Effect of Freeze-Drying on the Engineering, Cooking, and Functional Properties of Chickpea Cultivars Grown in an Indian Temperate Climate
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sample
2.2. Composition of Seeds
2.3. Engineering Properties of Seeds
2.3.1. Length, Width, and Thickness of Seeds (mm)
2.3.2. Diameter (mm)
2.3.3. Sphericity
2.3.4. Volume and Surface Area
2.3.5. Shape Factor
2.3.6. Thousand Kernal Mass
2.3.7. Bulk and True Density
2.3.8. Porosity
2.3.9. Compressibility Index and Hausner Ratio
2.4. Cooking Properties
2.4.1. Cooking Time
2.4.2. Gruel Solid Loss
2.4.3. Cooked Length/Breadth Ratio
2.4.4. Water Uptake Ratio of Cooked Seed
2.5. Functional Properties
2.5.1. Water and Oil Absorption Capacity
2.5.2. Emulsion Capacity and Stability
2.5.3. Foaming Capacity (FC) and Foaming Stability (FS)
2.5.4. Swelling and Solubility Indices
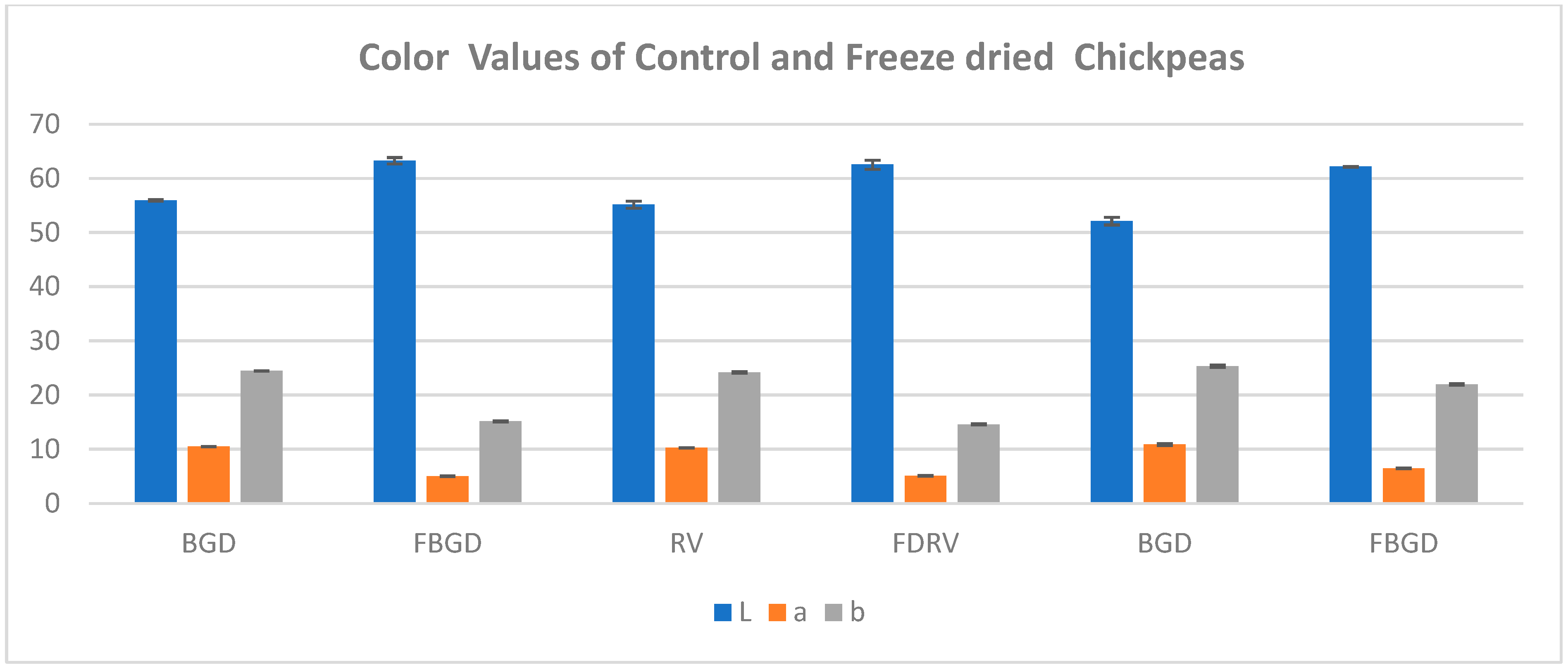
2.6. Color
2.7. Pasting Properties
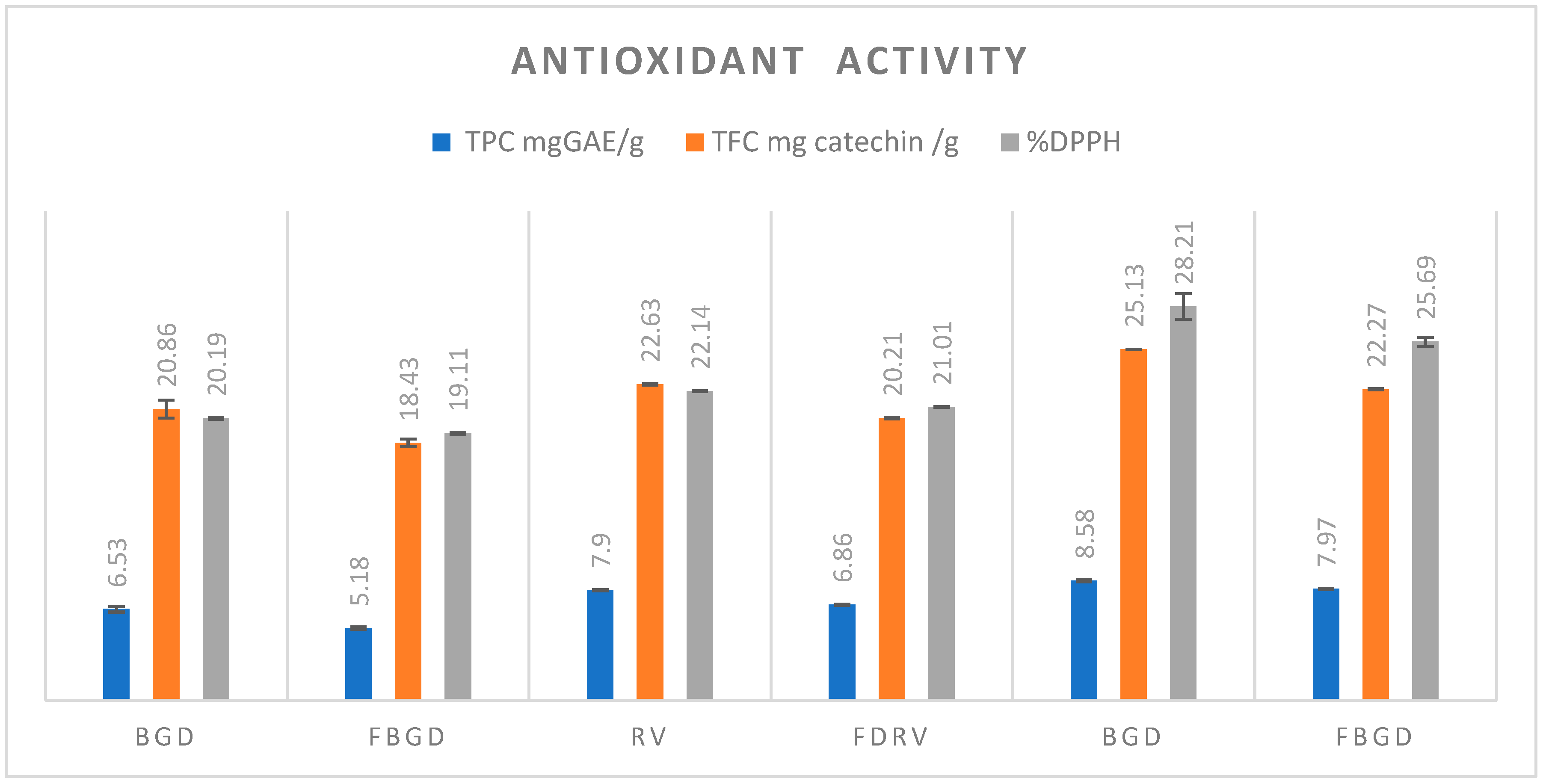
2.8. Anti-Oxidant Activity
2.8.1. Preparation of Extract
2.8.2. DPPH (1, 1-Diphenyl-2-Picrylhydrazyl) Radical Scavenging Assay
