Using Dried Crickets as a Nutrients and Bioactive Compounds Source in Crispy Vegetable Chips
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cricket Powder
2.2. Preparation of Cricket Protein-Enhanced Chips
2.3. Physical Analysis of Chips
2.3.1. Color Analysis
2.3.2. Hardness
2.4. Chemical Analysis of Cricket Powder, Vegetable Powder, and Chips
2.4.1. Composition Analysis
2.4.2. Antioxidant Activity of Cricket Powder, Vegetable Powder, and Chips
- g = weight of DPPH;
- m1 = DPPH concentration;
- v1 = required volume;
- Mw = molecular weight (394.4).
- AA% = antioxidant activity;
- Acontrol = control UV reading;
- Asample = sample UV reading.
2.4.3. Total Phenolic and Flavonoid Content of Vegetable Powder and Chips
2.4.4. Potassium Analysis of Kale Powder and Chips
2.5. Sensory Evaluation of Chips
2.6. Preservation Testing of Chips
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Analysis of Cricket Powder
3.2. Sensory Evaluation of Chips
3.3. Chemical Analysis of Vegetable Powder and Chips
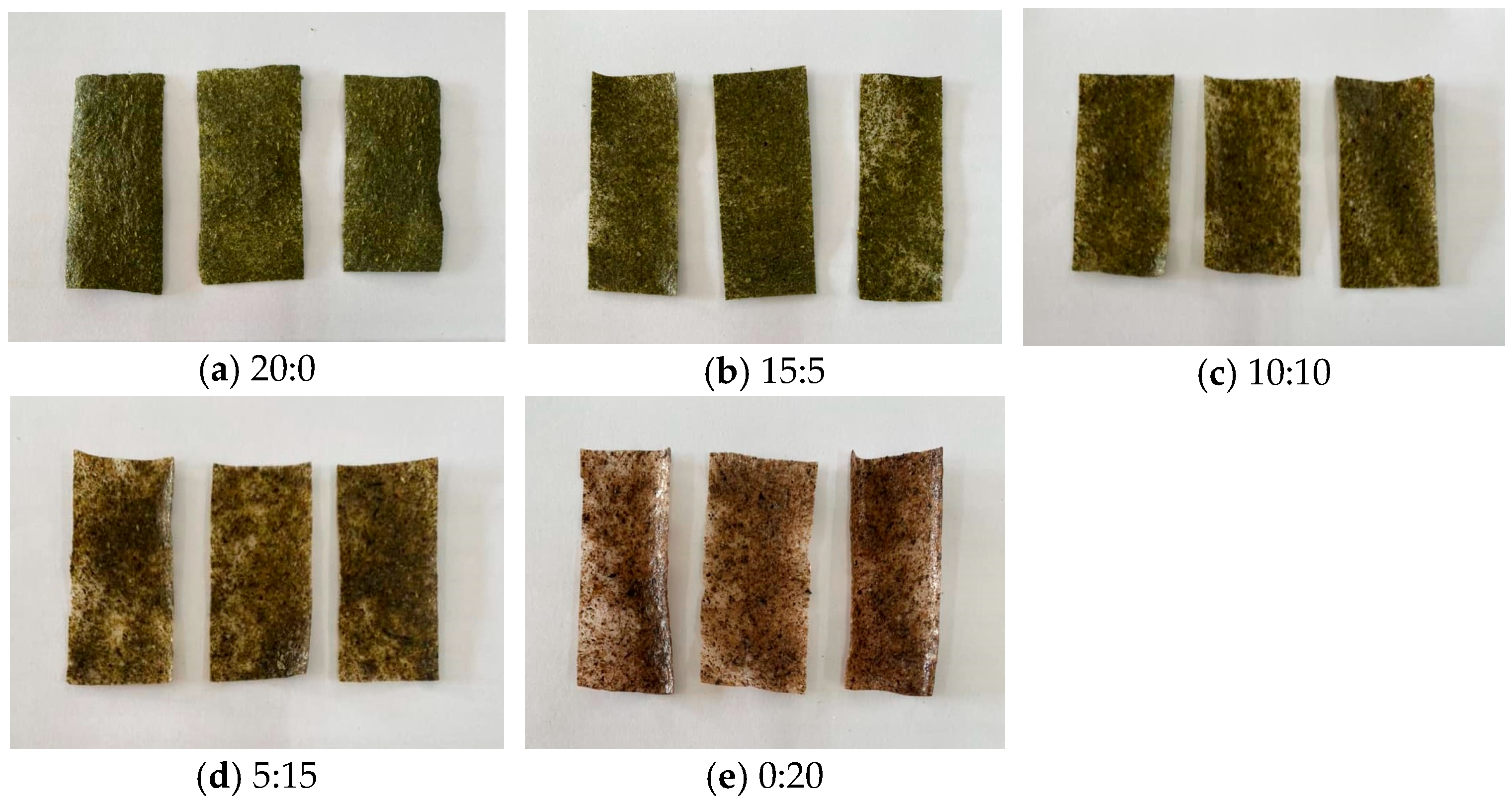
3.4. Physical Analysis of the Chips
3.5. Preservation Testing of the Chips
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giusti, A.; Spatola, G.; Mancini, S.; Nuvoloni, R.; Armani, A. Novel foods, old issues: Metabarcoding revealed mislabeling ib insect-based products sold bt e-commerce on the EU market. Food Res. Int. 2024, 184, 114268. [Google Scholar] [CrossRef]
- Frigerio, J.; Agostinetto, G.; Galimberti, A.; Mattia, F.D.; Labra, M.; Bruno, A. Tasting the differences: Microbiota analysis of different insect-based novel food. Food Res. Int. 2020, 137, 109426. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Santos, C.; Lima, R.C.; Pintado, M.E.; Cunha, L.M. Impact of defatting and drying methods on the overall liking and sensory profile of a cereal bar incorporating edible insect species. Future Foods 2022, 6, 100190. [Google Scholar] [CrossRef]
- Suga, N.; Tsumura, E.; Naito, Y.; Hamaguchi, I.; Matsuda, S.; Kawabata, K.; Sakamoto, K. Thermal stability of cricket powder and its effects on antioxidant activity, physical, and sensory properties of rice crackers. LWT 2023, 186, 115267. [Google Scholar] [CrossRef]
- Sanguanprasit, C. Available online: https://brandinside.asia/insect-business-future/ (accessed on 25 July 2024).
- Zafar, A.; Shaheen, M.; Tahir, A.B.; Silva, A.P.G.; Manzoor, H.Y.; Zia, S. Unraveling the nutritional, biofunctional, and sustainable food application of edible crickets: A comprehensive review. Trends Food Sci. Technol. 2024, 143, 104254. [Google Scholar] [CrossRef]
- Amoah, I.; Cobbinah, J.C.; Yeboah, J.A.; Essiam, F.A.; Lim, J.J.; Tandoh, M.A.; Rush, E. Edible insect powder for enrichment of bakery products—A review of nutritional, physical characteristics and acceptability of bakery products to consumers. Future Foods 2023, 8, 100251. [Google Scholar] [CrossRef]
