Measuring Global Dietary Diversity by Considering Nutritional Functional Dissimilarity and Dietary Guidelines
Abstract
1. Introduction
2. Methods
2.1. Concept and Measurement Principles of Dietary Diversity
2.2. A New Framework for Dietary Diversity Indices
2.2.1. Species-Neutral Indices
2.2.2. Functional Dissimilarity Indices
2.2.3. Dietary Guideline-Based Species-Neutral Indices
2.2.4. Dietary Guideline-Based Functional Dissimilarity Indices
3. Materials
3.1. Food Consumption Data
3.2. Nutrition Intake Data
3.3. Dietary Guideline Data
4. Results
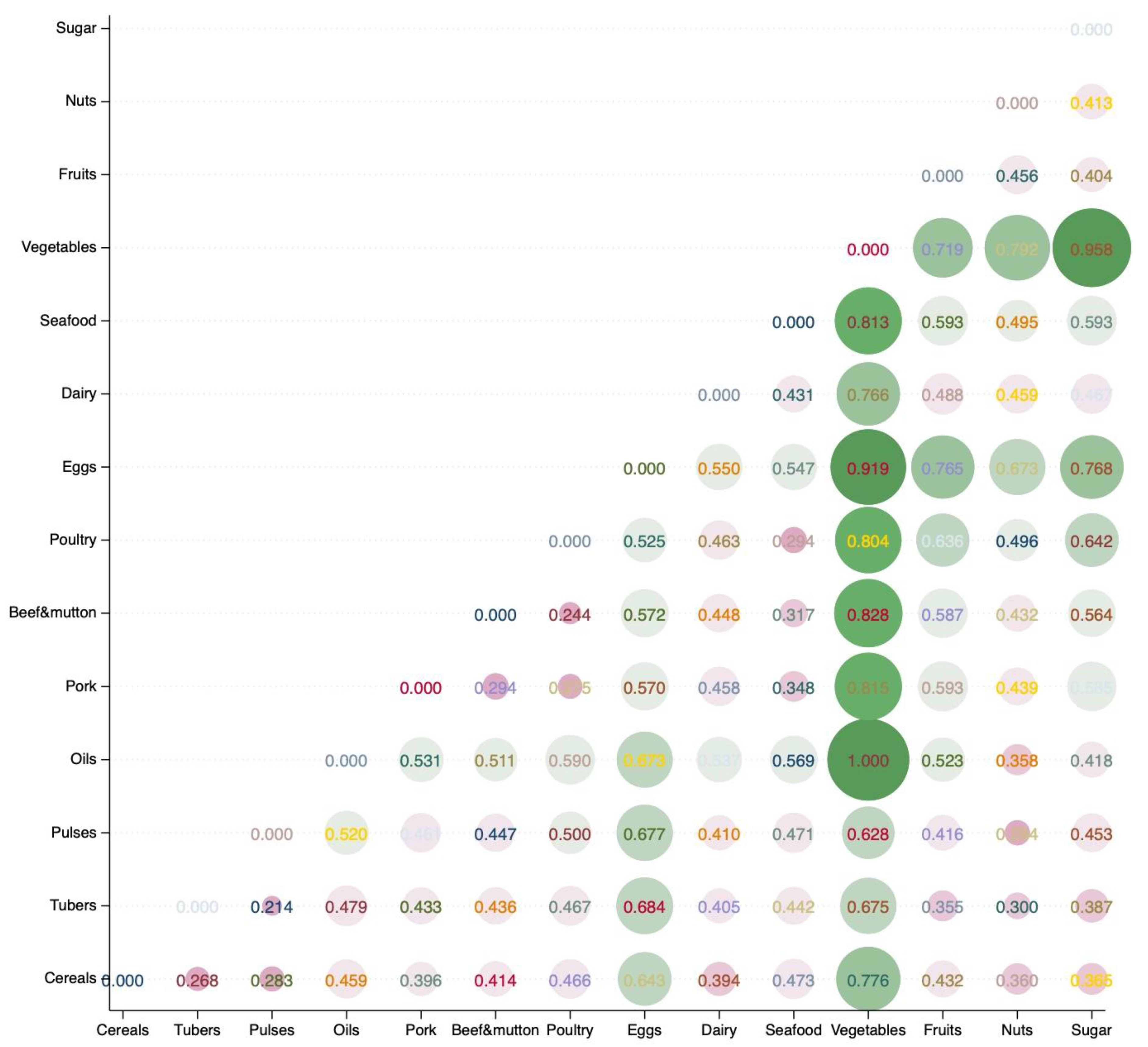
4.1. Characteristics of Global Food Consumption and Nutrition Functional Disparities
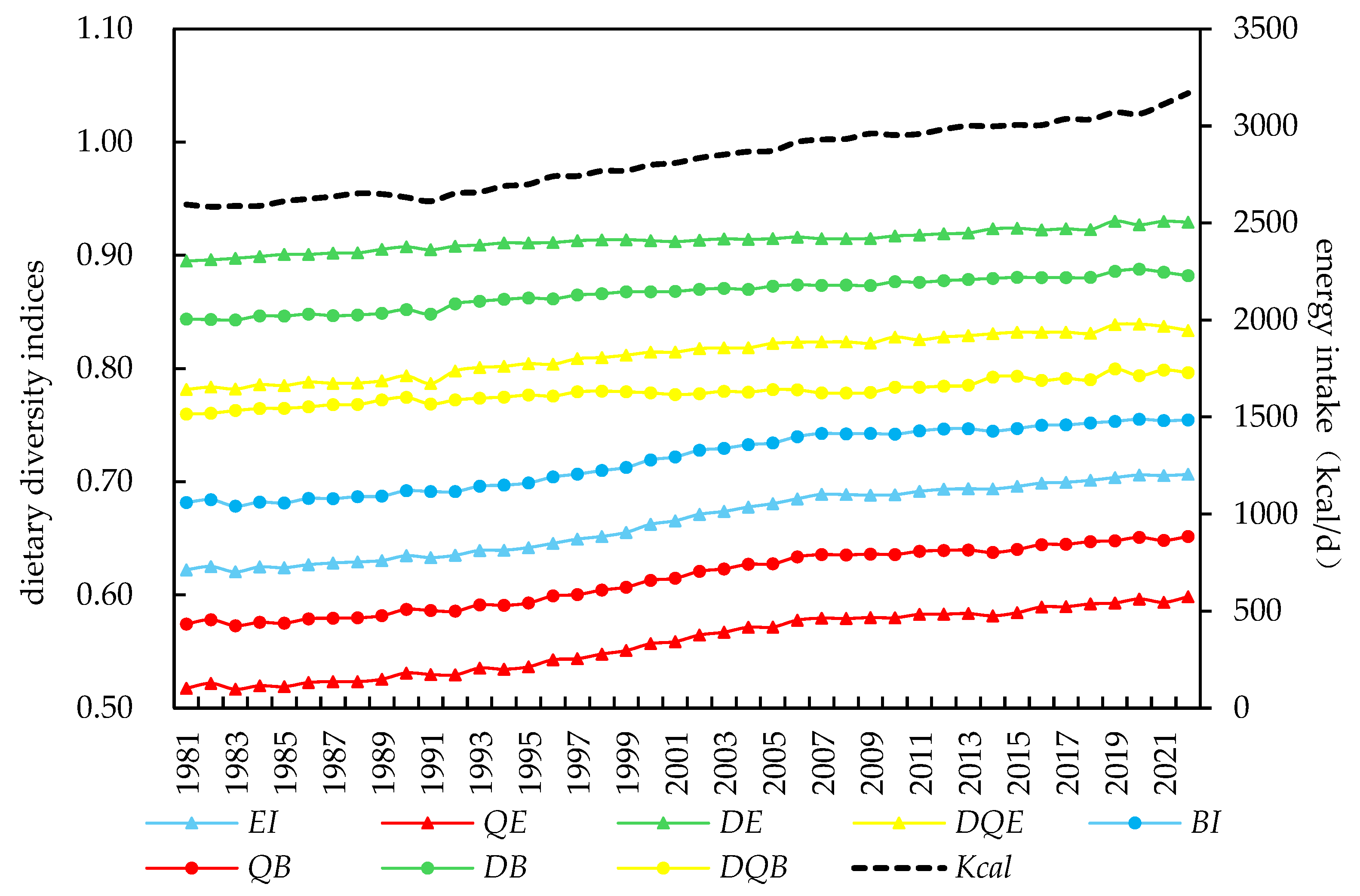
4.2. Characteristics of Global Dietary Diversity
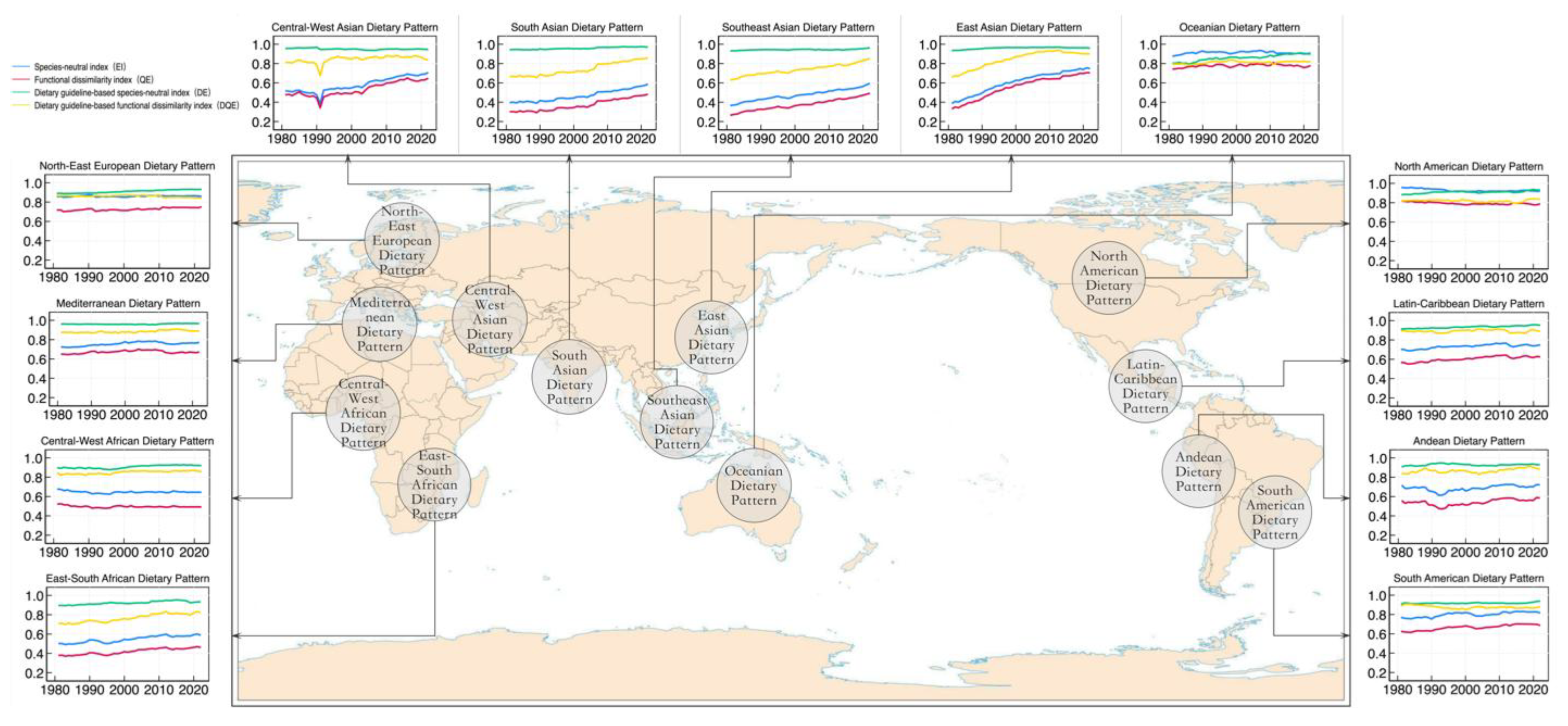
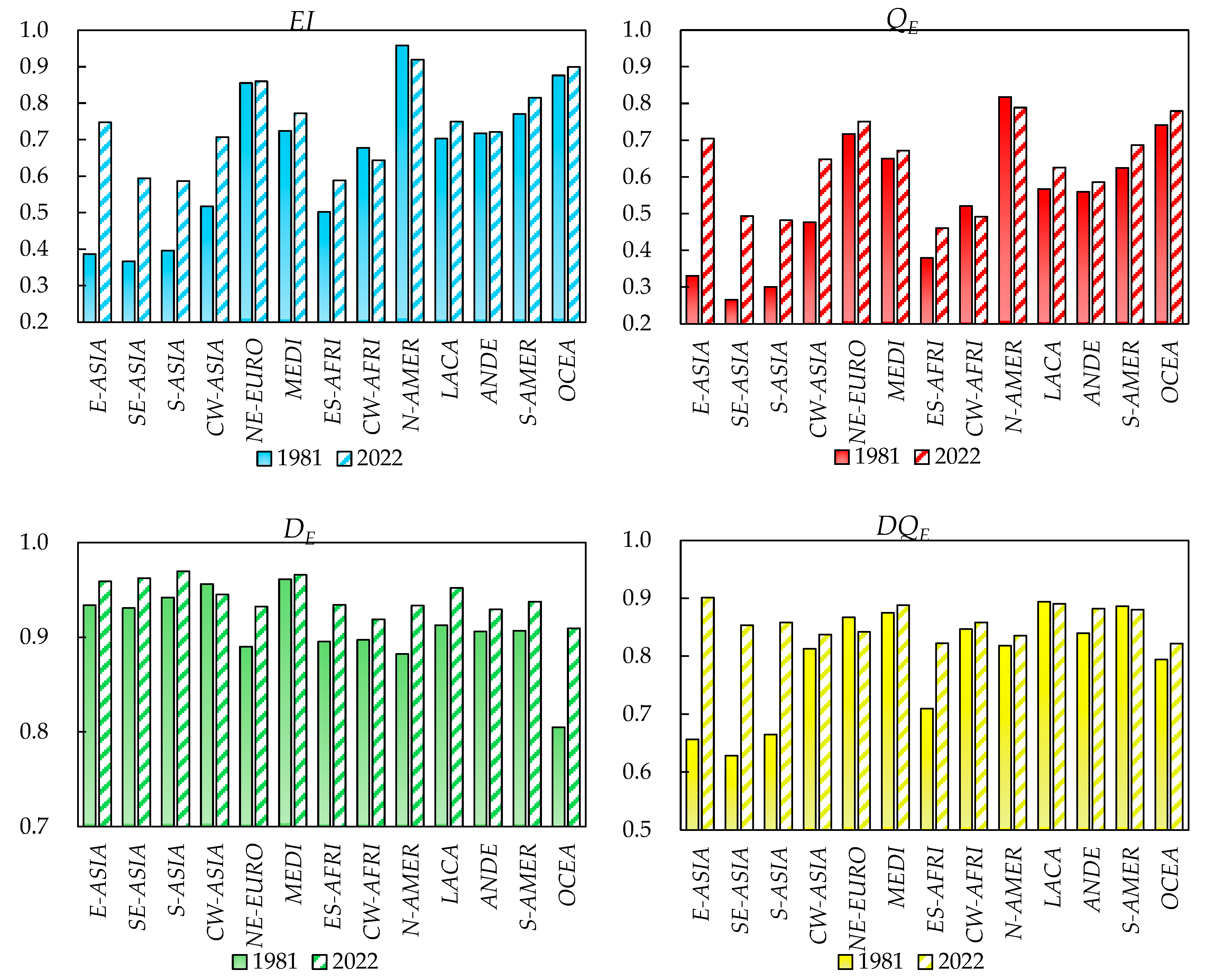
4.3. Characteristics of Global Dietary Diversity in Different Dietary Patterns
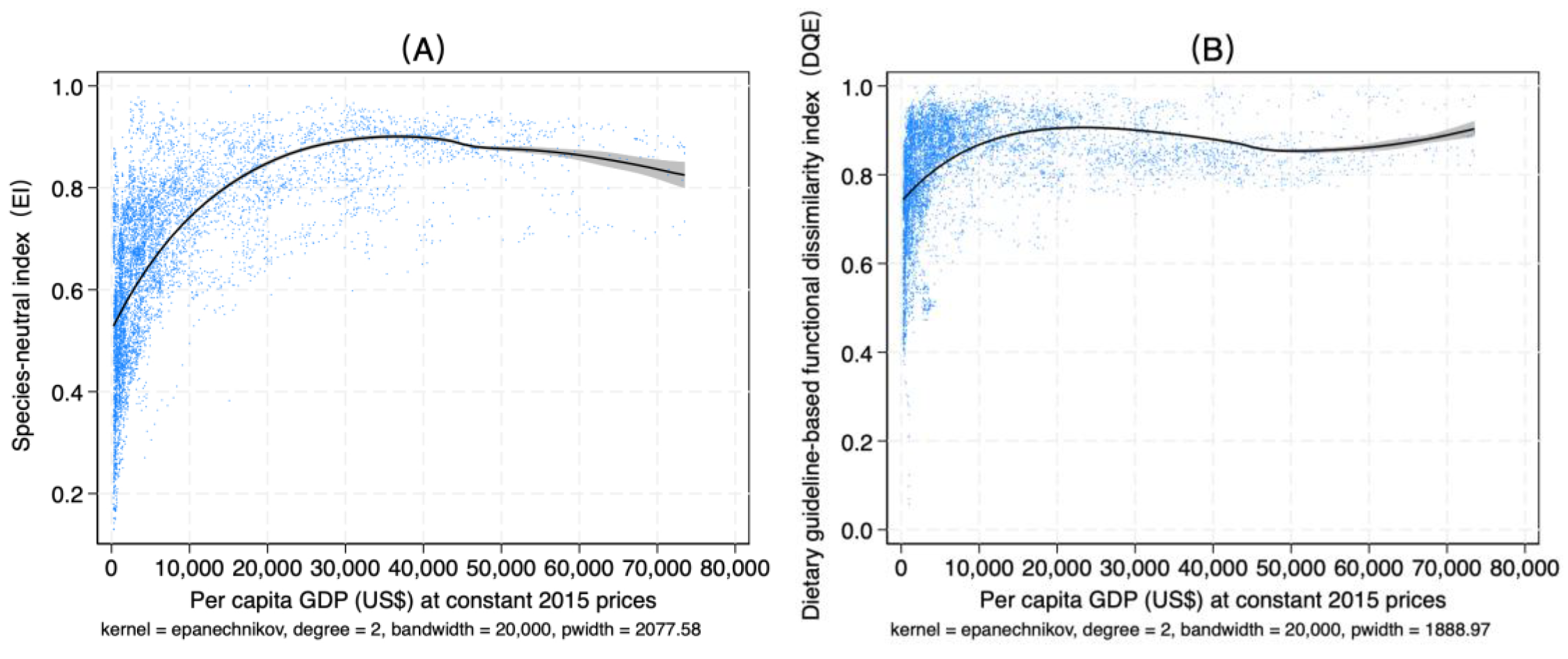
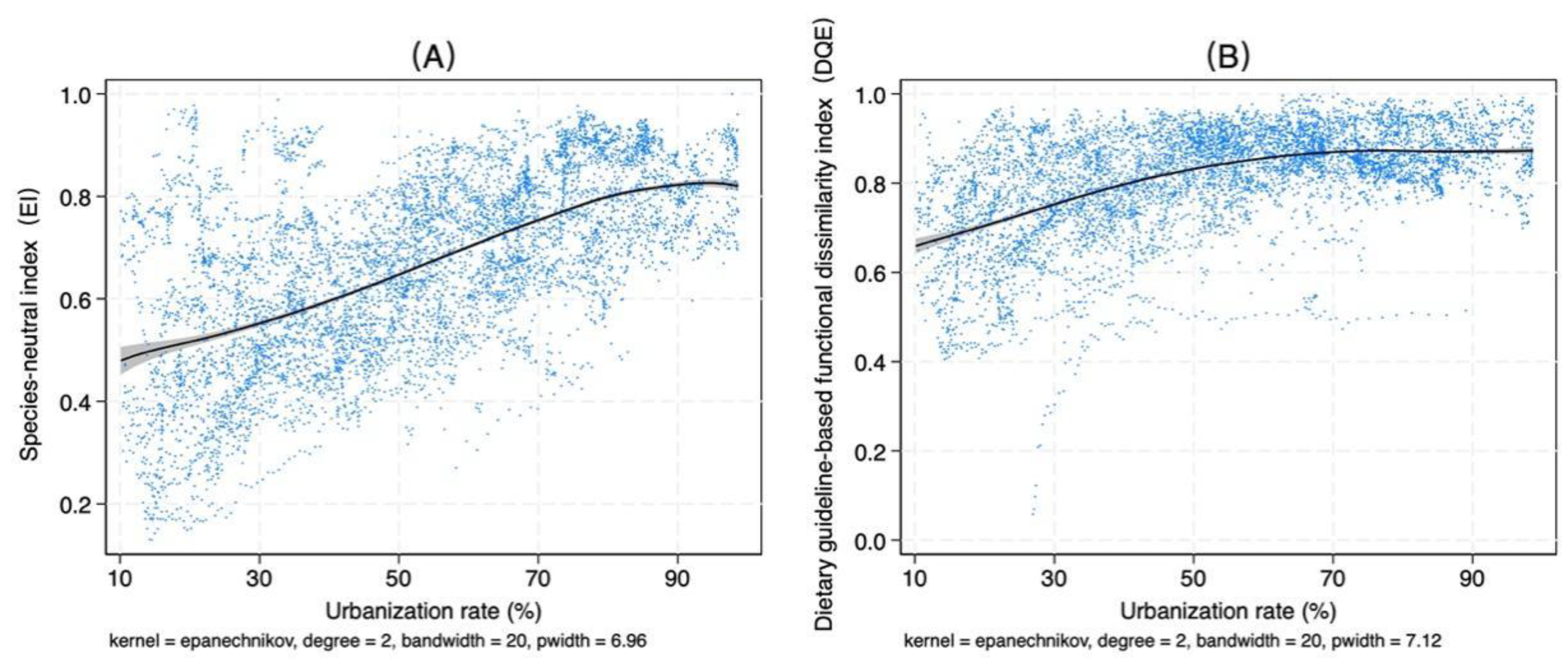
4.4. Relationships Among Dietary Diversity, Income, and Urbanization Rate
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| E-ASIA | East Asian Dietary Pattern |
| SE-ASIA | Southeast Asian Dietary Pattern |
| S-ASIA | South Asian Dietary Pattern |
| CW-ASIA | Central West Asian Dietary Pattern |
| NE-EURO | Northeast European Dietary Pattern |
| MEDI | Mediterranean Dietary Pattern |
| ES-AFRI | East South African Dietary Pattern |
| CW-AFRI | Central West African Dietary Pattern |
| N-AMER | North American Dietary Pattern |
| LACA | Latin Caribbean Dietary Pattern |
| ANDE | Andean Dietary Pattern |
| S-AMER | South American Dietary Pattern |
| OCEA | Oceanian Dietary Pattern |
Appendix A
| Dietary Pattern | Representative Countries | Diet Composition | Key Features |
|---|---|---|---|
| East Asian Dietary Pattern | China, Japan, South Korea | Primarily based on rice, noodles, and wheat, with protein from pork, chicken, fish, tofu, and eggs, alongside a variety of vegetables and light oils. | Diverse, vegetable-rich, low-fat. |
| South Asian Dietary Pattern | India, Pakistan | Focuses on rice, lentils, and flatbreads, with protein from pulses, chicken, mutton, fish, and dairy, heavily spiced with turmeric, cumin, and other spices. | Spice-heavy, vegetarian-influenced. |
