Data Analysis in Newly Developed Milk Sensor Platforms: Good Practices, Common Pitfalls, and Hard-Earned Lessons from Field Application
Abstract
1. Introduction
2. Materials and Methods
2.1. The ALERT Project
2.2. The MOLOKO Project
3. Results
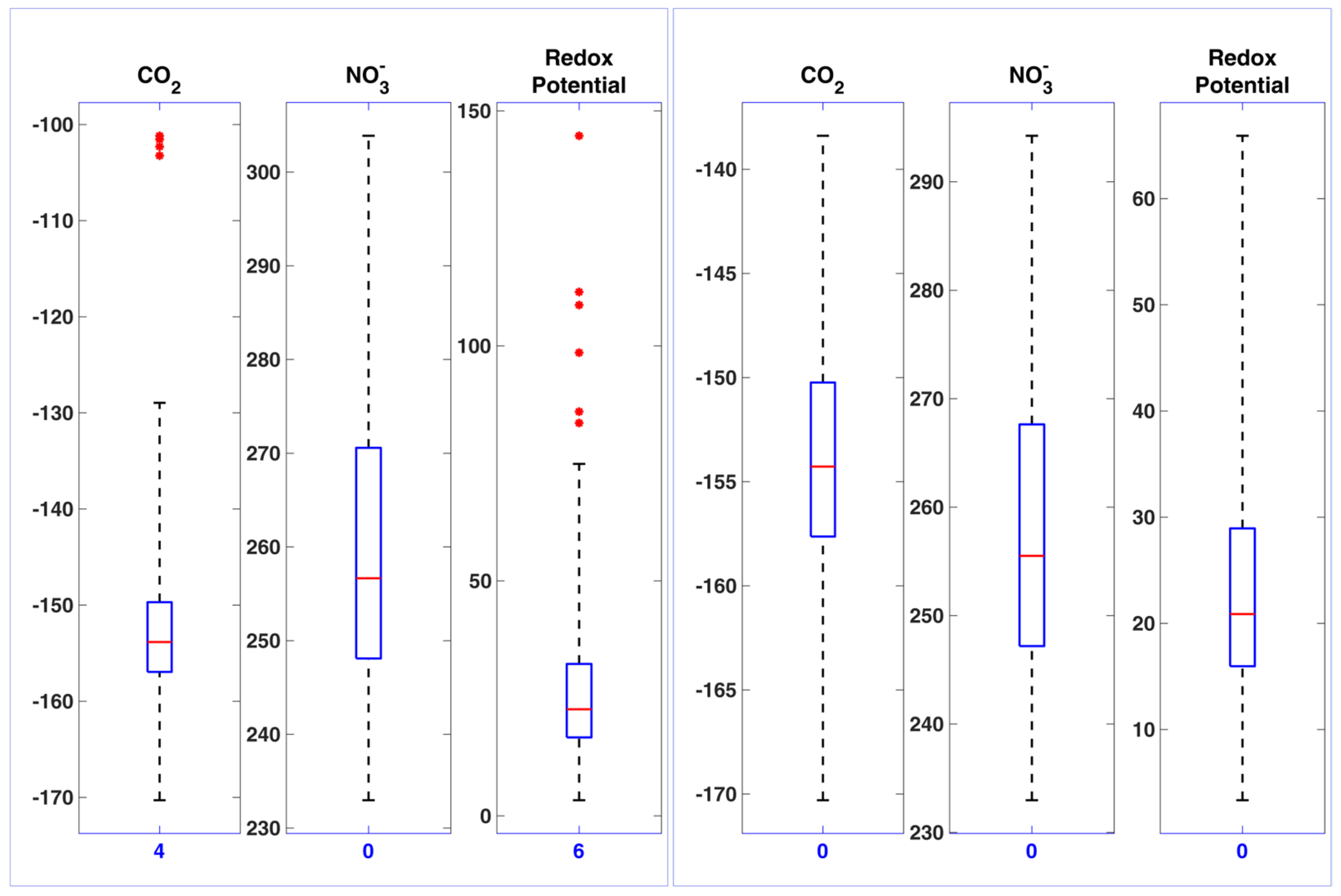
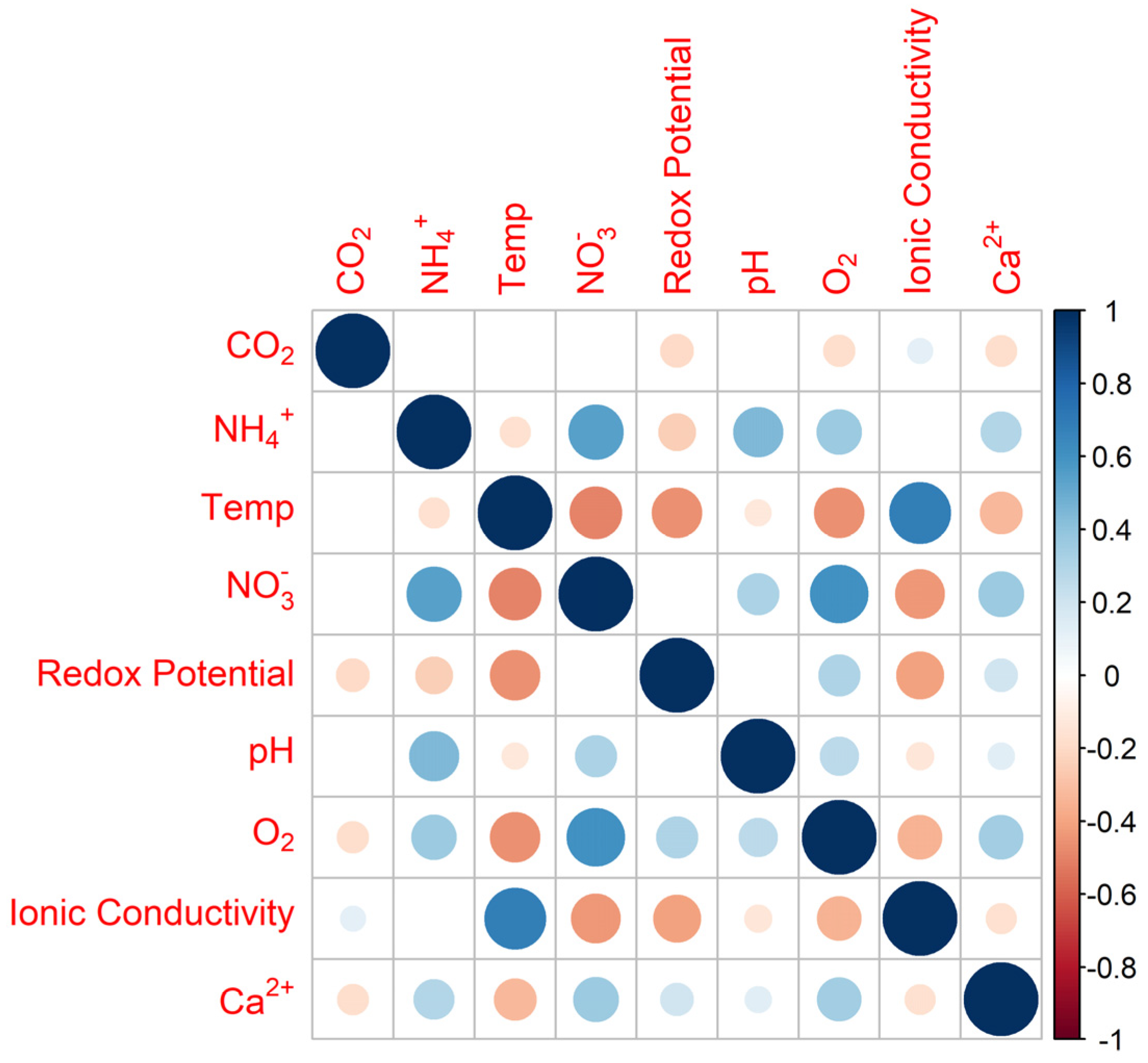
3.1. The Need for a Settled Knowledge of Sensors and Instrumentation Behavior
- Si(1..ni) ni individual milk daily sensor’s values for day i
- (i = 1.. 90, ni variable from 5 to 12, mean 9.09 sd = 2.02)
- Daily median sensor value over day i
- Median sensor value over all days i
- Daily detrended sensor values were calculated as:The Formula (1) can be read as follows: Daily detrended sensor values = Daily sensor values − Daily median sensor value + Overall median sensor value (three-month period).Di(1..ni) = Si(1..ni) − mi + M
3.2. The Need for a Predefined Lab Measurement Protocol Including an On-The Field Clear Sampling Plan and Strategy
3.3. Dealing with the Unexpected: Getting the Most from Instrument Prototypes
4. Discussion
- -
- A comprehensive understanding of sensors and instrumentation behavior (see Section 3.1): Specifications and guidelines provided by design and testing laboratories are essential; however, they are not sufficient on their own. Each sensor must be fully characterized under actual working conditions once installed in the field. This characterization should include, among other factors, calibration procedures, warm-up time, and chemical conditioning requirements.
- -