2.8.3. Total Phenolic Content (TPC)
2.9. Texture Profile Analysis
2.10. FTIR
2.11. Scanning Electron Microscopy
2.12. Statistical Analysis
3. Results
3.1. Proximate Composition
3.1.1. Engineering Parameters
3.1.2. Axial Dimensions
3.1.3. Diameter (mm)
3.1.4. Sphericity and Shape Factor
3.1.5. Volume and Surface Area
3.1.6. The 1000-Kernel Mass
3.1.7. Porosity
3.1.8. Bulk Density and Tapped Density
3.1.9. Hausner’s Ratio
3.1.10. Hydration Capacity
3.2. Cooking Properties
3.2.1. Cooking Time
3.2.2. Gruel Loss
3.2.3. Water Intake Capacity
3.2.4. Length/Breath Ratio
3.3. Functional Properties
3.3.1. Foaming Capacity and Stability
3.3.2. Water and Oil Absorption Capacity
3.3.3. Emulsion Capacity and Emulsion Stability
3.4. Pasting Properties
3.5. Swelling and Solubility Indices
3.6. Color
3.7. Antioxidant Activity
3.8. Textural Properties
3.9. ATR-FTIR Spectroscope Analysis
3.10. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT Statistical Database. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#compare (accessed on 12 June 2024).