| Southeast Asian Dietary Pattern | Thailand, Vietnam, Malaysia, Indonesia | Centered on rice and noodles, with protein from chicken, fish, and shrimp, seasoned with coconut, herbs, and chili, offering a mix of sweet, sour, and spicy flavors. | Sweet, sour, and spicy flavors. |
| Central West Asian Dietary Pattern | Kazakhstan, Uzbekistan, Iran | Relies on bread, rice, and lentils as staples, with protein from mutton, beef, chicken, fish, and yogurt, heavily flavored with spices and herbs. | Rich in spices and dairy. |
| Northeast European Dietary Pattern | Sweden, Germany, Russia, Poland | Features potatoes and rye bread as staples, with protein from pork, beef, fish, cheese, and dairy, alongside simple vegetable dishes. | High-protein, simple cooking. |
| Mediterranean Dietary Pattern | Italy, Greece, Spain, Turkey | Emphasizes whole-grain bread, pasta, and vegetables, with protein from fish, chicken, pulses, and nuts, seasoned with olive oil and herbs. | Olive oil- and fish-centric. |
| North American Dietary Pattern | USA, Canada | Includes bread, potatoes, and corn as staples, with high consumption of beef, chicken, pork, and processed foods like fast food and snacks. | High in processed foods. |
| Latin Caribbean Dietary Pattern | Mexico, Cuba, Colombia | Based on tortillas, rice, and beans, with protein from chicken, pork, and fish, heavily spiced with chili and accompanied by tropical fruits. | Corn and bean-based, spicy. |
| South American Dietary Pattern | Brazil, Argentina | Combines rice and black beans with protein from beef, chicken, and pork, often prepared as barbecue and served with coffee or mate tea. | Beef-heavy, barbecue culture. |
| Andean Dietary Pattern | Peru, Ecuador | Relies on quinoa, roots and tubers, and corn as staples, with protein from guinea pig, beef, chicken, and fish, complemented by regional herbs and spices. | Higher consumption of Roots and Tubers |
| Central West African Dietary Pattern | Nigeria, Ghana, Senegal | Built around cassava, yams, and plantains, with protein from fish, chicken, and mutton, seasoned with palm oil and spicy herbs. | Cassava-based, spicy. |
| East South African Dietary Pattern | Ethiopia, Uganda, South Africa | Primarily uses maize, cassava, and rice as staples, with protein from beef, mutton, chicken, fish, and dairy, seasoned with spices and butter. | Dairy and meat emphasis. |
