Quantitative Proteomics Reveals the Relationship between Protein Changes and Volatile Flavor Formation in Hunan Bacon during Low-Temperature Smoking
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Measurement of Basic Index
2.3. Electronic Nose (E-Nose) Analysis
2.4. GC-MS Analysis of Volatile Compounds
2.5. Protein Degradation and Oxidation
2.6. Detection of Free Amino Acid
2.7. SDS-PAGE Analysis of Sarcoplasmic and Myofibrillar Proteins
2.8. LFQ
2.8.1. Protein Extraction and Digestion
2.8.2. Nano LC-MS/MS Analysis
2.9. Statistical Analysis
3. Result and Discussion
3.1. Analysis of Physicochemical Properties
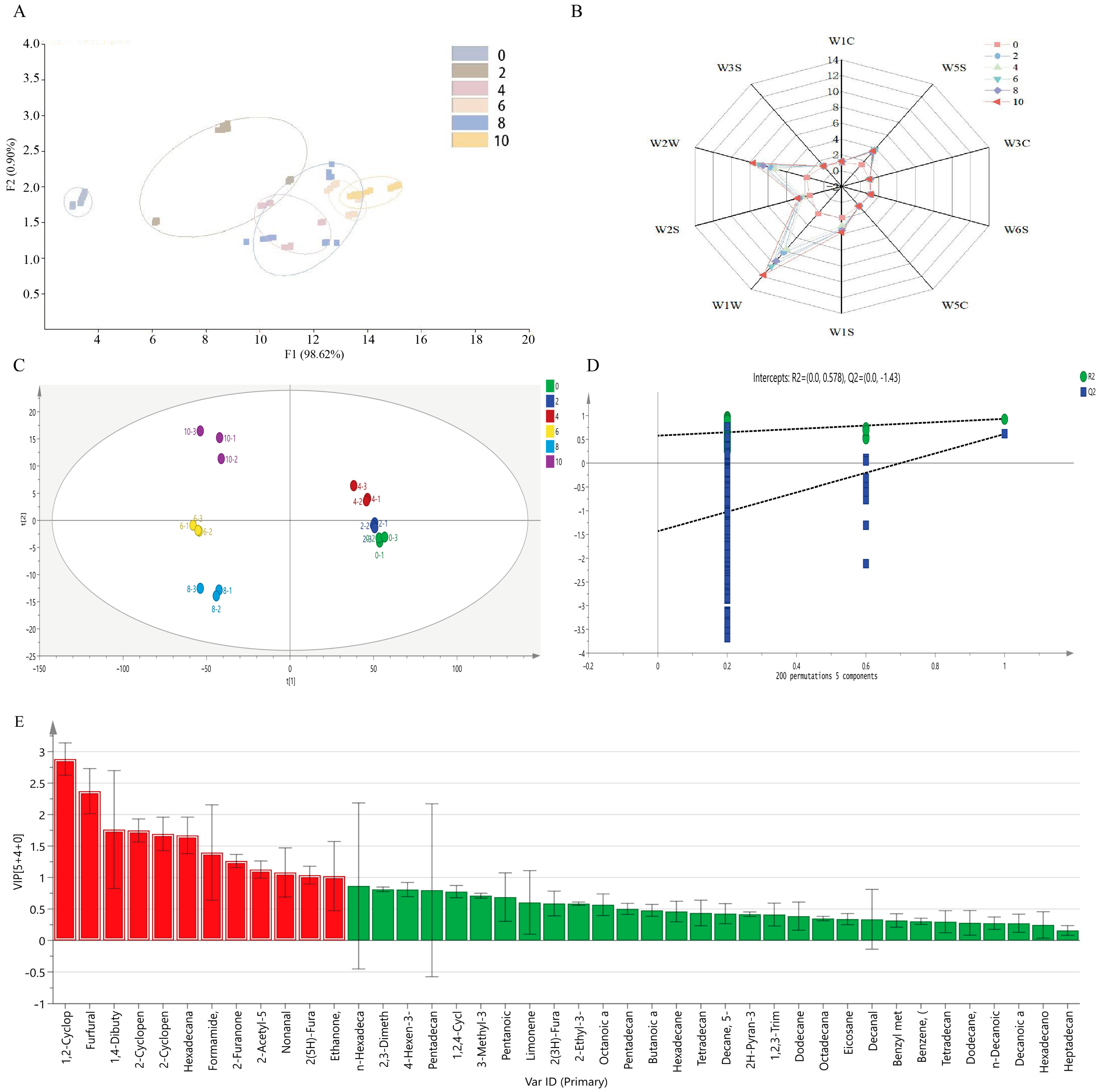
3.2. Changes in the Composition of Volatile Flavor Compounds in Bacon
3.3. Screening of Key Volatile Flavor Compounds in Bacon during LTS
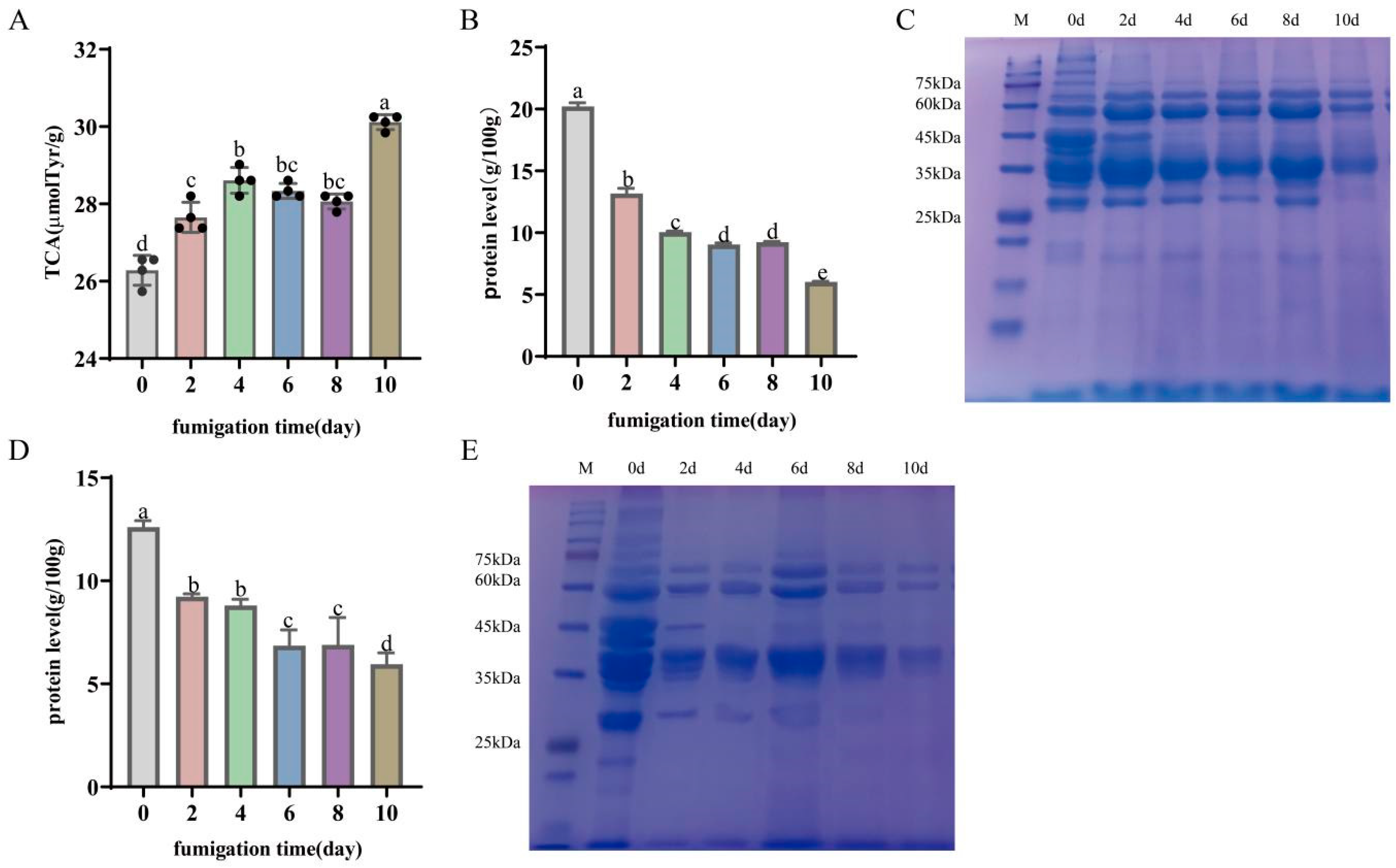
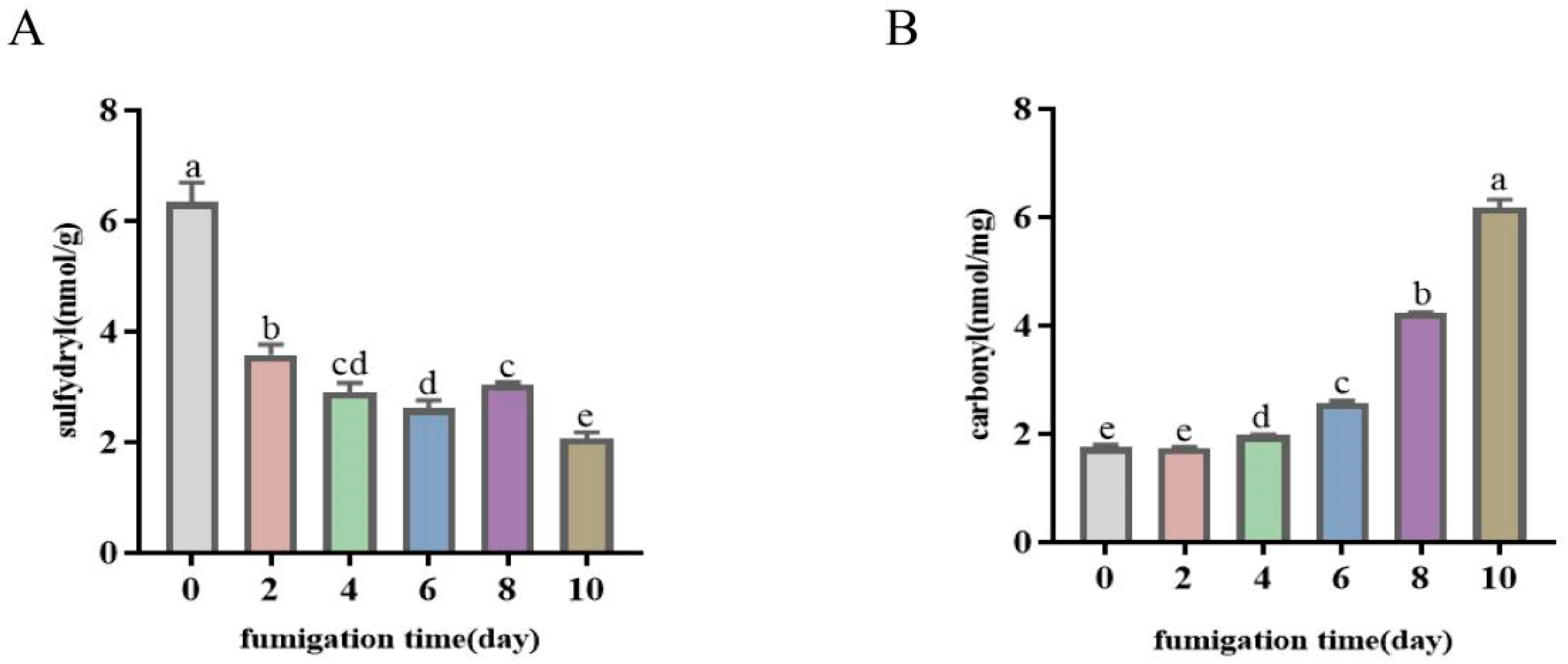
3.4. Degradation and Oxidation of Bacon Proteins during LTS
3.4.1. Protein Degradation
3.4.2. Protein Oxidation
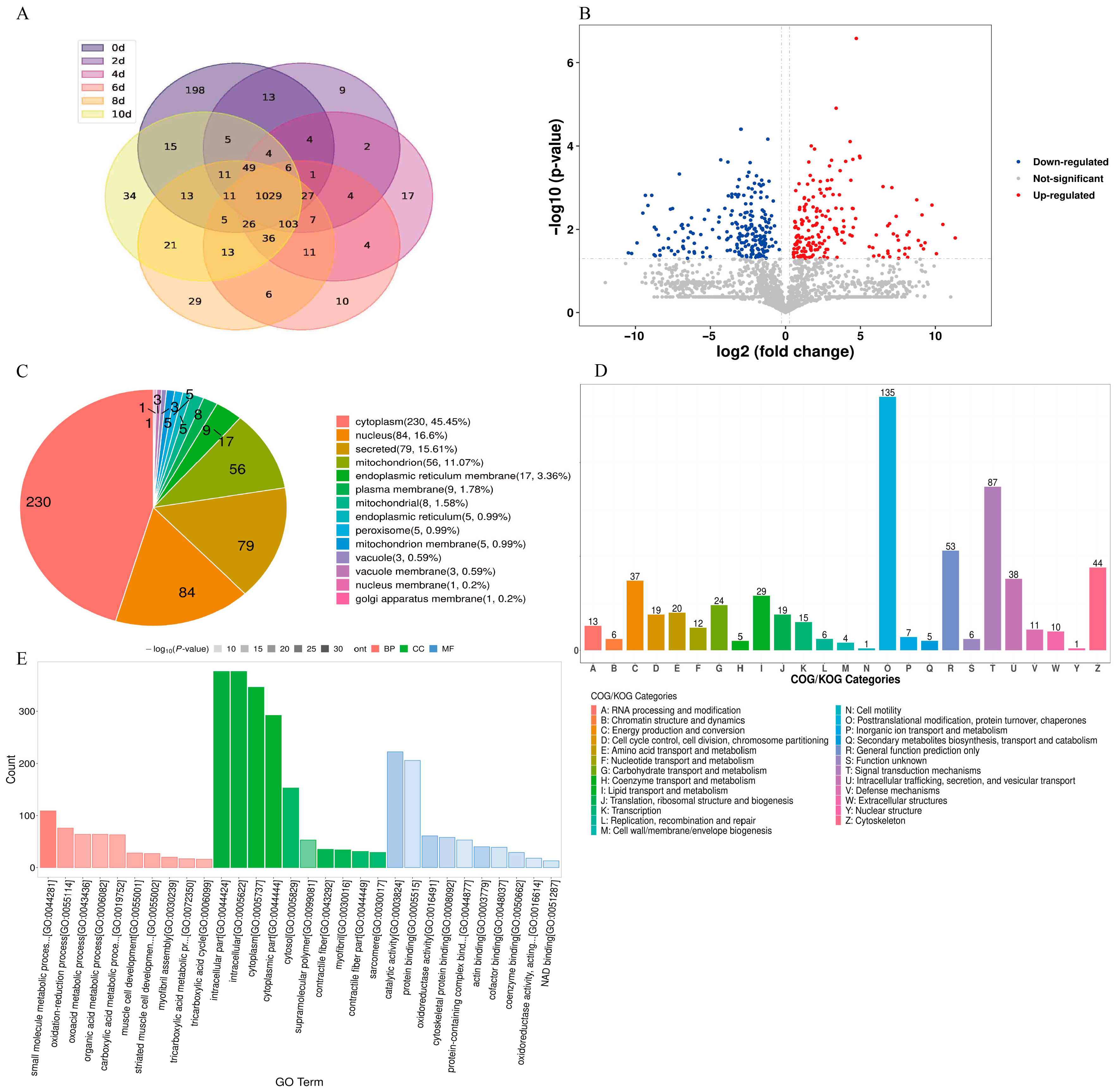
3.5. Qualitative and Quantitative Analyses of Changes in Bacon Proteins during LTS
3.6. Bioinformatic Analysis of DAPs in Bacon during LTS
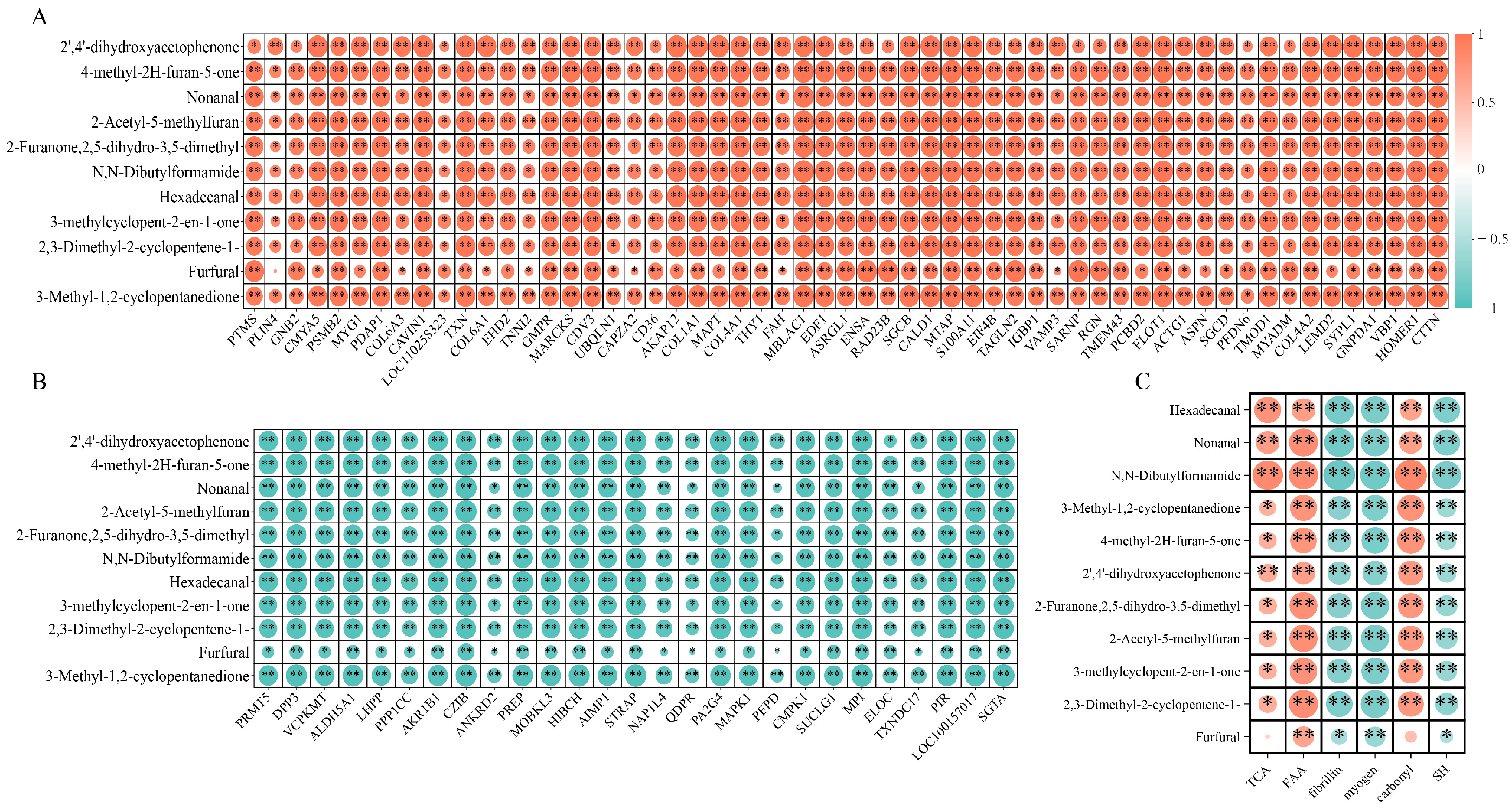