- The establishment of a predefined, detailed laboratory measurement protocol: Such a protocol would benefit both in-field measurements using sensor-based technologies and their integration with laboratory-based analytical techniques, such as MS. Sensor-based devices can complement gold-standard methods by serving as rapid, preliminary screening tools, thereby reducing the number of samples requiring labor-intensive and time-consuming MS analysis. This approach not only enhances the overall efficiency and accuracy of contaminant detection but also optimizes workflow, improves resource allocation, and accelerates decision-making in food safety assessments.
- -
- The inclusion of a clear sampling plan and field measurement strategy in the laboratory protocol (see Section 3.2): The protocol should be iteratively tested and validated in advance, and must be shared with, and agreed upon by, all stakeholders involved in data management and analysis. At a minimum, it should address key factors such as environmental conditions, ensuring that the sampling process is robust, reproducible, and suitable for integration with both field and laboratory procedures.
- -
- The development of a comprehensive sampling plan and associated operational framework: The sampling plan should clearly define the overall strategy, including sample size, selection of sampling locations, and sampling frequency. It must specify procedures for sample collection, handling, and storage to ensure consistency and integrity. In addition, the measurement methods to be used should be explicitly described. The protocol should also identify the personnel involved, detailing their roles and required training. Furthermore, data recording and management must be addressed through the design, construction, and testing of a structured database, accompanied by a user manual to ensure proper and consistent use.
