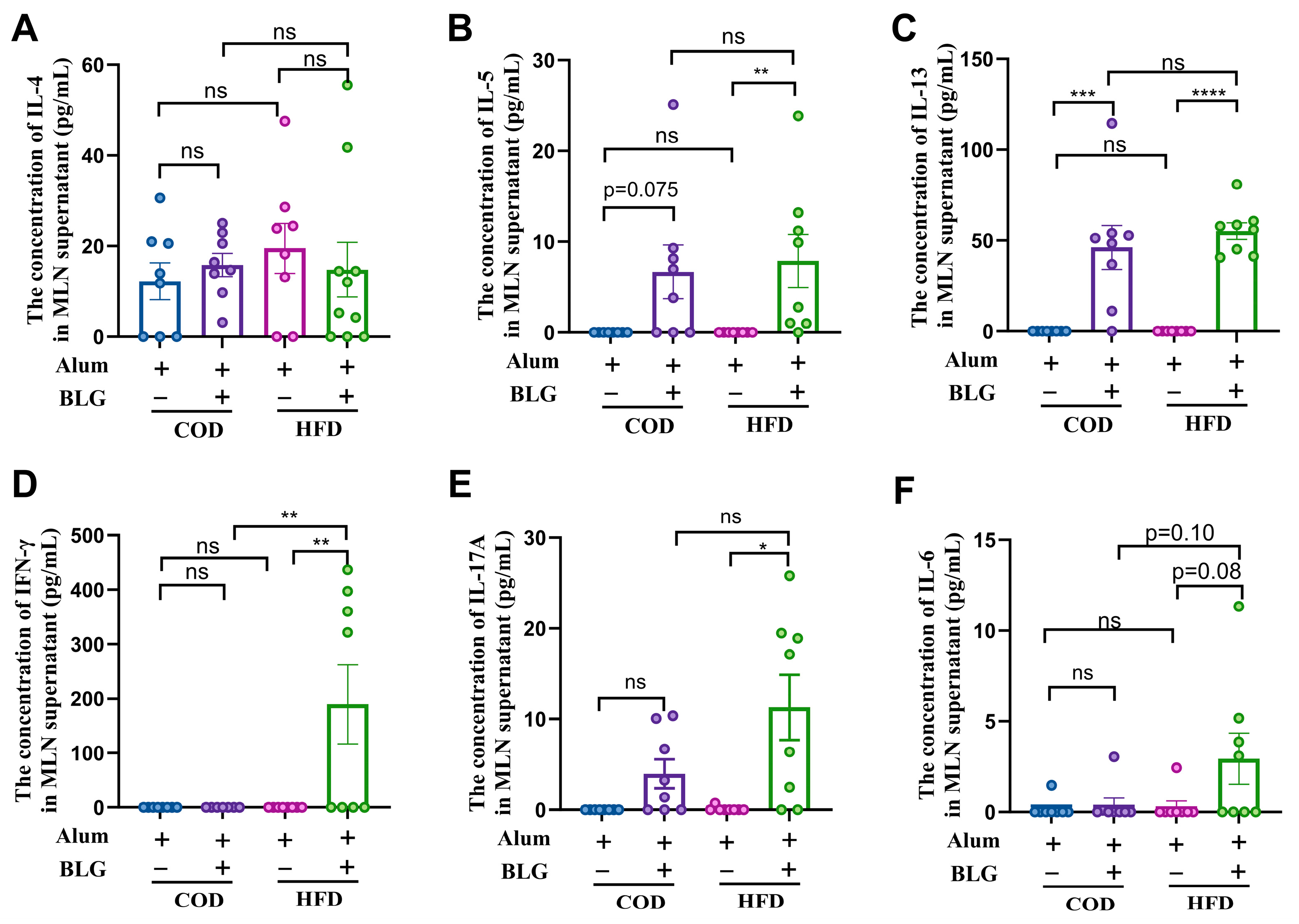
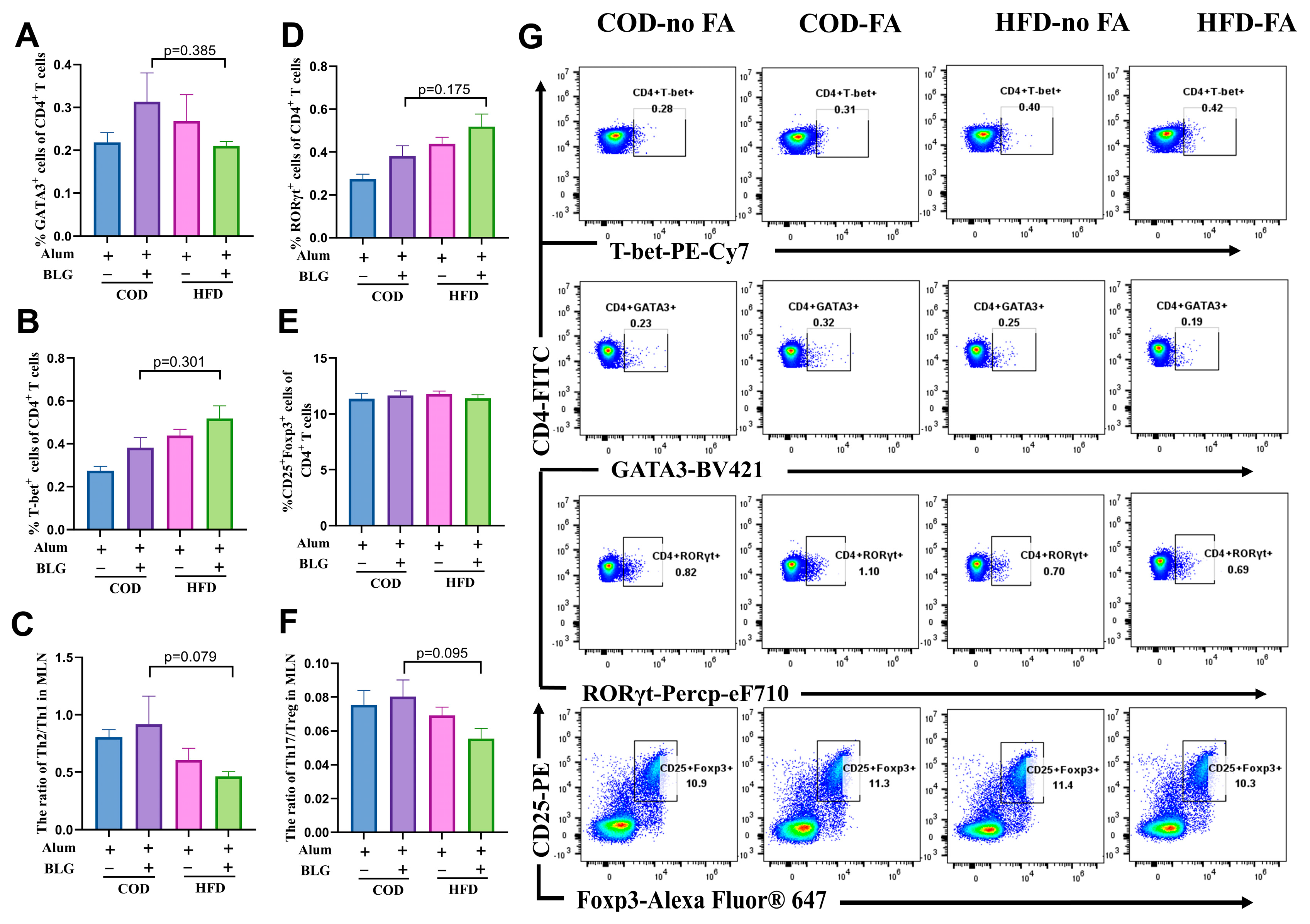
3.3. HFD Does Not Alter CD4+ T Cell Activation in the MLN
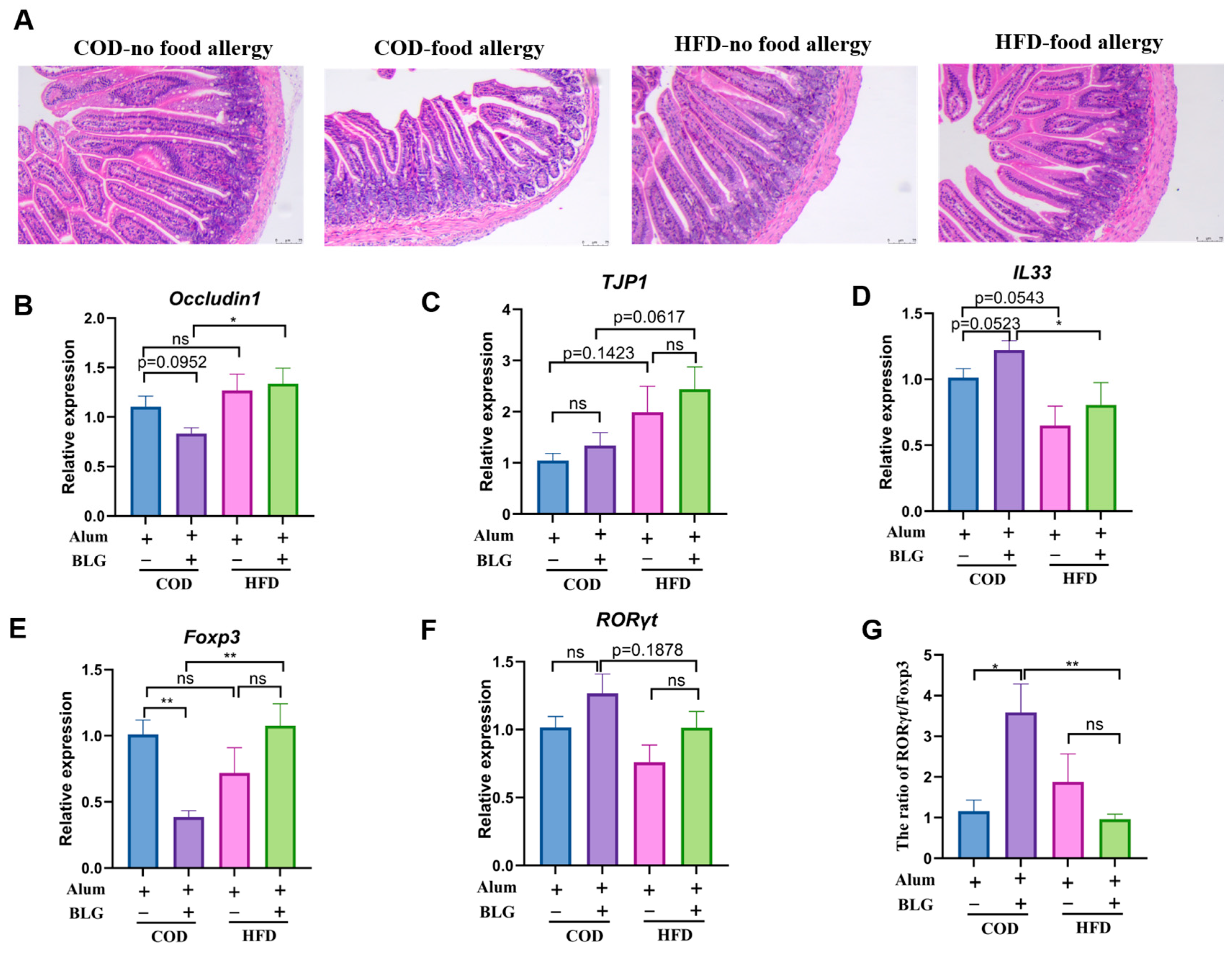
3.4. HFD Regulates Th17/Treg Balance in the Small Intestine and Contributes to Intestinal Homeostasis
4. Discussion
5. Conclusions
6. Limitations of This Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food allergy across the globe. J. Allergy Clin. Immunol. 2021, 148, 1347–1364. [Google Scholar] [CrossRef]
- Baseggio Conrado, A.; Ierodiakonou, D.; Gowland, M.H.; Boyle, R.J.; Turner, P.J. Food anaphylaxis in the United Kingdom: Analysis of national data, 1998–2018. BMJ 2021, 372, n251. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, L.; Xian, R.; Fang, H.; Wang, J.; Hu, Y.; Eigenmann, P. Time trends of childhood food allergy in China: Three cross-sectional surveys in 1999, 2009, and 2019. Pediatr. Allergy Immunol. 2021, 32, 1073–1079. [Google Scholar] [CrossRef]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. An evolutionary perspective on intestinal lymphatic fat absorption, the industrialization of food, and allergy. Ann. Allergy Asthma Immunol. 2014, 113, 339–342. [Google Scholar] [CrossRef]
- Feng, H.; Liu, Y.; Xiong, X.; Xu, Q.; Zhang, Z.; Wu, Y.; Lu, Y. Epidemiological survey of self-reported food allergy among university students in China. Medicine 2022, 101, e29606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Hussain, M.; Bonilla-Rosso, G.; Kwong Chung, C.K.C.; Bäriswyl, L.; Rodriguez, M.P.; Kim, B.S.; Engel, P.; Noti, M. High dietary fat intake induces a microbiota signature that promotes food allergy. J. Allergy Clin. Immunol. 2019, 144, 157–170.e158. [Google Scholar] [CrossRef]