3.7. Relationship between Protein Degradation or Oxidation and the Flavors of Bacon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Wang, Z.; Ji, L.; Zhang, J.; Zhao, Z.; Zhang, R.; Bai, T.; Hou, B.; Zhang, Y.; Liu, D.; et al. A Review: Microbial Diversity and Function of Fermented Meat Products in China. Front. Microbiol. 2021, 12, 645435. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, Z.; Yang, L.; Li, H. Volatile compounds comparison and mechanism exploration of non-smoked traditional Chinese bacon in Southwestern China and Eastern China. Food Res. Int. 2023, 169, 112834. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Wu, H.; Su, C.; He, Z. Quality relationship between smoked and air-dried bacon of Sichuan-Chongqing in China: Free amino acids, volatile compounds, and microbial diversity. Food Res. Int. 2023, 164, 112274. [Google Scholar] [CrossRef]
- Wang, S.; Guan, R.; Huang, H.; Yang, K.; Cai, M.; Chen, D. Effects of Different Smoking Materials and Methods on the Quality of Chinese Traditional Bacon (Larou). J. Food Prot. 2021, 84, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Q.; Chen, C.; Yu, H.; Xu, B. Effects of different smoking methods on sensory properties, free amino acids and volatile compounds in bacon. J. Sci. Food Agric. 2021, 101, 2984–2993. [Google Scholar] [CrossRef]
- Chen, L.; Teng, X.; Liu, Y.; Shi, H.; Li, Z.; Xue, C. The dynamic change of flavor characteristics in Pacific oyster (Crassostrea gigas) during depuration uncovered by mass spectrometry-based metabolomics combined with gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2024, 434, 137277. [Google Scholar] [CrossRef] [PubMed]