- Han, J.; Janz, J.A.M.; Gerlat, M. Development of gluten-free cracker snacks using pulse flours and fractions. Food Res. Int. 2010, 43, 627–633. [Google Scholar] [CrossRef]
- Lier, I.; Heuvel, E.; Mil, E.; Havemans, R.C. The value of food innovation with children: The case of insect snack balls for kids. Food Qual. Prefer. 2024, 118, 105199. [Google Scholar] [CrossRef]
- Bruttomesso, M.; Bianchi, F.; Pasqualoni, I.; Rizzi, C.; Simonato, B. Evaluation of the technological and compositional features of pancakes fortified with Acheta domesticus. LWT 2024, 199, 116073. [Google Scholar] [CrossRef]
- Dhakal, M.; Kemsawasd, V.; Whanmek, K.; Chathiran, W.; Intawong, S.; Srichamnong, W.; Suttisansanee, U.; Kittibunchakul, S. Physicochemical characteristics, volatile components and bioactivities of fermented seasoning sauce produced from cricket (Acheta domesticus) meal. Future Foods 2025, 11, 100505. [Google Scholar] [CrossRef]
- Pasini, G.; Cullere, M.; Vegro, M.; Simonato, B.; Zotte, A.D. Potentiality of protein fractions from the house cricket (Acheta domesticus) and yellow mealworm (Tenebrio molitor) for pasta formulation. LWT 2022, 164, 113638. [Google Scholar] [CrossRef]
- Bas, A.; El, S.N. Nutritional evaluation of biscuits enriched with cricket flour (Acheta domesticus). Int. J. Gastron. Food Sci. 2022, 29, 100583. [Google Scholar] [CrossRef]
- Igual, M.; García-Segovia, P.; Martínez-Monzo, J. Effect of Acheta domesticus (house cricket) addition on protein content, colour, texture, and extrusion parameters of extruded products. J. Food Eng. 2020, 282, 110032. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Cruz, T.M.P.; Silva, M.C.A. Cricket (Acheta domesticus) flour as meat replacer in frankfurters: Nutritional, technological, structural, and sensory characteristics. Innov. Food Sci. Emerg. Technol. 2023, 83, 103245. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Yu, T.; Yuan, B.; Huang, G.; Zhang, Y.; Ren, X.; Xiao, J.; Huang, D. Effects of ultrafine grinding on nutritional, physicochemical and protein composition of Acheta domesticus powder. Innov. Food Sci. Emerg. Technol. 2024, 97, 103816. [Google Scholar] [CrossRef]
- Ajdini, B.; Biancarosa, I.; Cardinaletti, G.; Illuminati, S.; Annibaldi, A.; Girolametti, F.; Fanelli, M.; Tulli, F.; Pinto, T.; Truzzi, C. Modulating the nutritional value of Acheta domesticus (house cricket) through the eco-sustainable Ascophyllum nodosum dietary supplementation. J. Food Compos. Anal. 2025, 140, 107263. [Google Scholar] [CrossRef]
- Brena-Melendez, A.; Garcia-Amezquita, L.E.; Liceaga, A.; Pascacio-Villafán, C.; Tejada-Ortigoza, V. Novel food ingredients: Evaluation of commercial processing conditions on nutritional and technological properties of edible cricket (Acheta domesticus) and its derived parts. Innov. Food Sci. Emerg. Technol. 2024, 92, 103589. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Bonaccorsi, G.; Lorini, C.; Cini, E. Assessment of the rheological properties and bread characteristics obtained by innovative protein sources (Cicer arietinum, Acheta domesticus, Tenebrio molitor): Novel food or potential improvers for wheat flour? LWT 2020, 118, 108867. [Google Scholar] [CrossRef]