- Tanaka, S.; Nemoto, Y.; Takei, Y.; Morikawa, R.; Oshima, S.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; Stutte, S.; et al. High-fat diet-derived free fatty acids impair the intestinal immune system and increase sensitivity to intestinal epithelial damage. Biochem. Biophys. Res. Commun. 2020, 522, 971–977. [Google Scholar] [CrossRef]
- Myles, I.A. Fast food fever: Reviewing the impacts of the Western diet on immunity. Nutr. J. 2014, 13, 61. [Google Scholar] [CrossRef]
- Hogenkamp, A.; van Vlies, N.; Fear, A.L.; van Esch, B.C.; Hofman, G.A.; Garssen, J.; Calder, P.C. Dietary fatty acids affect the immune system in male mice sensitized to ovalbumin or vaccinated with influenza. J. Nutr. 2011, 141, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Harbige, L.; Layward, L.; Morris-Downes, M.; Dumonde, D.; Amor, S. The protective effects of omega-6 fatty acids in experimental autoimmune encephalomyelitis (EAE) in relation to transforming growth factor-beta 1 (TGF-β 1) up-regulation and increased prostaglandin E2 (PGE2) production. Clin. Exp. Immunol. 2000, 122, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.-S.; Zhang, P.; Xie, R.-J.; Wang, M.; Wang, Z.-Y.; Zhou, Z.; Zhai, W.-J.; Feng, S.-Z.; Han, M.-Z. The regulation of CD4+ T cell immune responses toward Th2 cell development by prostaglandin E2. Int. Immunopharmacol. 2011, 11, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Tomicić, S.; Fälth-Magnusson, K.; Böttcher, M.F. Dysregulated Th1 and Th2 responses in food-allergic children--does elimination diet contribute to the dysregulation? Pediatr. Allergy Immunol. 2010, 21, 649–655. [Google Scholar] [CrossRef]
- Schmidt, V.; Hogan, A.E.; Fallon, P.G.; Schwartz, C. Obesity-Mediated Immune Modulation: One Step Forward, (Th)2 Steps Back. Front. Immunol. 2022, 13, 932893. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Zhang, Y.; Zhao, P.; Zhang, X. Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. J. Ethnopharmacol. 2017, 208, 199–206. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Yu, Q.; Shen, S.; Gao, Y.; Lin, X.; Zhang, W. Piper nigrum extract attenuates food allergy by decreasing Th2 cell response and regulating the Th17/Treg balance. Phytother. Res. 2021, 35, 3214–3225. [Google Scholar] [CrossRef]