- -
- A quality control preparedness to address unexpected instrument behavior and failures: In field campaigns—particularly when using internally developed prototypes or deploying new instrumentation in technologically challenging environments such as dairy farms—unexpected hardware failures can occur. To ensure the continuity and success of measurements, it is essential to maintain duplicates of entire measurement systems and/or critical hardware components. This redundancy supports rapid recovery from failures and minimizes disruptions during data collection.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| MS | Mass Spectrometry |
| MS/MS | tandem Mass Spectrometry |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GC | Gas Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| qPCR | quantitative Polymerase Chain Reaction |
| CCP | Critical Control Point |
| POC | Point of Care |
| POPA | Point of Particular Attention |
| SEA | Staphylococcus aureus enterotoxin A |
| ISE | Ion Selective Electrode |
| CSV | Comma Separated Values |
References
- Henke, K.A.; Alter, T.; Doherr, M.G.; Merle, R. From Stable to Table: Determination of German Consumer Perceptions of the Role of Multiple Aspects of Poultry Production on Meat Quality and Safety. J. Food Prot. 2021, 84, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lee, H.-Y.; Lin, Y.-T.; Liu, C.-W.; Tsai, P.F. Consumers’ Willingness to Pay for Organic Foods in China: Bibliometric Review for an Emerging Literature. Int. J. Environ. Res. Public Health 2019, 16, 1713. [Google Scholar] [CrossRef]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Logrieco, A.F.; Cho, G.-S.; Kabisch, J.; Böhnlein, C.; Franz, C.M.A.P. Microbial quality and safety of milk and milk products in the 21st century. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2013–2049. [Google Scholar] [CrossRef] [PubMed]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kang’ethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A review of the impact of mycotoxins on dairy cattle health: Challenges for food safety and dairy production in sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef]
- Akinyemi, M.O.; Ayeni, K.I.; Ogunremi, O.R.; Adeleke, R.A.; Oguntoyinbo, F.A.; Warth, B.; Ezekiel, C.N. A review of microbes and chemical contaminants in dairy products in sub-Saharan Africa. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1188–1220. [Google Scholar] [CrossRef]
- Duchenne-Moutien, R.A.; Neetoo, H. Climate Change and Emerging Food Safety Issues: A Review. J. Food Prot. 2021, 84, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics and Nanoplastics: Emerging Contaminants in Food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef]
- Gomes, M.P.; Rocha, D.C.; Moreira de Brito, J.C.; Tavares, D.S.; Marques, R.Z.; Soffiatti, P.; Sant’Anna-Santos, B.F. Emerging Contaminants in Water Used for Maize Irrigation: Economic and Food Safety Losses Associated with Ciprofloxacin and Glyphosate. Ecotoxicol. Environ. Saf. 2020, 196, 110549. [Google Scholar] [CrossRef]
- Grasso, G.; Zane, D.; Dragone, R. Field and Remote Sensors for Environmental Health and Food Safety Diagnostics: An Open Challenge. Biosensors 2022, 12, 285. [Google Scholar] [CrossRef]
- Xu, M.L.; Gao, Y.; Wang, X.; Han, X.X.; Zhao, B. Comprehensive strategy for sample preparation for the analysis of food contaminants and residues by GC–MS/MS: A review of recent research trends. Foods 2021, 10, 2473. [Google Scholar] [CrossRef]
- Domínguez, I.; Frenich, A.G.; Romero-González, R. Mass spectrometry approaches to ensure food safety. Anal. Methods 2020, 12, 1148–1162. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Tranchida, P.Q. (Ed.) Advanced Gas Chromatography in Food Analysis; Royal Society of Chemistry: Cambridge, UK, 2019; Volume 17. [Google Scholar] [CrossRef]
- Custodio-Mendoza, J.A.; Pokorski, P.; Aktaş, H.; Napiórkowska, A.; Kurek, M.A. Advances in chromatographic analysis of phenolic phytochemicals in foods: Bridging gaps and exploring new horizons. Foods 2024, 13, 2268. [Google Scholar] [CrossRef]
- Ren, W.; Sun, M.; Shi, X.; Wang, T.; Wang, Y.; Wang, C.; Li, M. Progress of Mass Spectrometry-Based Lipidomics in the Dairy Field. Foods 2023, 12, 2098. [Google Scholar] [CrossRef]
- Yeo, J.; Kang, J.; Kim, H.; Moon, C. A Critical Overview of HPLC-MS-Based Lipidomics in Determining Triacylglycerol and Phospholipid in Foods. Foods 2023, 12, 3177. [Google Scholar] [CrossRef]
- Grasso, G.; Caracciolo, L.; Cocco, G.; Frazzoli, C.; Dragone, R. Towards simazine monitoring in agro-zootechnical productions: A yeast cell bioprobe for real samples screening. Biosensors 2018, 8, 112. [Google Scholar] [CrossRef]
- Farag, M.A.; Tanios, M.; AlKarimy, S.; Ibrahim, H.; Guirguis, H.A. Biosensing approaches to detect potential milk contaminants: A comprehensive review. Food Addit. Contam. Part A 2021, 38, 1169–1192. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Geng, S.; Chandrawati, R. Food sensors: Challenges and opportunities. Adv. Mater. Technol. 2021, 6, 2001242. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging Point-of-Care Technologies for Food Safety Analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef]
- Sakthivel, K.; Balasubramanian, S.; Chang-Chien, G.-P.; Wang, S.-F.; Ahammad; Billey, W.; Platero, J.; Soundappan, T.; Sekhar, P. Editors’ Choice—Review—Advances in Electrochemical Sensors: Improving Food Safety, Quality, and Traceability. ECS Sens. Plus 2024, 3, 020605. [Google Scholar] [CrossRef]
- Fernández González, A.; Badía Laíño, R.; Costa-Fernández, J.M.; Soldado, A. Progress and Challenge of Sensors for Dairy Food Safety Monitoring. Sensors 2024, 24, 1383. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, H.; Mann, B.; Seth, R. Colorimetric determination of melamine in milk using unmodified silver nanoparticles. Spectrochim. Acta A 2016, 156, 89–97. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Sun, C. Chemical sensors and biosensors for the detection of melamine. RSC Adv. 2015, 5, 1125–1147. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Nanomaterial-Based Melamine Detection. Chemosensors 2019, 7, 9. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Anti-Aggregation-Enabled Colorimetric Sensing Applications of Gold and Silver Nanoparticles. Chemosensors 2022, 10, 536. [Google Scholar] [CrossRef]
- Sun, Q.; Dong, Y.; Wen, X.; Zhang, X.; Hou, S.; Zhao, W.; Yin, D. A review on recent advances in mass spectrometry analysis of harmful contaminants in food. Front. Nutr. 2023, 10, 1244459. [Google Scholar] [CrossRef]