- Augustin, M.A.; Chen, J.Y.; Ye, J.H. Processing to Improve the Sustainability of Chickpea as a Functional Food Ingredient. J. Sci. Food Agric. 2024, 104, 8397–8413. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, M.; Bai, Q.; de Souza, T.S.P.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Effects of Different Processing Methods on Pulses Phytochemicals: An Overview. Food Rev. Int. 2024, 40, 1138–1195. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Technological, Processing and Nutritional Aspects of Chickpea (Cicer arietinum)—A review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Aravindakshan, S.; Nguyen, T.H.A.; Kyomugasho, C.; Buvé, C.; Dewettinck, K.; Van Loey, A.; Hendrickx, M.E. The Impact of Drying and Rehydration on the Structural Properties and Quality Attributes of Pre-Cooked Dried Beans. Foods 2021, 10, 1665. [Google Scholar] [CrossRef]
- International, A. Official Methods of Analysis. Off. Methods Anal. AOAC Int. 2023, 1. [Google Scholar] [CrossRef]
- Mohsenin, N.; Mohsenin, N.N. Physical Properties of Plant and Animal Materials, 2nd ed.; Gordon and Breach Science Publishers: New York, NY, USA, 1986; Yahoo India Search Results; Available online: https://in.search.yahoo.com/search?fr=mcafee&type=E210IN1289G0&p=Mohsenin%2C+N.N.%2C+1986.+Physical+Properties+of+Plant+and+Animal+Materials%2C+second+Edn.+Gordon+and+Breach+Science+Publishers%2C+New+York (accessed on 11 July 2024).
- Mohsenin, N. Physical Properties of Plant and Animial Materials. Vol. 1. Structure, Physical Characterisitics and Mechanical Properties. 1970. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19720401916 (accessed on 23 March 2024).
- Jain, R.; Jain, R.K.; Bal, D. Properties of Pearl Millet. J. Agric. Eng. Res. 1997, 66, 85–91. [Google Scholar] [CrossRef]
- Mccabe, W.L.; Smith, J.C.; Harriott, P.; Rhodes, F.H.; York, N.; San, S.L.; Auckland, F.; Caracas, B.; London, L.; Mexico, M.; et al. Unit Operations of Chemical Engineering. 1993. Available online: https://thuvienso.hoasen.edu.vn/handle/123456789/8475 (accessed on 18 July 2024).
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physicochemical and Functional Properties of Flours from Three Black Gram (Phaseolus mungo L.) Cultivars. Int. J. Food Sci. Technol. 2013, 48, 771–777. [Google Scholar] [CrossRef]
- Carr, R. Evaluating Flow Properties of Solids. 1965. Available online: https://www.sid.ir/paper/594421/en (accessed on 22 May 2024).