- Żbikowska-Gotz, M.; Pałgan, K.; Gawrońska-Ukleja, E.; Kuźmiński, A.; Przybyszewski, M.; Socha, E.; Bartuzi, Z. Expression of IL-17A concentration and effector functions of peripheral blood neutrophils in food allergy hypersensitivity patients. Int. J. Immunopathol. Pharmacol. 2016, 29, 90–98. [Google Scholar] [CrossRef]
- Winer, S.; Paltser, G.; Chan, Y.; Tsui, H.; Engleman, E.; Winer, D.; Dosch, H.M. Obesity predisposes to Th17 bias. Eur. J. Immunol. 2009, 39, 2629–2635. [Google Scholar] [CrossRef]
- Endo, Y.; Asou Hikari, K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, J.D.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015, 12, 1042–1055. [Google Scholar] [CrossRef]
- Dhuban, K.B.; d’Hennezel, E.; Ben-Shoshan, M.; McCusker, C.; Clarke, A.; Fiset, P.; Mazer, B.; Piccirillo, C.A. Altered T helper 17 responses in children with food allergy. Int. Arch. Allergy Immunol. 2013, 162, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Shao, H.; Zhang, X.; Yang, F.; Wang, J.; Tan, S.; Chen, H.; Li, X. Comparison of cow’s milk allergy models highlighted higher humoral and Th2 immune responses in BALB/c than C3H/HeNCrl mice. Food Chem. Toxicol. 2024, 184, 114315. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, F.; Bai, T.; Wu, Y.; Zheng, S.; Tong, P.; Chen, H.; Li, X. 2’-FL in Dairy Matrices Attenuates Allergic Symptoms in Mice by Reducing BLG Hypersensitivity and Modulating Gut Microecology. J. Agric. Food Chem. 2025, 73, 9606–9617. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, E.; Sugiura, T.; Zeki, K.; Yoshida, Y.; Yamashita, U. Sensitivity difference to the suppressive effect of prostaglandin E2 among mouse strains: A possible mechanism to polarize Th2 type response in BALB/c mice. J. Immunol. 2000, 164, 2386–2395. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Chen, C.; Sun, S.; Liu, G.; Liu, M.; Hao, M.; Che, H. Gender differences in food allergy depend on the PPAR γ/NF-κB in the intestines of mice. Life Sci. 2021, 278, 119606. [Google Scholar] [CrossRef]
- Toyoshima, S.; Wakamatsu, E.; Ishida, Y.; Obata, Y.; Kurashima, Y.; Kiyono, H.; Abe, R. The spleen is the site where mast cells are induced in the development of food allergy. Int. Immunol. 2017, 29, 31–45. [Google Scholar] [CrossRef]
- Yoshida, N.; Comte, D.; Mizui, M.; Otomo, K.; Rosetti, F.; Mayadas, T.N.; Crispín, J.C.; Bradley, S.J.; Koga, T.; Kono, M.; et al. ICER is requisite for Th17 differentiation. Nat. Commun. 2016, 7, 12993. [Google Scholar] [CrossRef]
- Knight, A.K.; Blázquez, A.B.; Zhang, S.; Mayer, L.; Sampson, H.A.; Berin, M.C. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1234–G1243. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.-P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef]
- Lim, J.J.; Reginald, K.; Say, Y.-H.; Liu, M.H.; Chew, F.T. A Dietary Pattern for High Estimated Total Fat Amount Is Associated with Enhanced Allergy Sensitization and Atopic Diseases among Singapore/Malaysia Young Chinese Adults. Int. Arch. Allergy Immunol. 2023, 184, 975–984. [Google Scholar] [CrossRef]
- Yang, F.; Bai, T.; Zhang, X.; Meng, X.; Yuan, J.; Wu, Z.; Yang, A.; Chen, H.; Li, X. High-fat diet-induced obesity accelerates the progression of food allergy through synergistic effect of gut microbiota and lipid metabolism. Food Biosci. 2025, 68, 106588. [Google Scholar] [CrossRef]