- Weihs, F.; Anderson, A.; Trowell, S.; Caron, K. Resonance Energy Transfer-Based Biosensors for Point-of-Need Diagnosis-Progress and Perspectives. Sensors 2021, 21, 660. [Google Scholar] [CrossRef]
- Sridhar, A.; Kapoor, A.; Kumar, P.S.; Ponnuchamy, M.; Sivasamy, B.; Vo, D.-V.N. Lab-on-a-Chip Technologies for Food Safety, Processing, and Packaging Applications: A Review. Environ. Chem. Lett. 2022, 20, 901–927. [Google Scholar] [CrossRef]
- Arreguin-Campos, R.; Jiménez-Monroy, K.L.; Diliën, H.; Cleij, T.J.; van Grinsven, B.; Eersels, K. Imprinted Polymers as Synthetic Receptors in Sensors for Food Safety. Biosensors 2021, 11, 46. [Google Scholar] [CrossRef]
- Wu, G.; Li, H.; Chen, J.; Lai, K.W.; Xiong, L.; Yang, R. Microfluidics Enhanced Biosensor Technology for Advancing Food Safety Monitoring. Trends Food Sci. Technol. 2024, 151, 104556. [Google Scholar] [CrossRef]
- Camarca, A.; Varriale, A.; Capo, A.; Pennacchio, A.; Calabrese, A.; Giannattasio, C.; Murillo Almuzara, C.; D’Auria, S.; Staiano, M. Emergent Biosensing Technologies Based on Fluorescence Spectroscopy and Surface Plasmon Resonance. Sensors 2021, 21, 906. [Google Scholar] [CrossRef] [PubMed]
- Curulli, A. Electrochemical Biosensors in Food Safety: Challenges and Perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.; Hu, C.; Wang, Y.; Wang, L.; Peng, F.; Geng, P.; Guan, M. Recent Advancements in Microfluidic Chip Biosensor Detection of Foodborne Pathogenic Bacteria: A Review. Anal. Bioanal. Chem. 2022, 414, 2883–2902. [Google Scholar] [CrossRef] [PubMed]
- Lambrini, K.; Aikaterini, F.; Konstantinos, K.; Christos, I.; Ioanna, P.V.; Areti, T. Milk Nutritional Composition and Its Role in Human Health. J. Pharm. Pharmacol. 2021, 9, 8–13. [Google Scholar] [CrossRef]
- Chiesa, L.M.; DeCastelli, L.; Nobile, M.; Martucci, F.; Mosconi, G.; Fontana, M.; Castrica, M.; Arioli, F.; Panseri, S. Analysis of antibiotic residues in raw bovine milk and their impact toward food safety and on milk starter cultures in cheese-making process. LWT 2020, 131, 109783. [Google Scholar] [CrossRef]
- Borda, D.; Wilson, L.; Banu, I.; Bleoanca, I.; Ballabio, D.; Mannino, S.; Zheleva, I.; Todorov, T.; Bozkurt, H.; Göğüş, F. Statistical Process Control (SPC). In Statistics in Food and Biotechnology; Schleining, G., Mannino, S., Suwannaporn, P., Eds.; Integrating Food Science and Engineering Knowledge into the Food Chain; Springer: Cham, Switzerland, 2024; Volume 16, pp. 67–91. [Google Scholar] [CrossRef]
- Lin, P.-H.; Li, B.-R. Antifouling Strategies in Advanced Electrochemical Sensors and Biosensors. Analyst 2020, 145, 1110–1120. [Google Scholar] [CrossRef]
- Martelli, F.; Giacomozzi, C.; Dragone, R.; Boselli, C.; Amatiste, S.; Brajon, G.; Grasso, G.; Mantovani, A.; Frazzoli, C. Official Control and Self-Monitoring: Data Agreement Report in the Integrated Food Safety System of an Italian Dairy Chain. Int. Dairy J. 2019, 97, 185–190. [Google Scholar] [CrossRef]
- Ozella, L.; Brotto Rebuli, K.; Forte, C.; Giacobini, M. A Literature Review of Modeling Approaches Applied to Data Collected in Automatic Milking Systems. Animals 2023, 13, 1916. [Google Scholar] [CrossRef]
- Lombardo, A.; Boselli, C.; Amatiste, S.; Ninci, S.; Frazzoli, C.; Dragone, R.; De Rossi, A.; Grasso, G.; Mantovani, A.; Brajon, G. From Invention to Innovation: Risk Analysis to Integrate One Health Technology in the Dairy Farm. Front. Public Health 2017, 5, 302. [Google Scholar] [CrossRef]
- Frazzoli, C.; Mantovani, A.; Campanella, L.; Dragone, R. Technological Integrated Bioelectronic System and Relevant Control Charting for Early Intervention on Food Chain and the Environment/System for Environment Diagnostic and Monitoring and Self-Control of Food Chain (BEST). WO2010001432A1, 2010. Available online: https://patents.google.com/patent/WO2010001432A1/en?q=G01N33%2f02&inventor=frazzoli (accessed on 8 May 2025).
- Lewis, M.J. Physical and Physicochemical Properties of Milk and Milk Products. In Advanced Dairy Chemistry; McSweeney, P.L.H., O’Mahony, J.A., Kelly, A.L., Eds.; Springer: Cham, Switzerland, 2022; pp. 617–664. [Google Scholar] [CrossRef]
- Abraham, S.; Cachon, R.; Jeanson, S.; Ebel, B.; Michelon, D.; Aubert, C.; Rojas, G.; Feron, E.; Beuvier, P.; Gervais, A. A procedure for reproducible measurement of redox potential (eh) in dairy processes. Dairy Sci. Technol. 2013, 93, 675–690. [Google Scholar] [CrossRef]
- Alwazeer, D. Importance of Consideration of Oxidoreduction Potential as a Critical Quality Parameter in Food Industries. Food Res. Int. 2020, 132, 109108. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Physical properties of milk. In Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 321–343. [Google Scholar] [CrossRef]
- Aydogdu, T.; O’Mahony, J.A.; McCarthy, N.A. pH, the fundamentals for milk and dairy processing: A review. Dairy 2023, 4, 395–409. [Google Scholar] [CrossRef]
- Lewis, M.J. The measurement and significance of ionic calcium in milk—A review. Int. J. Dairy Technol. 2011, 64, 1–13. [Google Scholar] [CrossRef]
- Gaucheron, F.; Le Graet, Y. Determination of ammonium in milk and dairy products by ion chromatography. J. Chromatogr. A 2000, 893, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Indyk, H.E.; Woollard, D.C. Contaminants of Milk and Dairy Products: Nitrates and Nitrites as Contaminants. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 833–839. [Google Scholar] [CrossRef]
- Jóźwik, A.; Strzałkowska, N.; Bagnicka, E.; Grzybek, W.; Krzyżewski, J.; Poławska, E.; Kołataj, A.; Horbańczuk, J.O. Relationship between milk yield, stage of lactation, and some blood serum metabolic parameters of dairy cows. Czech J. Anim. Sci. 2012, 57, 353–360. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Salts of Milk. In Dairy Chemistry and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 241–270. [Google Scholar] [CrossRef]
- Stocco, G.; Summer, A.; Cipolat-Gotet, C.; Malacarne, M.; Cecchinato, A.; Amalfitano, N.; Bittante, G. The mineral profile affects the coagulation pattern and cheese-making efficiency of bovine milk. J. Dairy Sci. 2021, 104, 8439–8453. [Google Scholar] [CrossRef]
- ICAR (International Committee for Animal Recording) ICAR Guidelines, Section 11: Guidelines for Testing, Approval and Checking of Milk Recording Devices. 2012. Available online: https://www.icar.org (accessed on 5 December 2024).