| Oceanian Dietary Pattern | Australia, New Zealand | Features bread, potatoes, and rice as staples, with protein from beef, lamb, chicken, fish, and dairy, influenced by Western and Asian cuisines. | Meat-heavy, Western influence. |
References
- Zhu, W.; Han, X.; Liu, Y.; Li, G.; Wen, J. Sustainable Healthy Diets in China: A Multidimensional Framework and Assessment. Front. Sustain. Food Syst. 2024, 8, 1464965. [Google Scholar] [CrossRef]
- Moscatelli, F.; De Maria, A.; Marinaccio, L.A.; Monda, V.; Messina, A.; Monacis, D.; Toto, G.; Limone, P.; Monda, M.; Messina, G. Assessment of Lifestyle, Eating Habits and the Effect of Nutritional Education among Undergraduate Students in Southern Italy. Nutrients 2023, 15, 2894. [Google Scholar] [CrossRef]
- Balana, B.B.; Ogunniyi, A.; Oyeyemi, M.; Fasoranti, A.; Edeh, H.; Andam, K. COVID-19, Food Insecurity and Dietary Diversity of Households: Survey Evidence from Nigeria. Food Sec. 2023, 15, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Baye, K.; Laillou, A.; Chitekwe, S. Empowering Women Can Improve Child Dietary Diversity in Ethiopia. Matern. Child Nutr. 2024, 20, e13285. [Google Scholar] [CrossRef]
- Ukonu, I.C.; Wallace, C.A.; Lowe, N.M. Household Food Security and Dietary Diversity in South-eastern Nigeria. Matern. Child Nutr. 2024, 20, e13599. [Google Scholar] [CrossRef]
- Rani, V.; Arends, D.E.; Brouwer, I.D. Dietary Diversity as an Indicator of Micronutrient Adequacy of the Diet of Five to Eight Year Old Indian Rural Children. Nutr. Food Sci. 2010, 40, 466–476. [Google Scholar] [CrossRef]
- Vogliano, C.; Raneri, J.E.; Coad, J.; Tutua, S.; Wham, C.; Lachat, C.; Burlingame, B. Dietary Agrobiodiversity for Improved Nutrition and Health Outcomes within a Transitioning Indigenous Solomon Island Food System. Food Sec. 2021, 13, 819–847. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, T. Can Non-Farm Employment Improve Dietary Diversity of Left-Behind Family Members in Rural China? Foods 2024, 13, 1818. [Google Scholar] [CrossRef]
- Krivonos, E.; Kuhn, L. Trade and Dietary Diversity in Eastern Europe and Central Asia. Food Policy 2019, 88, 101767. [Google Scholar] [CrossRef]
- Miyamoto, K.; Kawase, F.; Imai, T.; Sezaki, A.; Shimokata, H. Dietary Diversity and Healthy Life Expectancy—An International Comparative Study. Eur. J. Clin. Nutr. 2019, 73, 395–400. [Google Scholar] [CrossRef]