- Singh, U.; Kherdekar, M.S.; Sharma, D.; Saxena, K.B. Cooking Quality and Chemical Composition of Some Early, Medium and Late Maturing Cultivars of Pigeon Pea (Cajanus cajan (L.) Mill). J. Food Sci. Technol. 1984, 21, 367–372. [Google Scholar]
- Wani, I.A.; Andrabi, S.N.; Sogi, D.S.; Hassan, I. Comparative Study of Physicochemical and Functional Properties of Flours from Kidney Bean (Phaseolus vulgaris L.) and Green Gram (Vigna radiata L.) Cultivars Grown in Indian Temperate Climate. Legum. Sci. 2019, 2, e11. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Shivhare, U.S.; Gill, B.S. Physico-Chemical and Functional Properties of Native and Hydrolyzed Kidney Bean (Phaseolus vulgaris L.) Protein Isolates. Food Res. Int. 2015, 76, 11–18. [Google Scholar] [CrossRef]
- Wani, I.A.; Jabeen, M.; Geelani, H.; Masoodi, F.A.; Saba, I.; Muzaffar, S. Effect of Gamma Irradiation on Physicochemical Properties of Indian Horse Chestnut (Aesculus indica Colebr.) Starch. Food Hydrocoll 2014, 35, 253–263. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.D.; Oomah, B.D.D.; Mazza, G. Wheat Quality: Antioxidant Activity of Wheat Millstreams. Wheat Qual. Elucidation 2002, 1, 219–231. [Google Scholar]
- Sofi, S.A.; Rafiq, S.; Singh, J.; Mir, S.A.; Sharma, S.; Bakshi, P.; McClements, D.J.; Khaneghah, A.M.; Dar, B. Impact of Germination on Structural, Physicochemical, Techno-Functional, and Digestion Properties of Desi Chickpea (Cicer arietinum L.) Flour. Food Chem. 2022, 405, 135011. [Google Scholar] [CrossRef]
- Deo, M.M.; Verma, P.; Praharaj, C.S.; Prakash, O.; Kumar, N. Physical and Engineering Properties of Chickpea (Cicer arietnum L.) for Planter Design. J. Food Legumes 2023, 36, 66–71. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N.; Sodhi, N.S. Physicochemical, Cooking, Textural and Roasting Characteristics of Chickpea (Cicer arietinum L.) Cultivars. J. Food Eng. 2005, 69, 511–517. [Google Scholar] [CrossRef]
- Sofi, S.A.; Singh, J.; Muzaffar, K.; Mir, S.A.; Dar, B.N. Effect of Germination Time on Physico-Chemical, Functional, Pasting, Rheology and Electrophoretic Characteristics of Chickpea Flour. J. Food Meas. Charact. 2020, 14, 2380–2392. [Google Scholar] [CrossRef]
- Dash, B.B.; Gardia, P.; Sahoo, A.; Lenka, C. Physico-chemical Properties, Nutritional Quality and Potential Utilization of Chickpeas: A Review. Acta Sci. Nutr. Health 2024, 8, 11–21. [Google Scholar] [CrossRef]
- Mune, M.A.M.; Sogi, D.S. Emulsifying and Foaming Properties of Protein Concentrates Prepared from Cowpea and Bambara Bean Using Different Drying Methods. Int. J. Food Prop. 2016, 19, 371–384. [Google Scholar] [CrossRef]