- Bolognesi, M.; Prosa, M.; Toerker, M.; Lopez Sanchez, L.; Wieczorek, M.; Giacomelli, C.; Benvenuti, E.; Pellacani, P.; Elferink, A.; Morschhauser, A.; et al. A Fully Integrated Miniaturized Optical Biosensor for Fast and Multiplexing Plasmonic Detection of High-and Low-Molecular-Weight Analytes. Adv. Mater. 2023, 35, e2208719. [Google Scholar] [CrossRef]
- Elferink, A.J.W.; Entiriwaa, D.; Bulgarelli, P.; Smits, N.G.E.; Peters, J. Development of a Microsphere-Based Immunoassay Authenticating A2 Milk and Species Purity in the Milk Production Chain. Molecules 2022, 27, 3199. [Google Scholar] [CrossRef]
- Martelli, F.; Giacomozzi, C.; Fadda, A.; Frazzoli, C. Understanding Seasonal Changes to Improve Good Practices in Livestok Management. Front. Public Health 2018, 6, 175. [Google Scholar] [CrossRef]
- Osorio-Avalos, J.; Garza-Camargo, D.M.; Robles-Jimenez, L.E.; Plaza, J.; Abecia, J.A.; Palacios, C.; Gonzalez5Ronquillo, M. Influence of Mediterranean Climate and Lunar Calendar on Milk Production in Lacaune Breed Ewes. Int. J. Biometeorol. 2022, 66, 1191–1197. [Google Scholar] [CrossRef]
- Maksymiuk, K.; Stelmach, E.; Michalska, A. Unintended changes of ion-selective membranes composition—Origin and effect on analytical performance. Membranes 2020, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Motshakeri, M.; Sharma, M.; Phillips, A.R.; Kilmartin, P.A. Electrochemical methods for the analysis of milk. J. Agric. Food Chem. 2022, 70, 2427–2449. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Lee, W.C.; Kung, C.T.; Li, L.C.; Lee, C.T.; Fu, L.M. Recent advances in microfluidic devices for contamination detection and quality inspection of milk. Micromachines 2021, 12, 558. [Google Scholar] [CrossRef] [PubMed]
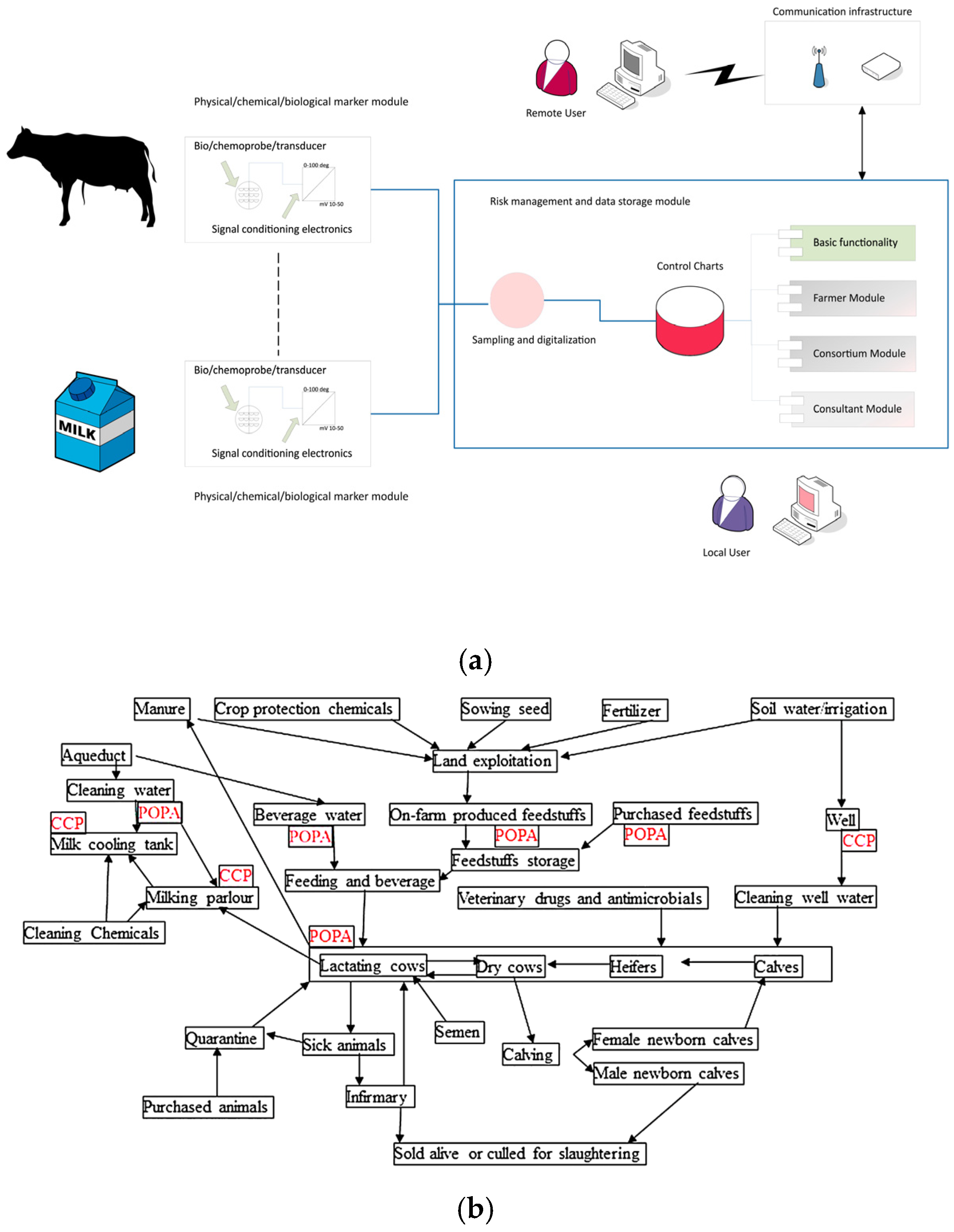
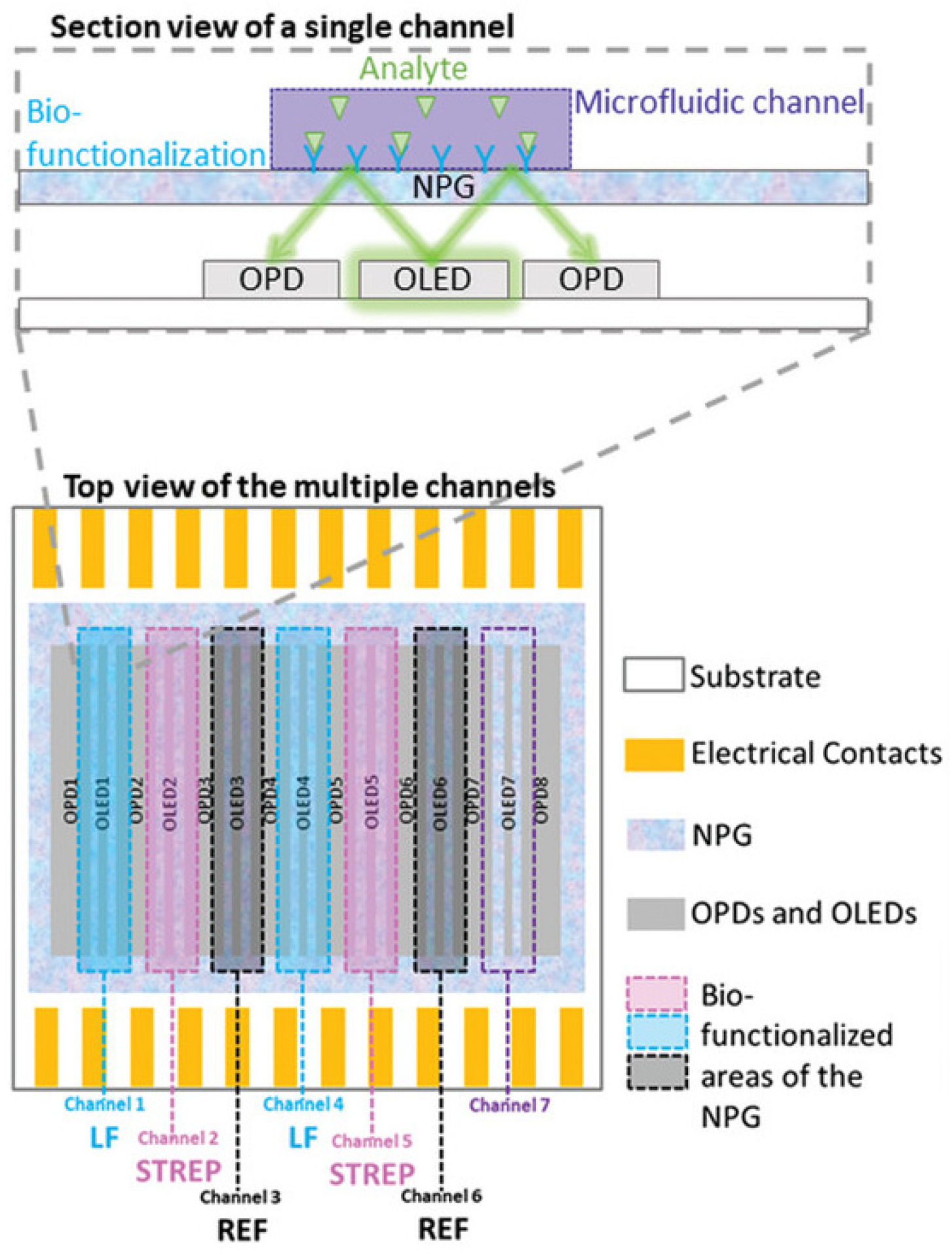
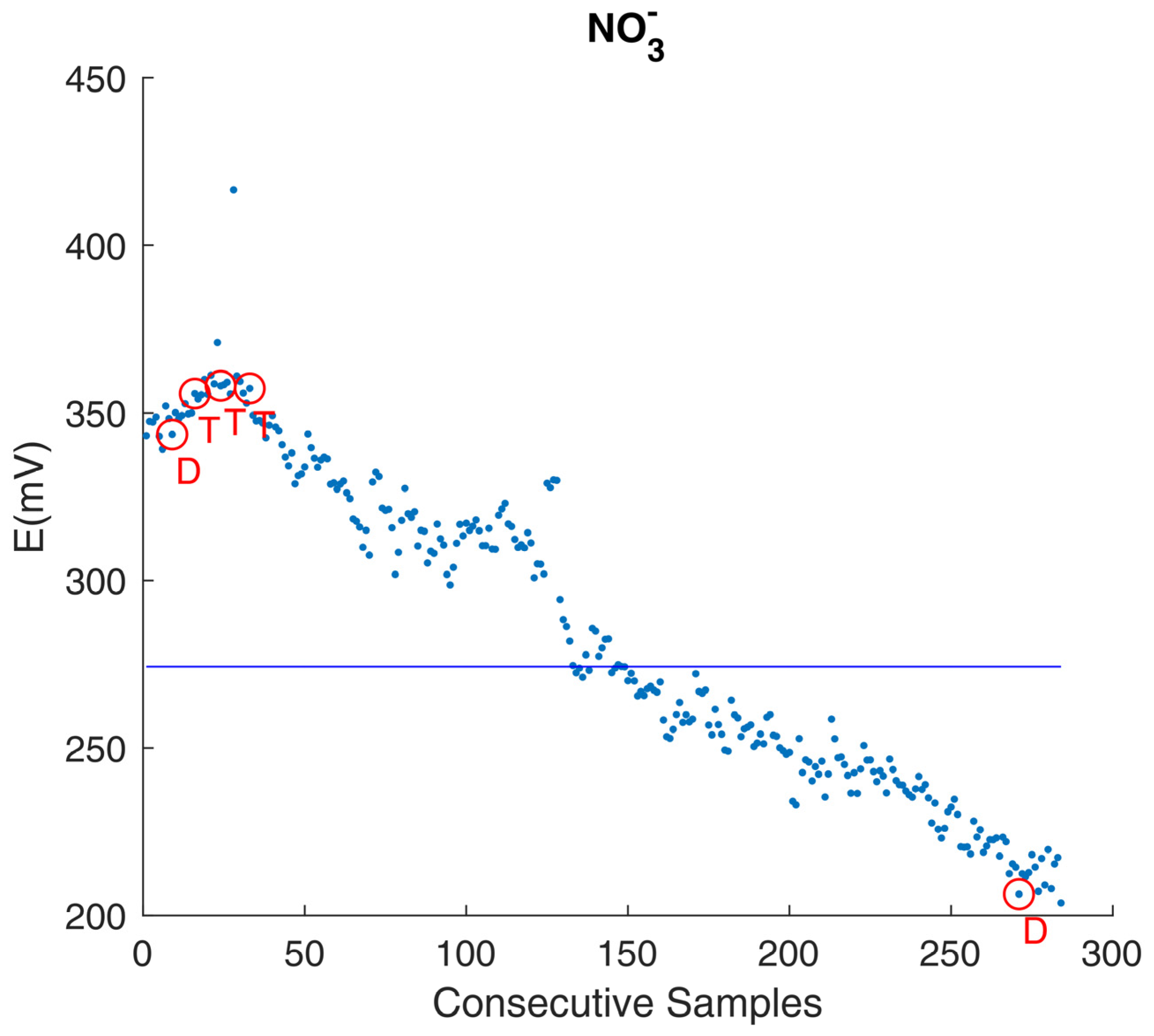
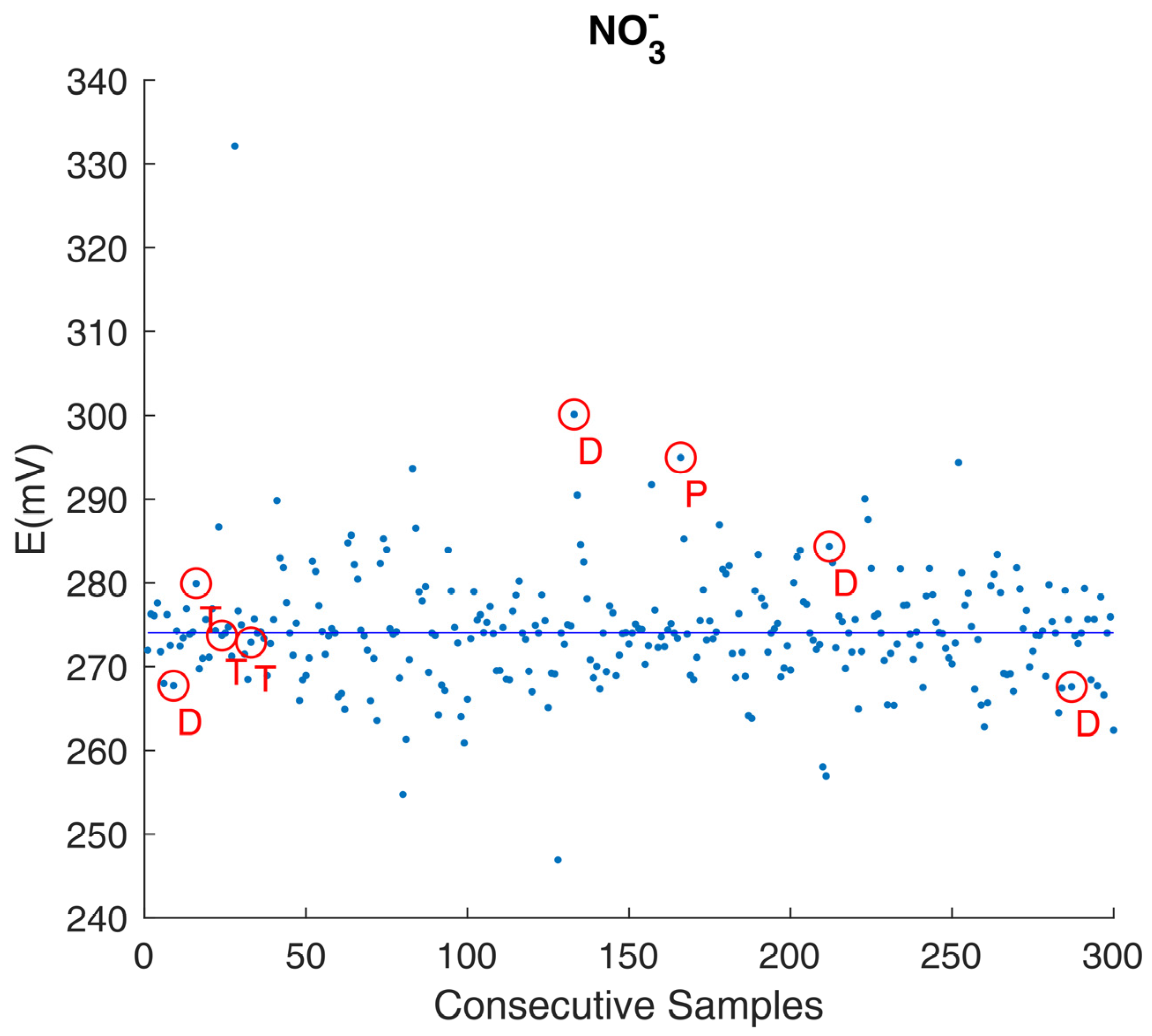
| Sensor | Measurement Range * | Other Specifications * |