- Saldana, E.; Saldarriaga, L.; Cabrera, J.; Siche, R.; Behrens, J.H.; Selani, M.M.; de Almeida, M.A.; Silva, L.D.; Silva Pinto, J.S.; Contreras-Castillo, C.J. Relationship between volatile compounds and consumer-based sensory characteristics of bacon smoked with different Brazilian woods. Food Res. Int. 2019, 119, 839–849. [Google Scholar] [CrossRef]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2021, 371, 131–139. [Google Scholar] [CrossRef]
- Li, R.; Geng, C.; Xiong, Z.; Cui, Y.; Liao, E.; Peng, L.; Jin, W.; Wang, H. Evaluation of protein degradation and flavor compounds during the processing of Xuan’en ham. J. Food Sci. 2022, 87, 3366–3385. [Google Scholar] [CrossRef]
- Merlo, T.C.; Lorenzo, J.M.; Saldana, E.; Patinho, I.; Oliveira, A.C.; Menegali, B.S.; Selani, M.M.; Dominguez, R.; Contreras-Castillo, C.J. Relationship between volatile organic compounds, free amino acids, and sensory profile of smoked bacon. Meat Sci. 2021, 181, 108596. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, R.; Liu, L.; Zhu, Z.; Mo, H.; Xu, M.; Shi, L.; Zhang, H. Proteomics analysis to investigate the impact of diversified thermal processing on meat tenderness in Hengshan goat meat. Meat Sci. 2022, 183, 108655. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Shi, Q.; Zhang, R.; Shi, L.; Chu, X. Unraveling proteome changes of irradiated goat meat and its relationship to off-flavor analyzed by high-throughput proteomics analysis. Food Chem. 2021, 337, 127806. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Pateiro, M.; Kumar, M.; Franco, D.; Capanoglu, E.; Dhama, K.; Lorenzo, J.M. The potential of proteomics in the study of processed meat products. J. Proteom. 2023, 270, 104744. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.3-2016; China Food Safety National Standard. GB Determination of Moisture Content in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Gao, T.; Li, J.; Zhang, L.; Jiang, Y.; Ma, R.; Song, L.; Gao, F.; Zhou, G. Effect of different tumbling marination treatments on the quality characteristics of prepared pork chops. Asian-Australas. J. Anim. Sci. 2015, 28, 260. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, Q.; Liu, Q.; Wang, Y.; Kong, B. Evaluation of flavor characteristics of bacon smoked with different woodchips by HS-SPME-GC-MS combined with an electronic tongue and electronic nose. Meat Sci. 2021, 182, 108626. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Kong, X.; Hu, C.; Zhou, B.; Wang, C.; Shen, Q.W. Fatty Acid Content, Flavor Compounds, and Sensory Quality of Pork Loin as Affected by Dietary Supplementation with l-arginine and Glutamic Acid. J. Food Sci. 2019, 84, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Visessanguan, W.; Benjakul, S.; Riebroy, S.; Thepkasikul, P. Changes