- Torres, L.; Camila Gonçalves Miranda, M.; Dantas Martins, V.; Caixeta, F.; de Almeida Oliveira, M.; Martins Trindade, L.; Carvalho de Assis, H.; Nascimento, V.; Pinheiro Rosa, N.; Gomes, E.; et al. Obesity-induced hyperglycemia impairs oral tolerance induction and aggravates food allergy. Mucosal Immunol. 2023, 16, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.I.; Wagner, J.-J.; Goedicke-Fritz, S.; Rogosch, T.; Coccejus, V.; Laudenbach, V.; Nikolaizik, W.; Härtel, C.; Maier, R.F.; Kerzel, S.; et al. TH17 Cell Frequency in Peripheral Blood Is Elevated in Overweight Children without Chronic Inflammatory Diseases. Front. Immunol. 2017, 8, 1543. [Google Scholar] [CrossRef]
- Bapat, S.P.; Whitty, C.; Mowery, C.T.; Liang, Y.; Yoo, A.; Jiang, Z.; Peters, M.C.; Zhang, L.-J.; Vogel, I.; Zhou, C.; et al. Obesity alters pathology and treatment response in inflammatory disease. Nature 2022, 604, 337–342. [Google Scholar] [CrossRef]
- Berin, M.C.; Lozano-Ojalvo, D.; Agashe, C.; Baker, M.G.; Bird, J.A.; Nowak-Wegrzyn, A. Acute FPIES reactions are associated with an IL-17 inflammatory signature. J. Allergy Clin. Immunol. 2021, 148, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yan, J.; Xiang, Q.; Wang, F.; Dai, H.; Huang, K.; Fang, L.; Yao, H.; Wang, L.; Zhang, W. Fructooligosaccharides protect against OVA-induced food allergy in mice by regulating the Th17/Treg cell balance using tryptophan metabolites. Food Funct. 2021, 12, 3191–3205. [Google Scholar] [CrossRef]
- Hong, C.-P.; Park, A.; Yang, B.-G.; Yun, C.H.; Kwak, M.-J.; Lee, G.-W.; Kim, J.-H.; Jang, M.S.; Lee, E.-J.; Jeun, E.-J. Gut-specific delivery of T-helper 17 cells reduces obesity and insulin resistance in mice. Gastroenterology 2017, 152, 1998–2010. [Google Scholar] [CrossRef]