- Kita, A.; Lisińska, G.; Gołubowska, G. The effects of oils and frying temperatures on the texture and fat content of potato crisps. Food Chem. 2007, 102, 1–5. [Google Scholar] [CrossRef]
- La Fuente, C.I.A.; Lopes, C.C. HTST puffing in order to produce crispy banana—The effect of the step-down treatment prior to air-drying. LWT 2018, 92, 324–329. [Google Scholar] [CrossRef]
- Zotarelli, M.F.; Porciuncula, B.D.A.; Laurindo, J.B. A convective multi-flash drying process for producing dehydrated crispy fruits. J. Food Eng. 2012, 108, 523–531. [Google Scholar] [CrossRef]
- Jirukkakul, N. The production and development of tomato crisp from tomato pomace. Asia Pac. J. Sci. Technol. 2017, 22, 120–130. [Google Scholar]
- Chobot, M.; Kozlowska, M.; Ignaczak, A.; Kowalska, H. Development of drying and roasting processed for the production of plant-based pro-healthy snacks in the light of nutritional trends and sustainable techniques. Trends Food Sci. Technol. 2024, 149, 104553. [Google Scholar] [CrossRef]
- Susmitha, A.; Sasikumar, K.; Rajan, D.; M, A.P.; Nampoothiri, K.M. Development and characterization of corn starch-gelatin based edible films incorporated with mango and pineapple for active packaging. Food Biosci. 2021, 41, 100977. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Liu, H.; Li, M.; Ma, Z. Barrier and mechanical properties of carrot puree films. Food Bioprod. Process. 2011, 89, 149–156. [Google Scholar] [CrossRef]
- Saencom, S.; Chiewchan, N.; Devahastin, S. Production of dried ivy gourd sheet as a health snack. Food Bioprod. Process. 2011, 8, 414–421. [Google Scholar] [CrossRef]
- Oliveira, E.F.R.; Bonfim, K.S.; Aouada, F.A.; Azeredo, H.M.C.; Mour, M.R. A sustainable approach on the potential use of kale puree in edible wraps. Appl. Food Res. 2023, 3, 100261. [Google Scholar] [CrossRef]
- Korus, A.; Lisiewska, Z. Effect of preliminary processing and method of preservation on the content of selected antioxidative compounds in kale (Brassica oleracea L. var. acephala) leaves. Food Chem. 2011, 129, 149–154. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Jirukkakul, N. Improvement of physical properties and phenolic compounds of egg noodles by banana pulp and peel flour fortification. Food Res. 2021, 5, 14–20. [Google Scholar] [CrossRef]
- Lasunon, P.; Phonkerd, N.; Pariwat, S.; Sengkhamparn, N. Effect of Soaking Conditions and Fuzzy Analytical Method for Producing the Quick-Cooking Black Jasmine Rice. Molecules 2022, 27, 3615. [Google Scholar] [CrossRef]
- Russamee, B.; Jirukakul, N. Physical and antioxidant properties of pre-gelatinized black jasmine rice flour film incorporated with marigold flower extract. Food Appl. Biosci. J. 2024; in press. [Google Scholar]