- Lachat, C.; Raneri, J.E.; Smith, K.W.; Kolsteren, P.; Van Damme, P.; Verzelen, K.; Penafiel, D.; Vanhove, W.; Kennedy, G.; Hunter, D.; et al. Dietary Species Richness as a Measure of Food Biodiversity and Nutritional Quality of Diets. Proc. Natl. Acad. Sci. USA 2018, 115, 127–132. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Fan, H.; Tian, X. Rising Food Accessibility Contributed to the Increasing Dietary Diversity in Rural and Urban China. Asia Pac. J. Clin. Nutr. 2017, 26, 738–747. [Google Scholar] [PubMed]
- Borkotoky, K.; Unisa, S.; Gupta, A.K. State-Level Dietary Diversity as a Contextual Determinant of Nutritional Status of Children in India: A Multilevel Approach. J. Biosoc. Sci. 2018, 50, 26–52. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Chih, T.Y.; Oddy, W.H. Food Variety at 2 Years of Age Is Related to Duration of Breastfeeding. Nutrients 2012, 4, 1464–1474. [Google Scholar] [CrossRef]
- Zimmer, M.H.; Hart, L.C.; Manning-Courtney, P.; Murray, D.S.; Bing, N.M.; Summer, S. Food Variety as a Predictor of Nutritional Status Among Children with Autism. J. Autism Dev. Disord. 2012, 42, 549–556. [Google Scholar] [CrossRef]
- Drescher, L.S.; Thiele, S.; Mensink, G.B. A New Index to Measure Healthy Food Diversity Better Reflects a Healthy Diet than Traditional Measures, 2. J. Nutr. 2007, 137, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Vadiveloo, M.; Dixon, L.B.; Mijanovich, T.; Elbel, B.; Parekh, N. Development and Evaluation of the US Healthy Food Diversity Index. Br. J. Nutr. 2014, 112, 1562–1574. [Google Scholar] [CrossRef]
- Verger, E.O.; Le Port, A.; Borderon, A.; Bourbon, G.; Moursi, M.; Savy, M.; Mariotti, F.; Martin-Prevel, Y. Dietary Diversity Indicators and Their Associations with Dietary Adequacy and Health Outcomes: A Systematic Scoping Review. Adv. Nutr. 2021, 12, 1659–1672. [Google Scholar] [CrossRef]
- Marshall, S.; Burrows, T.; Collins, C.E. Systematic Review of Diet Quality Indices and Their Associations with Health-related Outcomes in Children and Adolescents. J. Hum. Nutr. Diet. 2014, 27, 577–598. [Google Scholar] [CrossRef]
- Hanley-Cook, G.T.; Argaw, A.; de Kok, B.; Toe, L.C.; Dailey-Chwalibóg, T.; Ouédraogo, M.; Kolsteren, P.; Huybregts, L.; Lachat, C. Seasonality and Day-to-Day Variability of Dietary Diversity: Longitudinal Study of Pregnant Women Enrolled in a Randomized Controlled Efficacy Trial in Rural Burkina Faso. J. Nutr. 2022, 152, 2145–2154. [Google Scholar] [CrossRef]