- Shevkani, K.; Kaur, R.; Singh, N.; Hlanze, D.P. Colour, Composition, Digestibility, Functionality and Pasting Properties of Diverse Kidney Beans (Phaseolus vulgaris) Flours. Curr. Res. Food Sci. 2022, 5, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Parimalavalli, R.; Babu, A.; Rao, J. A Study on Comparison between Cereal (Wheat) and Non Cereal (Chickpea) Flour Characteristics. Int. J. Curr. Trend Res. 2014, 3, 70–76. [Google Scholar]
- Yildirim, A.; Öner, M.D.; Bayram, M. Effect of Soaking and Ultrasound Treatments on Texture of Chickpea. J. Food Sci. Technol. 2011, 50, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, S.; Udipi, S.A.; Ghugre, P. Polyphenols and Tannins in Indian Pulses: Effect of Soaking, Germination and Pressure Cooking. Food Res. Int. 2010, 43, 526–530. [Google Scholar] [CrossRef]
- Boateng, J.; Verghese, M.; Walker, L.; Ogutu, S. Effect of Processing on Antioxidant Contents in Selected Dry Beans (Phaseolus spp. L.). LWT 2008, 41, 1541–1547. [Google Scholar] [CrossRef]
- Guerrero, P.; Kerry, J.P.; de la Caba, K. FTIR Characterization of Protein–Polysaccharide Interactions in Extruded Blends. Carbohydr. Polym. 2014, 111, 598–605. [Google Scholar] [CrossRef]
- Sofi, P.A.; Mir, R.R.; Zargar, S.M.; Rani, S.; Fatima, S.; Shafi, S.; Zaffar, A. What Makes the Beans (Phaseolus vulgaris L.) Soft: Insights into the Delayed Cooking and Hard to Cook Trait. Proc. Indian Natl. Sci. Acad. 2022, 88, 142–159. [Google Scholar] [CrossRef]
- Aguilera, Y.; Benítez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Influence of Dehydration Process in Castellano Chickpea: Changes in Bioactive Carbohydrates and Functional Properties. Plant Foods Hum. Nutr. 2011, 66, 391–400. [Google Scholar] [CrossRef]
- Karunarathna, S.; Wickramasinghe, I.; Brennan, C.; Truong, T.; Navaratne, S.; Chandrapala, J. Investigating the Impact of Boiling and Pressure Cooking on Resistant Starch Levels in Food. Int. J. Food Sci. Technol. 2024, 59, 3907–3917. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal Processing Effects on the Functional Properties and Microstructure of Lentil, Chickpea, and Pea Flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]

| Cultivars | L (mm) | B (mm) | T (mm) | AMD (mm) | GMD (mm) | SMD (mm) | ED (mm) | Sphericity | V (mm3) | SA | SF | BD | TD | HR | P% | M (1000) g | HC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BGD | 8.76 ± 0.67 a | 6.28 ± 0.32 a | 6.23 ± 0.22 a | 7.09 ± 0.41 a | 6.86 ± 0.36 a | 12.19 ± 0.66 a | 8.71 ± 0.47 a | 0.78 ± 0.54 ab | 177.51 ± 0.03 a | 155.18 ± 0.53 a | 0.18 ± 0.18 b | 1.62 ± 0.02 d | 1.68 ± 0.01 d | 1.03 ± 1.24 a | 3.27 ± 1.24 a | 390.33 ± 13.23 b | 0.36 ± 0.02 a |
| FDBGD | 10.96 ± 0.22 c | 8.11 ± 0.91 cd | 7.33 ± 0.13 b | 8.80 ± 0.42 cd | 8.48 ± 0.30 cd | 15.12 ± 0.59 cd | 10.80 ± 0.43 cd | 0.77 ± 1.35 a | 337.02 ± 0.01 cd | 238.28 ± 0.32 cd | 0.17 ± 0.05 ab | 0.45 ± 0.00 ab | 0.49 ± 0.00 b | 1.09 ± 0.00 c | 8.51 ± 0.00 c | 386.26 ± 1.20 b | 0.59 ± 0.58 d |