- Pilco-Romero, G.; Chisaguano-Tonato, A.M.; Herrera-Fontana, M.E.; Chimbo-Gandara, L.F.; Sharifi-Rad, M.; Giampieri, F.; Battino, M.; Vernaza, M.G.; Alvarez-Suarez, J.M. House cricket (Acheta domesticus): A review based on its nutritional composition, quality, and potential uses in the food industry. Trends Food Sci Technol. 2023, 142, 104226. [Google Scholar] [CrossRef]
- Machado, C.R.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Cheng, K.M.; Leong, K.N.; Chan, S.W. Cricket as an alternative source of protein in the development of nutritious baked chips. Food Res. 2022, 6 (Suppl. S2), 74–82. [Google Scholar] [CrossRef]
- Akullo, J.O.; Kiage-Mokua, B.N.; Nakimbugwe, D.; Kwetegyeka, J.; Nganga, J.; Kinyuru, J. Fatty acid composition and lipid stability of cricket (Gryllus bimaculatus) flour preserved using ginger and garlic extracts. Future Foods 2025, 11, 100570. [Google Scholar] [CrossRef]
- Jirukkakul, N. Physical and Antioxidant Properties of Gelatin Film Mixed with Sesame Oil, Rice Bran Oil and Coconut Oil. Int. Food Res. J. 2022, 29, 1020–1031. [Google Scholar] [CrossRef]
- Chen, J.; Venkitasamy, C.; Shen, Q.; McHugh, T.H.; Zhang, R.; Pan, Z. Development of healthy crispy carrot snacks using sequential infrared blanching and hot air drying method. LWT 2018, 97, 469–475. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzynska, A.; Galus, S.; Janowicz, M. Freeze-dried snacks obtained from frozen vegetable by-products and apple pomace—Selected properties, energy consumption and carbon footprint. Innov. Food Sci. Emerg. Technol. 2022, 77, 102949. [Google Scholar] [CrossRef]
- Ciurzynska, A.; Marczak, W.; Lenart, A.; Janowicz, M. Production of innovative freeze-dried vegetable snack with hydrocolloids in terms of technological process and carbon footprint calculation. Food Hydrocoll. 2020, 108, 105993. [Google Scholar] [CrossRef]
- Ciurzynska, A.; Galus, S.; Karwacka, M.; Janowicz, M. The sorption properties, structure and shrinkage of freeze-dried multi-vegetable snack bars in the aspect of the environmental water activity. LWT 2022, 171, 114090. [Google Scholar] [CrossRef]
- Santosh, O.; Bajwa, H.K.; Bisht, M.S.; Chongtham, N. Antioxidant activity and sensory evaluation of crispy salted snacks fortified with bamboo shoot rich in bioactive compounds. Appl. Food Res. 2021, 1, 100018. [Google Scholar] [CrossRef]
- Tulamandi, S.; Rangarajan, V.; Rizvi, S.S.H.; Singhal, R.S.; Chattopadhyay, S.K.; Sah, N.C. A biodegradable and edible packaging film based on papaya puree, gelatin, and defatted soy protein. Food Packag. Shelf Life 2016, 10, 60–71. [Google Scholar] [CrossRef]
- Mam, S.; Rudra, S.G.; Kundu, A.; Singh, S.; Joshi, A.; Bhardwaj, R.; Kumar, D. Crisps from red cabbage: Process standardization for nutrients retention. Food Humanit. 2025, 4, 1000555. [Google Scholar] [CrossRef]
- TCPS 515/2547; Roasted Seaweed. Thai Industrial Standards Institute (TISI): Bangkok, Thailand, 2004.