- Hanley-Cook, G.T.; Daly, A.J.; Remans, R.; Jones, A.D.; Murray, K.A.; Huybrechts, I.; De Baets, B.; Lachat, C. Food Biodiversity: Quantifying the Unquantifiable in Human Diets. Crit. Rev. Food Sci. Nutr. 2023, 63, 7837–7851. [Google Scholar] [CrossRef]
- Pla, L.; Casanoves, F.; Di Rienzo, J. Functional Diversity Indices. In Quantifying Functional Biodiversity; SpringerBriefs in Environmental Science; Springer: Dordrecht, The Netherlands, 2012; pp. 27–51. ISBN 978-94-007-2647-5. [Google Scholar]
- Luckett, B.G.; DeClerck, F.A.; Fanzo, J.; Mundorf, A.R.; Rose, D. Application of the Nutrition Functional Diversity Indicator to Assess Food System Contributions to Dietary Diversity and Sustainable Diets of Malawian Households. Public Health Nutr. 2015, 18, 2479–2487. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Akbari, F.; Bellissimo, N.; Azadbakht, L. Dietary Diversity Score and Obesity: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Clin. Nutr. 2016, 70, 1–9. [Google Scholar] [CrossRef]
- Ricotta, C.; Szeidl, L. Towards a Unifying Approach to Diversity Measures: Bridging the Gap between the Shannon Entropy and Rao’s Quadratic Index. Theor. Popul. Biol. 2006, 70, 237–243. [Google Scholar] [CrossRef]
- Patil, G.P.; Taillie, C. Diversity as a Concept and Its Measurement. J. Am. Stat. Assoc. 1982, 77, 548–561. [Google Scholar] [CrossRef]
- Rao, C.R. Diversity and Dissimilarity Coefficients: A Unified Approach. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Rao, C.R. Diversity: Its Measurement, Decomposition, Apportionment and Analysis. Sankhyā Indian J. Stat. Ser. A 1982, 44, 1–22. [Google Scholar]
- Green, A.; Nemecek, T.; Smetana, S.; Mathys, A. Reconciling Regionally-Explicit Nutritional Needs with Environmental Protection by Means of Nutritional Life Cycle Assessment. J. Clean. Prod. 2021, 312, 127696. [Google Scholar] [CrossRef]
- Wang, X.-W.; Hu, Y.; Menichetti, G.; Grodstein, F.; Bhupathiraju, S.N.; Sun, Q.; Zhang, X.; Hu, F.B.; Weiss, S.T.; Liu, Y.-Y. Nutritional Redundancy in the Human Diet and Its Application in Phenotype Association Studies. Nat. Commun. 2023, 14, 4316. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Hirvonen, K.; Bai, Y.; Headey, D.; Masters, W.A. Affordability of the EAT–Lancet Reference Diet: A Global Analysis. Lancet Glob. Health 2020, 8, e59–e66. [Google Scholar] [CrossRef]