- Sugimura, N.; Otani, K.; Watanabe, T.; Nakatsu, G.; Shimada, S.; Fujimoto, K.; Nadatani, Y.; Hosomi, S.; Tanaka, F.; Kamata, N.; et al. High-fat diet-mediated dysbiosis exacerbates NSAID-induced small intestinal damage through the induction of interleukin-17A. Sci. Rep. 2019, 9, 16796. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Xing, Y.; Sidhu, I.; Subudhi, I.; Mansfield, K.P.; Hsieh, B.; Biancur, D.E.; Larsen, S.B.; Cammer, M.; Li, D.; et al. Interleukin-17 governs hypoxic adaptation of injured epithelium. Science 2022, 377, eabg9302. [Google Scholar] [CrossRef]
- Martínez-López, M.; Iborra, S.; Conde-Garrosa, R.; Mastrangelo, A.; Danne, C.; Mann, E.R.; Reid, D.M.; Gaboriau-Routhiau, V.; Chaparro, M.; Lorenzo, M.P.; et al. Microbiota Sensing by Mincle-Syk Axis in Dendritic Cells Regulates Interleukin-17 and-22 Production and Promotes Intestinal Barrier Integrity. Immunity 2019, 50, 446–461.e449. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Wiatrak, B.; Dzimira, S.; Merwid-Ląd, A.; Szczukowski, Ł.; Świątek, P.; Szeląg, A. Targeting lineage-specific transcription factors and cytokines of the Th17/Treg axis by novel 1, 3, 4-oxadiazole derivatives of pyrrolo [3, 4-d] Pyridazinone attenuates TNBS-induced experimental colitis. Int. J. Mol. Sci. 2022, 23, 9897. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Hasan, A.; Al-Ghimlas, F.; Warsame, S.; Al-Hubail, A.; Ahmad, R.; Bennakhi, A.; Al-Arouj, M.; Behbehani, K.; Dehbi, M.; Dermime, S. IL-33 is negatively associated with the BMI and confers a protective lipid/metabolic profile in non-diabetic but not diabetic subjects. BMC Immunol. 2014, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, N.; Feng, X.; Yang, Y.; Fang, Y.; Zhuang, S.; Dai, Y.; Liu, M.; Tang, L. Circulating levels of IL-33 are elevated by obesity and positively correlated with metabolic disorders in Chinese adults. J. Transl. Med. 2021, 19, 52. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Dwivedi, P.D. Pathophysiology of IL-33 and IL-17 in allergic disorders. Cytokine Growth Factor Rev. 2017, 38, 22–36. [Google Scholar] [CrossRef]