in composition and functional properties of proteins and their contributions to Nham characteristics. Meat Sci. 2004, 66, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Ganhao, R.; Morcuende, D.; Estevez, M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage. Meat Sci. 2010, 85, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Wu, Z.; Jiao, Y.; Liu, G.; Wang, Z.; He, Z.; Tao, G.; Qin, F.; Zeng, M.; Chen, J. Exploring the relationship between potato components and Maillard reaction derivative harmful products using multivariate statistical analysis. Food Chem. 2021, 339, 127853. [Google Scholar] [CrossRef]
- Han, M.; Wu, Y.; Wang, P.; Xu, X.; Zhou, G. The changes and relationship of structure and functional properties of rabbit myosin during heat-induced gelation. CyTA-J. Food 2014, 13, 63–68. [Google Scholar] [CrossRef]
- Galla, N.R.; Pamidighantam, P.R.; Akula, S.; Karakala, B. Functional properties and in vitro antioxidant activity of roe protein hydrolysates of Channa striatus and Labeo rohita. Food Chem. 2012, 135, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Q.; Liu, Q.; Chen, Q.; Liu, H.; Xu, M.; Kong, B. Heterocyclic aromatic amine contents and quality characteristics of bacon as influenced by NaCl concentration of brine. J. Food Sci. 2022, 87, 2527–2537. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yu, Q.Q.; Fu, Y.; Tian, X.J.; Jia, F.; Li, X.M.; Dai, R.T. Towards muscle-specific meat color stability of Chinese Luxi yellow cattle: A proteomic insight into post-mortem storage. J. Proteom. 2016, 147, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.S. Effect of Proteolytic Enzymes and Ginger Extract on Tenderization of M. pectoralis profundus from Holstein Steer. Korean J. Food Sci. Anim. Resour. 2018, 38, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, Y.; Wang, Z.; Akhtar, K.H.; Saleh, A.S.M.; Li, W.; Zhang, D. Insights into flavor formation of braised chicken: Based on E-nose, GC–MS, GC-IMS, and UPLC-Q-Exactive-MS/MS. Food Chem. 2024, 448, 138972. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bi, Y.; Du, R.; Yuan, H.; Hou, Y.; Luo, R. The impact of freezing methods on the quality, moisture distribution, microstructure, and flavor profile of hand-grabbed mutton during long-term frozen storage. Food Res. Int. 2023, 173, 113346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, X.; Tian, Y.; Wang, Q.; Li, X.; An, F.; Luo, Z.; Shang, P.; Liu, Z.; Huang, Q. Mechanisms of cooking methods on flavor formation of Tibetan pork. Food Chem. X 2023, 19, 100873. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Franco, D.; Carballo, J. Effect of the inclusion of chestnut in the finishing diet on volatile compounds during the manufacture of dry-cured “Lacon” from Celta pig breed. Meat Sci. 