- Van Selm, B.; Frehner, A.; De Boer, I.J.; Van Hal, O.; Hijbeek, R.; Van Ittersum, M.K.; Talsma, E.F.; Lesschen, J.P.; Hendriks, C.M.; Herrero, M. Circularity in Animal Production Requires a Change in the EAT-Lancet Diet in Europe. Nat. Food 2022, 3, 66–73. [Google Scholar] [CrossRef]
- Cai, H.; Talsma, E.F.; Chang, Z.; Wen, X.; Fan, S.; Van’t Veer, P.; Biesbroek, S. Health Outcomes, Environmental Impacts, and Diet Costs of Adherence to the EAT-Lancet Diet in China in 1997–2015: A Health and Nutrition Survey. Lancet Planet. Health 2024, 8, e1030–e1042. [Google Scholar] [CrossRef]
- Springmann, M.; Spajic, L.; Clark, M.A.; Poore, J.; Herforth, A.; Webb, P.; Rayner, M.; Scarborough, P. The Healthiness and Sustainability of National and Global Food Based Dietary Guidelines: Modelling Study. BMJ 2020, 370, m2322. [Google Scholar] [CrossRef]
- Theil, H.; Finke, R. The Consumer’s Demand for Diversity. Eur. Econ. Rev. 1983, 23, 395–400. [Google Scholar] [CrossRef]
- Pingali, P. Westernization of Asian Diets and the Transformation of Food Systems: Implications for Research and Policy. Food Policy 2007, 32, 281–298. [Google Scholar] [CrossRef]
- Popkin, B.M. Nutrition, Agriculture and the Global Food System in Low and Middle Income Countries. Food Policy 2014, 47, 91–96. [Google Scholar] [CrossRef]
- Hovhannisyan, V.; Devadoss, S. Effects of Urbanization on Food Demand in China. Empir. Econ. 2020, 58, 699–721. [Google Scholar] [CrossRef]
- Springmann, M.; Clark, M.A.; Rayner, M.; Scarborough, P.; Webb, P. The Global and Regional Costs of Healthy and Sustainable Dietary Patterns: A Modelling Study. Lancet Planet. Health 2021, 5, e797–e807. [Google Scholar] [CrossRef]
- Miller, V.; Webb, P.; Cudhea, F.; Shi, P.; Zhang, J.; Reedy, J.; Erndt-Marino, J.; Coates, J.; Mozaffarian, D. Global Dietary Quality in 185 Countries from 1990 to 2018 Show Wide Differences by Nation, Age, Education, and Urbanicity. Nat. Food 2022, 3, 694–702. [Google Scholar] [CrossRef]
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef] [PubMed]
| Considering the Nutrition Functional Dissimilarity | |||
|---|---|---|---|
| NO | YES | ||