- Appiakannan, H.S.; Rasimowicz, M.L.; Harrison, C.B.; Weber, E.T. Differential effects of high-fat diet on glucose tolerance, food intake, and glucocorticoid regulation in male C57BL/6J and BALB/cJ mice. Physiol. Behav. 2020, 215, 112773. [Google Scholar] [CrossRef]
- Nishikawa, S.; Yasoshima, A.; Doi, K.; Nakayama, H.; Uetsuka, K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp. Anim. 2007, 56, 263–272. [Google Scholar] [CrossRef]
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd ElHafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Jensen-Jarolim, E. Gender aspects in food allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 249–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Xie, R.; Huang, M.; Hu, C.; Wu, Y.; Li, X.; Chen, H. High-Fat Diet-Induced Mild Obesity Alters the Activation of T Cells and Maintains Intestinal Homeostasis in Food Allergy Animal Model. Foods 2025, 14, 1852. https://doi.org/10.3390/foods14111852
Yang F, Xie R, Huang M, Hu C, Wu Y, Li X, Chen H. High-Fat Diet-Induced Mild Obesity Alters the Activation of T Cells and Maintains Intestinal Homeostasis in Food Allergy Animal Model. Foods. 2025; 14(11):1852. https://doi.org/10.3390/foods14111852
Chicago/Turabian StyleYang, Fan, Ruofan Xie, Meijia Huang, Chunqiu Hu, Yong Wu, Xin Li, and Hongbing Chen. 2025. "High-Fat Diet-Induced Mild Obesity Alters the Activation of T Cells and Maintains Intestinal Homeostasis in Food Allergy Animal Model" Foods 14, no. 11: 1852. https://doi.org/10.3390/foods14111852
APA StyleYang, F., Xie, R., Huang, M., Hu, C., Wu, Y., Li, X., & Chen, H. (2025). High-Fat Diet-Induced Mild Obesity Alters the Activation of T Cells and Maintains Intestinal Homeostasis in Food Allergy Animal Model. Foods, 14(11), 1852. https://doi.org/10.3390/foods14111852