| Ingredients (g) | Kale Powder/Cricket Powder | ||||
|---|---|---|---|---|---|
| 20:0 | 15:5 | 10:10 | 5:15 | 0:20 | |
| Kale powder | 20 | 15 | 10 | 5 | 0 |
| Cricket powder | 0 | 5 | 10 | 15 | 20 |
| Water | 200 | 200 | 200 | 200 | 200 |
| Soy sauce | 1 | 1 | 1 | 1 | 1 |
| Sugar | 26 | 26 | 26 | 26 | 26 |
| Cassava starch | 20 | 20 | 20 | 20 | 20 |
| Pepper powder | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Salt | 1 | 1 | 1 | 1 | 1 |
| Composition | Hot Air Cricket Powder | Vacuum-Dried Cricket Powder | Microwaved Cricket Powder | Freeze-Dried Cricket Powder |
|---|---|---|---|---|
| Moisture content (wb) | 2.87 ± 0.03 b | 3.26 ± 0.52 a | 1.42 ± 0.25 c | 3.24 ± 0.06 a |
| Fat (db) | 26.94 ± 0.19 b | 26.85 ± 0.24 b | 28.98 ± 0.32 a | 27.13 ± 0.61 b |
| Protein (db) | 53.45 ± 0.02 c | 55.80 ± 0.08 b | 51.44 ± 0.67 d | 58.51 ± 0.12 a |
| Fiber (db) | 12.31 ± 0.24 b | 10.34 ± 0.20 d | 12.73 ± 0.07 a | 10.84 ± 0.11 c |
| Ash (db) | 3.21 ± 0.02 c | 3.31 ± 0.02 b | 3.04 ± 0.04 d | 3.40 ± 0.01 a |
| Carbohydrate (db) | 4.09 ± 0.28 a | 3.70 ± 0.39 b | 3.81 ± 0.28 a | 0.12 ± 0.03 c |
| Sample | DPPH (%) |
|---|---|
| Hot air oven cricket powder | 76.22 ± 0.37 |
| Vacuum-dried cricket powder | 76.56 ± 0.54 |
| Microwaved cricket powder | 76.04 ± 0.61 |
| Freeze-dried cricket powder | 76.74 ± 0.80 |
| Kale–Cricket | Appearance | Color | Odor | Flavor | Texture | Overall |
|---|---|---|---|---|---|---|
| 20:00 | 7.17 ± 1.21 a | 7.17 ± 1.09 a | 6.10 ± 1.27 a | 5.57 ± 1.52 b | 5.50 ± 1.74 c | 5.77 ± 1.48 a |
| 15:05 | 7.00 ± 0.95 a | 6.67 ± 0.92 ab | 5.90 ± 1.37 ab | 5.63 ± 1.59 b | 6.33 ± 1.95 abc | 5.77 ± 1.72 a |
| 10:10 | 6.90 ± 0.92 a | 7.00 ± 1.10 ab | 6.53 ± 1.38 a | 6.63 ± 1.71 a | 7.07 ± 1.57 a | 6.57 ± 1.65 a |
| 5:15 | 6.90 ± 1.03 a | 6.47 ± 1.25 b | 6.17 ± 1.15 a | 6.33 ± 1.69 ab | 6.60 ± 1.59 ab | 6.37 ± 1.47 a |
| 0:20 | 6.03 ± 1.45 b | 5.57 ± 1.57 c | 5.40 ± 1.28 b | 5.50 ± 1.55 b | 5.90 ± 1.75 bc | 5.67 ± 1.49 a |
| Kale–Cricket | Moisture Content (wb) | Protein (db) | Fat (db) | Ash (db) | Fiber (db) |
|---|---|---|---|---|---|
| 20:0 | 0.95 ± 0.20 a | 9.13 ± 0.24 c | 0.16 ± 0.12 c | 7.22 ± 0.28 a | 3.37 ± 0.09 a |
| 10:10 | 1.01 ± 0.07 a | 13.27 ± 0.31 b | 1.15 ± 0.21 b | 5.14 ± 0.05 b | 2.98 ± 0.11 b |