|---|---|---|
| Ca2+ ISE | 0.02–4010.00 mg/L | Sensitivity: +25 to +30 mV per decade change in ion activity |
| NH4+ ISE | 0.9–9000.0 mg/L | Sensitivity: +54 to +60 mV per decade change in ion activity |
| NO3− ISE | 0.4–62,000.0 mg/L | Sensitivity: +52 to +58 mV per decade change in ion activity |
| Cl− ISE | 1–35,500 mg/L | Sensitivity: +54 to +60 mV per decade change in ion activity |
| CO2 gas-permeable membrane electrode | 8.80–880.20 mg/L | Accuracy: ±1.95 mg/L |
| O2 luminescent gas-sensitive probe | 8 µg/L to saturation | Accuracy: ±(1% of the reading + 8 µg/L) |
| pH sensor | 0 to 14 pH units | Accuracy (pH units): ±0.1 pH units. |
| Redox sensor | −2000 to +2000 mV | Accuracy (mV): ±10 |
| Ionic conductivity sensor | 0 to 20 mS/cm | Accuracy ± 0.5% of the reading |
| Accuracy (°C): ±0.10 °C | ||
| Temperature sensor | −100 to +500 °C |
| Feature | Alert Project | MOLOKO Project |
|---|---|---|
| Primary focus and scope | Monitoring the quality, health, and traceability of bovine milk | Development of a miniaturized optical biosensor for fast and multiplex plasmonic detection of milk quality and safety parameters. |
| Technology focus and analytical approach | Using an array of targeted selective detection surfaces and integrated optical and electrochemical sensors (the patented BEST system) for the continuous monitoring of various chemical and physico-chemical parameters in milk, including inorganic ions and gases. | Miniaturized optical biosensor focus on specific analytes using plasmonic sensing by integrating organic optoelectronic devices and nanostructured plasmonic grating; use of a multiplex immunoassay and recombinant antibodies |
| Parameters measured | Free inorganic ion fraction (Ca2+, NH4+, NO3−, Cl−), dissolved gases (O2, CO2), ionic conductivity, pH, redox potential, temperature. | Lactoferrin, streptomycin; β-Casein A2 vs. β-Casein A1 (discriminated using a multiplex immunoassay); Staphylococcus aureus Enterotoxin A (using recombinant antibodies—patent pending) |