| Considering the dietary guidelines | (1) Species-neutral indices | (2) Functional dissimilarity indices | |
| NO | |||
| (3) Dietary guideline-based species-neutral indices | (4) Dietary guideline-based functional dissimilarity indices | ||
| YES | |||
| Foods | 1981–2022 | 1981–2000 | 2001–2022 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantity (kg/year) | Energy (kcal/d) | Quantity (kg/year) | Energy (kcal/d) | Quantity (kg/year) | Energy (kcal/d) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Cereals | 146.8 | 54.3 | 1501.6 | 555.6 | 143.9 | 56.9 | 1471.9 | 581.8 | 149.2 | 52.0 | 1526.1 | 531.9 |
| Tubers | 56.3 | 63.8 | 121.9 | 137.9 | 54.5 | 71.3 | 118.0 | 154.2 | 57.8 | 56.8 | 125.1 | 122.8 |
| Pulses | 6.8 | 6.4 | 18.4 | 17.4 | 6.7 | 6.3 | 18.2 | 17.1 | 6.8 | 6.5 | 18.6 | 17.6 |
| Oils | 12.3 | 6.8 | 302.9 | 167.7 | 11.2 | 6.7 | 274.9 | 165.5 | 13.2 | 6.7 | 326.0 | 165.9 |
| Pork | 11.7 | 15.0 | 44.0 | 56.1 | 10.2 | 14.6 | 38.2 | 54.9 | 13.0 | 15.1 | 48.8 | 56.6 |
| Beef and mutton | 14.7 | 13.6 | 55.7 | 51.5 | 15.3 | 15.2 | 57.9 | 57.5 | 14.2 | 12.1 | 53.9 | 45.8 |
| Poultry | 15.5 | 15.3 | 40.9 | 40.3 | 11.0 | 12.0 | 29.0 | 31.5 | 19.3 | 16.7 | 50.8 | 44.0 |
| Eggs | 6.3 | 5.3 | 22.9 | 19.2 | 5.6 | 5.0 | 20.3 | 18.2 | 6.9 | 5.4 | 25.1 | 19.7 |
| Dairy | 88.1 | 73.9 | 161.6 | 135.6 | 79.7 | 70.4 | 146.1 | 129.1 | 95.1 | 76.0 | 174.3 | 139.4 |
| Seafood | 15.0 | 15.3 | 42.1 | 43.0 | 14.2 | 15.6 | 40.0 | 43.7 | 15.6 | 15.1 | 43.8 | 42.4 |
| Vegetables | 84.1 | 64.9 | 62.8 | 48.5 | 72.1 | 58.7 | 53.9 | 43.9 | 93.9 | 67.9 | 70.2 | 50.8 |
| Fruits | 80.4 | 56.5 | 110.6 | 77.7 | 73.4 | 54.1 | 101.0 | 74.4 | 86.1 | 57.7 | 118.5 | 79.4 |
| Nuts | 5.1 | 5.7 | 59.2 | 66.0 | 3.9 | 4.7 | 45.2 | 54.3 | 6.1 | 6.2 | 70.8 | 72.3 |
| Sugar | 35.0 | 21.5 | 291.1 | 179.2 | 30.6 | 18.6 | 254.7 | 155.2 | 38.6 | 23.0 | 321.2 | 191.7 |
| Energy | 2835.7 | 2669.2 | 2973.2 | |||||||||
| Obs | 104,314 | 47,166 | 57,148 | |||||||||
| EI | QE | DE | DQE | BI | QB | DB | DQB | |
|---|---|---|---|---|---|---|---|---|
| EI | 1 | |||||||
| QE | 0.967 *** | 1 | ||||||
| DE | −0.150 *** | −0.147 *** | 1 | |||||
| DQE | 0.550 *** | 0.521 *** | 0.515 *** | 1 | ||||
| BI | 0.981 *** | 0.953 *** | −0.238 *** | 0.527 *** | 1 | |||
| QB | 0.975 *** | 0.997 *** | −0.168 *** | 0.530 *** | 0.972 *** | 1 | ||