| 0:20 | 1.03 ± 0.02 a | 16.39 ± 0.32 a | 2.36 ± 0.12 a | 2.70 ± 0.05 c | 2.96 ± 0.03 b |
| Kale–Cricket | DPPH (%) | Phenolic Content (mg GAE/mg Sample) | Flavonoid Content (mg QE/mg Sample) | Potassium (%) |
|---|---|---|---|---|
| 20:0 | 10.09 ± 0.63 c | 3.76 ± 0.23 c | 12.15 ± 0.26 a | 1.66 ± 0.30 a |
| 10:10 | 60.90 ± 2.23 b | 6.25 ± 0.46 b | 11.16 ± 0.19 b | 0.66 ± 0.01 b |
| 0:20 | 93.13 ± 0.31 a | 8.43 ± 0.32 a | 6.88 ± 0.10 c | 0 ± 0.00 c |
| Kale powder | 32.62 ± 0.58 | 8.57 ± 0.63 | 21.25 ± 0.43 | 5.34 ± 1.58 |
| Kale–Cricket | L* | a* | b* | Hardness (N) |
|---|---|---|---|---|
| 20:0 | 31.86 ± 1.00 a | 0.28 ± 0.11 c | 16.48 ± 0.95 a | 2.56 ± 0.02 c |
| 10:10 | 28.64 ± 2.16 b | 0.95 ± 10.09 b | 13.34 ± 1.19 b | 5.28 ± 0.03 b |
| 0:20 | 27.07 ± 2.30 b | 3.33 ± 0.27 a | 5.74 ± 0.68 c | 14.42 ± 0.27 a |
| Storage Time (Month) | L* | a* | b* | TBARS Increase (%) |
|---|---|---|---|---|
| 0 | 29.00 ± 0.20 b | 2.88 ± 0.06 a | 14.33 ± 0.22 a | 0 ± 0.00 e |
| 1 | 29.85 ± 1.02 b | 1.66 ± 0.22 b | 11.67 ± 0.87 b | 4.53 ± 1.23 d |
| 2 | 28.18 ± 1.89 b | 1.16 ± 0.15 c | 10.96 ± 1.40 b | 11.73 ± 2.48 c |
| 3 | 30.29 ± 0.26 b | 0.92 ± 0.01 d | 11.56 ± 0.03 b | 23.50 ± 0.27 b |
| 4 | 29.15 ± 0.33 b | 0.47 ± 0.02 e | 9.89 ± 0.40 c | 37.70 ± 11.58 a |
| 5 | 31.54 ± 0.05 a | 0.25 ± 0.01 f | 12.31 ± 0.03 b | 40.90 ± 2.31 a |
| 6 | 31.13 ± 0.01 a | 0.10 ± 0.01 g | 8.75 ± 0.02 d | 45.13 ± 5.49 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirukkakul, N.; Phoolklang, A. Using Dried Crickets as a Nutrients and Bioactive Compounds Source in Crispy Vegetable Chips. Foods 2025, 14, 1810. https://doi.org/10.3390/foods14101810
Jirukkakul N, Phoolklang A. Using Dried Crickets as a Nutrients and Bioactive Compounds Source in Crispy Vegetable Chips. Foods. 2025; 14(10):1810. https://doi.org/10.3390/foods14101810
Chicago/Turabian StyleJirukkakul, Natcharee, and Areeya Phoolklang. 2025. "Using Dried Crickets as a Nutrients and Bioactive Compounds Source in Crispy Vegetable Chips" Foods 14, no. 10: 1810. https://doi.org/10.3390/foods14101810
APA StyleJirukkakul, N., & Phoolklang, A. (2025). Using Dried Crickets as a Nutrients and Bioactive Compounds Source in Crispy Vegetable Chips. Foods, 14(10), 1810. https://doi.org/10.3390/foods14101810