| Innovation | Continuous monitoring from farm level for early anomaly identification. Definition of integrated markers for specific condition assessment. HACCP-like multi-sensor early warning system for identification and management of anomalies. | Integration of plasmonic sensing with organic optoelectronics; miniaturized optical biosensor for real-setting analysis. |
| Application and outcomes | ||
| Self-control activity of the milk supply chain; support for precision livestock farming; building control charts; risk management and traceability. | Potential for point-of-care (POC) systems; accurate and low-cost analytical detection; integration of MOLOKO sensor into milking machines & milking process. |
| Elements of a Measurement Protocol | Meaning and Examples |
|---|---|
| Environmental conditions | Conditions under which measurements should be taken considering the use case and including any environmental control or recording of environmental variables necessary during the measurements |
| Sampling plan | Overall strategy, including sample size, locations and frequency, how samples should be collected, handled, and stored |
| Measurement methods | Techniques and instruments to be used for measurements, including calibration and conditioning procedures |
| Personnel and training | Identification of the laboratory (for offline analyses), personnel/operator responsible for carrying out the measurements, the training and qualifications required for personnel |
| Data record and management | Guidelines for recording data, including formats and units of measurement, the procedures for data storage, organization, management, and traceability |
| Quality control | Measures to be implemented to ensure data integrity, including procedures for routine checks and calibration of instruments. |
| Parameter (Unit) | N | Mean | SD | CV | Median | IQR | IQR/Median | Normality |