| DB | 0.453 *** | 0.435 *** | 0.575 *** | 0.950 *** | 0.445 *** | 0.447 *** | 1 | |
| DQB | −0.313 *** | −0.347 *** | 0.826 *** | 0.451 *** | −0.365 *** | −0.357 *** | 0.449 *** | 1 |
| (1) Species-Neutral Indices | (2) Functional Dissimilarity Indices | (3) Dietary Guideline-Based Species-Neutral Indices | (4) Dietary Guideline-Based Functional Dissimilarity Indices | |||||
|---|---|---|---|---|---|---|---|---|
| EI | BI | QE | QB | DE | DB | DQE | DQB | |
| Mean | 0.666 | 0.721 | 0.559 | 0.615 | 0.914 | 0.867 | 0.813 | 0.779 |
| SD | 0.173 | 0.175 | 0.171 | 0.171 | 0.082 | 0.093 | 0.117 | 0.118 |
| CV | 0.260 | 0.243 | 0.306 | 0.278 | 0.090 | 0.107 | 0.144 | 0.151 |
| Skewness | 0.484 | 0.834 | 0.379 | 0.547 | 3.505 | 1.861 | 1.362 | 2.359 |
| Kurtosis | 2.599 | 3.132 | 2.524 | 2.724 | 21.047 | 9.308 | 5.261 | 11.248 |
| 7409 | 7409 | 7409 | 7409 | 7409 | 7409 | 7409 | 7409 | |
| Dietary Diversity Indices | Geographical Variation Tests | Temporal Trend Tests | ||||||
|---|---|---|---|---|---|---|---|---|
| Bartlett’s Equal Variances Test | ANOVA | Fixed Effects | Random Effects | |||||
| χ2 | p-Value | F | p-Value | Coef | p-Value | Inter-Regional Variance | p-Value | |
| EI | 608.338 | 0.000 | 446.640 | 0.000 | 0.0024 | 0.000 | 0.0242 | 0.000 |
| QE | 574.357 | 0.000 | 363.580 | 0.000 | 0.0026 | 0.000 | 0.0204 | 0.000 |
| DE | 310.340 | 0.000 | 163.880 | 0.000 | 0.0008 | 0.000 | 0.0008 | 0.000 |
| DQE | 566.338 | 0.000 | 74.950 | 0.000 | 0.0018 | 0.000 | 0.0027 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, S.; Zhu, W. Measuring Global Dietary Diversity by Considering Nutritional Functional Dissimilarity and Dietary Guidelines. Foods 2025, 14, 1759. https://doi.org/10.3390/foods14101759
Quan S, Zhu W. Measuring Global Dietary Diversity by Considering Nutritional Functional Dissimilarity and Dietary Guidelines. Foods. 2025; 14(10):1759. https://doi.org/10.3390/foods14101759
Chicago/Turabian StyleQuan, Shiwen, and Wenbo Zhu. 2025. "Measuring Global Dietary Diversity by Considering Nutritional Functional Dissimilarity and Dietary Guidelines" Foods 14, no. 10: 1759. https://doi.org/10.3390/foods14101759
APA StyleQuan, S., & Zhu, W. (2025). Measuring Global Dietary Diversity by Considering Nutritional Functional Dissimilarity and Dietary Guidelines. Foods, 14(10), 1759. https://doi.org/10.3390/foods14101759