| RV | 9.84 ± 0.46 b | 8.53 ± 0.87 d | 7.36 ± 0.44 b | 8.58 ± 0.54 c | 8.33 ± 0.56 c | 14.80 ± 1.00 c | 10.57 ± 0.72 c | 0.84 ± 1.21 bc | 310.78 ± 0.10 c | 223.59 ± 1.06 c | 0.16 ± 0.10 a | 1.63 ± 0.00 d | 1.69 ± 0.00 d | 1.03 ± 0.00 a | 3.11 ± 0.35 a | 492.73 ± 17.60 d | 0.63 ± 0.01 e |
| FDRV | 10.26 ± 0.20 bc | 9.10 ± 0.52 d | 8.20 ± 0.17 c | 9.18 ± 0.30 d | 8.94 ± 0.27 d | 15.88 ± 0.48 d | 11.34 ± 0.35 d | 0.87 ± 1.30 c | 381.43 ± 0.01 d | 255.71 ± 0.25 d | 0.16 ± 0.00 a | 0.45 ± 0.00 b | 0.49 ± 0.00 b | 1.07 ± 0.00 b | 7.28 ± 0.92 b | 441.86 ± 1.71 c | 0.76 ± 0.01 f |
| SH | 8.08 ± 0.43 a | 6.51 ± 0.24 ab | 6.05 ± 0.52 a | 6.88 ± 0.40 a | 6.70 ± 0.38 a | 11.87 ± 0.67 a | 8.48 ± 0.48 a | 0.82 ± 0.90 abc | 162.27 ± 0.03 a | 145.28 ± 0.47 a | 0.17 ± 0.25 ab | 0.79 ± 0.00 c | 0.82 ± 0.00 c | 1.03 ± 0.00 a | 3.65 ± 0.02 a | 296.30 ± 12.4 a | 0.42 ± 0.02 b |
| FDSH | 9.57 ± 0.27 b | 7.39 ± 0.22 bc | 6.68 ± 0.57 ab | 7.88 ± 0.24 b | 7.62 ± 0.27 b | 13.56 ± 0.46 b | 9.69 ± 0.32 b | 0.79 ± 0.03 ab | 243.11 ± 23.42 b | 190.91 ± 11.40 b | 0.17 ± 0.00 ab | 0.43 ± 0.00 a | 0.45 ± 0.00 a | 1.04 ± 0.00 a | 3.98 ± 0.01 a | 284.9 ± 0.65 a | 0.52 ± 0.01 c |
| Cultivars | Foaming Capacity (%) | Foaming Stability (%) | Water Absorption Capacity (g/g) | Oil Absorption Capacity (g/g) | Emulsion Capacity (%) | Emulsion Stability (%) |
|---|---|---|---|---|---|---|
| BGD | 42.58 ± 0.119 a | 36.18 ± 0.07 a | 0.97 ± 0.03 ab | 0.73 ± 0.02 a | 42.35 ± 1.206 a | 74.31 ± 1.07 c |
| FD BGD | 44.37 ± 0.05 b | 37.58 ± 0.55 b | 1.24 ± 0.01 c | 0.91 ± 0.02 bc | 46.59± 0.60 b | 84.91 ± 0.14 a |
| RV | 45.16 ± 0.04 c | 37.49 ± 0.62 b | 0.98 ± 0.02 ab | 0.97± 0.04 d | 44.84 ± 0.69 b | 80.00 ± 0.00 b |
| FD RV | 47.20 ± 0.12 d | 38.80 ± 0.14 c | 1.19 ± 0.00 c | 0.98 ± 0.01 bc | 50.69 ± 1.58 c | 83.88 ± 0.50 c |
| SH | 42.58 ± 0.119 a | 36.79 ± 0.04 a | 0.84 ± 0.18 a | 0.85± 0.05 b | 42.58 ± 0.12 b | 73.80 ± 3.07 a |
| FDSH | 46.76 ± 0.67 d | 37.62 ± 0.24 b | 1.05 ± 0.02 b | 0.96 ± 0.01 cd | 54.49 ± 1.62 d | 85.34 ± 0.11 c |
| Cultivars | Peak Viscosity (cP) | Trough Viscosity (cP) | Breakdown Viscosity (cP) | Final Viscosity (cP) | Setback Viscosity (cP) | Pasting Time. (min.) | Pasting Temperature (°C) |
|---|---|---|---|---|---|---|---|
| BGD | 1178 ± 49.51 b | 944.33 ± 37.16 abc | 235 ± 71.58 a | 1113± 43.31 a | 168.66 ± 8.08 b | 6.95 ± 0.075 d | 79.31 ± 1.23 ab |
| FDBGD | 1382 ± 21.00 d | 1128.66 ± 7.50 cd | 253.66 ± 13.50 a | 1622 ± 35.51 c | 493.00 ± 28.00 d | 6.23 ± 0.10 a | 79.9 ± 0.05 ab |
| RV | 1064.33 ± 9.29 a | 898.66 ± 3.51 ab | 165.66 ± 12.66 a | 1025.33 ± 4.04 a | 126.66 ± 3.05 a | 6.86 ± 0.06 cd | 80.8 ± 0.00 ab |
| FDRV | 1437.66 ± 6.50 e | 1173.66 ± 12.50 d | 264.00 ± 6.00 a | 1673 ± 24.00 c | 524.66 ± 11.50 d | 6.14 ± 0.14 a | 79.93 ± 0.05 ab |
| SH | 1217.33 ± 15.5 b | 874.33 ± 0.71 a | 345.33 ± 19.79 a | 1034.33 ± 10.60 a | 160.00 ± 9.89 ab | 6.69 ± 0.18 bc | 82.63 ± 0.56 b |