|---|---|---|---|---|---|---|---|---|
| CO2 (mV) | 188 | −153.78 | 6.11 | −0.04 | −154.27 | 7.4 | 0.05 | yes |
| NH4+ (mV) | 188 | 253.43 | 2.92 | 0.01 | 253.4 | 4.64 | 0.02 | yes |
| Temperature (°C) | 188 | 29.42 | 1.91 | 0.07 | 29.08 | 3.09 | 0.11 | no |
| NO3− (mV) | 188 | 258.11 | 14.23 | 0.06 | 255.51 | 20.45 | 0.08 | no |
| Redox potential (mV) | 188 | 23.62 | 10.98 | 0.46 | 20.88 | 12.98 | 0.62 | no |
| pH (pH units) | 188 | 6.56 | 0.08 | 0.01 | 6.56 | 0.11 | 0.02 | yes |
| O2 | 188 | 5.69 | 0.25 | 0.04 | 5.73 | 0.33 | 0.06 | no |
| Ionic conductivity (mS/cm) | 188 | 6.44 | 0.49 | 0.08 | 6.42 | 0.61 | 0.09 | yes |
| Ca2+ (mV) | 188 | 409.46 | 3.81 | 0.01 | 409.31 | 5.82 | 0.01 | yes |
| Cl− (mV) | 188 | 103.11 | 6.68 | 0.06 | 102.28 | 8.96 | 0.09 | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martelli, F.; Giacomozzi, C.; Dragone, R.; Frazzoli, C.; Grasso, G. Data Analysis in Newly Developed Milk Sensor Platforms: Good Practices, Common Pitfalls, and Hard-Earned Lessons from Field Application. Foods 2025, 14, 1724. https://doi.org/10.3390/foods14101724
Martelli F, Giacomozzi C, Dragone R, Frazzoli C, Grasso G. Data Analysis in Newly Developed Milk Sensor Platforms: Good Practices, Common Pitfalls, and Hard-Earned Lessons from Field Application. Foods. 2025; 14(10):1724. https://doi.org/10.3390/foods14101724
Chicago/Turabian StyleMartelli, Francesco, Claudia Giacomozzi, Roberto Dragone, Chiara Frazzoli, and Gerardo Grasso. 2025. "Data Analysis in Newly Developed Milk Sensor Platforms: Good Practices, Common Pitfalls, and Hard-Earned Lessons from Field Application" Foods 14, no. 10: 1724. https://doi.org/10.3390/foods14101724
APA StyleMartelli, F., Giacomozzi, C., Dragone, R., Frazzoli, C., & Grasso, G. (2025). Data Analysis in Newly Developed Milk Sensor Platforms: Good Practices, Common Pitfalls, and Hard-Earned Lessons from Field Application. Foods, 14(10), 1724. https://doi.org/10.3390/foods14101724