| FDSH | 1288.66 ± 23.50 c | 1106.66 ± 0.58 bcd | 175.33 ± 15.14 a | 1367.66 ± 2.51 b | 261.00 ± 2.00 c | 6.63 ± 0.04 b | 76.32 ± 0.35 a |
| Swelling Index | BGD | RV | SH | FDBGD | FDRV | FDSH |
|---|---|---|---|---|---|---|
| 50 °C | 1.63 ± 0.10 b | 1.75 ± 0.1 b | 1.43 ± 0.15 a | 2.12 ± 0.07 c | 2.44 ± 0.14 d | 2.82 ± 0.06 e |
| 60 °C | 2.08 ± 0.16 a | 2.00 ± 0.13 a | 2.10 ± 0.08 a | 2.76 ± 0.03 b | 2.96 ± 0.05 c | 3.36 ± 0.04 d |
| 70 °C | 4.35 ± 0.81 a | 4.38 ± 0.81 a | 3.93 ± 0.07 a | 4.56 ± 0.05 a | 5.79 ± 0.74 b | 4.27 ± 0.02 a |
| 80 °C | 5.23 ± 0.26 a | 5.78 ± 0.25 b | 5.33 ± 0.10 a | 5.83 ± 0.87 b | 5.71 ± 0.22 b | 5.94 ± 0.03 b |
| 90 °C | 6.68 ± 0.20 a | 6.68 ± 0.17 a | 7.31 ± 0.55 b | 7.11 ± 0.11 ab | 7.28 ± 0.05 b | 7.56 ± 0.06 b |
| Solubility Index | ||||||
| 50 °C | 0.30 ± 0.03 c | 0.20 ± 0.02 a | 0.24 ± 0.03 b | 0.42 ± 0.01 d | 0.29 ± 0.01 c | 0.31 ± 0.04 c |
| 60 °C | 0.36 ± 0.04 bc | 0.30 ± 0.22 a | 0.32 ± 0.05 ab | 0.47 ± 0.02 a | 0.36 ± 0.01 bc | 0.38 ± 0.01 c |
| 70 °C | 0.45 ± 0.02 c | 0.37 ± 0.02 a | 0.37 ± 0.02 a | 0.48 ± 0.01 d | 0.41 ± 0.01 b | 0.44 ± 0.02 c |
| 80 °C | 0.32 ± 0.00 b | 0.27 ± 0.00 a | 0.32 ± 0.01 b | 0.42 ± 0.01 e | 0.39 ± 0.00 c | 0.41 ± 0.03 d |
| 90 °C | 0.34 ± 0.03 a | 0.35 ± 0.011 a | 0.41 ± 0.01 b | 0.49 ± 0.03 c | 0.41 ± 0.01 b | 0.47 ± 0.05 c |
| Cultivars | Hardness (kg) | Adhesiveness (kg/s) | Cohesiveness | Chewiness |
|---|---|---|---|---|
| BGD | 3.91 ± 0.82 d | 0.24 ± 0.04 a | 0.22 ± 0.06 b | 2.62 ± 0.24 bc |
| FDBGD | 2.05 ± 0.04 ab | 0.55 ± 0.02 d | 0.18 ± 0.05 ab | 2.18 ± 0.11 ab |
| RV | 3.33 ± 0.36 c | 0.38 ± 0.10 bc | 0.15 ± 0.03 a | 3.76 ± 1.16 d |
| FDRV | 1.99 ± 0.03 a | 0.47 ± 0.01 cd | 0.12 ± 0.01 a | 2.16 ± 0.04 a |
| SH | 2.72 ± 0.39 bc | 0.31 ± 0.01 ab | 0.18 ± 0.01 ab | 3.40 ± 0.46 cd |
| FDSH | 1.48 ± 0.11 ab | 0.47 ± 0.06 cd | 0.14 ± 0.01 a | 1.42 ± 0.25 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, N.; Wani, I.A. Effect of Freeze-Drying on the Engineering, Cooking, and Functional Properties of Chickpea Cultivars Grown in an Indian Temperate Climate. Foods 2025, 14, 1687. https://doi.org/10.3390/foods14101687
Farooq N, Wani IA. Effect of Freeze-Drying on the Engineering, Cooking, and Functional Properties of Chickpea Cultivars Grown in an Indian Temperate Climate. Foods. 2025; 14(10):1687. https://doi.org/10.3390/foods14101687
Chicago/Turabian StyleFarooq, Najeebah, and Idrees Ahmed Wani. 2025. "Effect of Freeze-Drying on the Engineering, Cooking, and Functional Properties of Chickpea Cultivars Grown in an Indian Temperate Climate" Foods 14, no. 10: 1687. https://doi.org/10.3390/foods14101687
APA StyleFarooq, N., & Wani, I. A. (2025). Effect of Freeze-Drying on the Engineering, Cooking, and Functional Properties of Chickpea Cultivars Grown in an Indian Temperate Climate. Foods, 14(10), 1687. https://doi.org/10.3390/foods14101687