2014, 96, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Zhang, J.; Wu, R.; Wang, T.; Ding, W. Characterization of the Volatile Compounds of Zhenba Bacon at Different Process Stages Using GC-MS and GC-IMS. Foods 2021, 10, 2869. [Google Scholar] [CrossRef]
- Poljanec, I.; Marusic Radovcic, N.; Petricevic, S.; Karolyi, D.; Listes, E.; Medic, H. Proteolysis and protein oxidation throughout the smoked dry-cured ham process. Food Chem 2021, 362, 130207. [Google Scholar] [CrossRef]
- Zhang, Y.; Magro, A.; Puolanne, E.; Zotte, A.D.; Ertbjerg, P. Myofibrillar protein characteristics of fast or slow frozen pork during subsequent storage at −3 °C. Meat Sci. 2021, 176, 108468. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, G.; Xu, X.; Zhang, W.; Li, C. Contribution of cathepsin B and L to endogenous proteolysis in the course of modern Jinhua ham processing. Food Control 2022, 135, 108584. [Google Scholar] [CrossRef]
- Qu, Z.; Feng, C.; Li, R.; Liu, N.; Zheng, S. Characteristic and effect analysis of protein and peptide in Cantonese cured meat processing. Food Sci. Hum. Wellness 2022, 11, 1392–1401. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Wang, Y.; Cao, J.X.; Chen, Y.J.; Liu, Y.; Sun, Y.Y.; Pan, D.D.; Ou, C.R. The effect of dry-cured salt contents on accumulation of non-volatile compounds during dry-cured goose processing. Poult. Sci. 2016, 95, 2160–2166. [Google Scholar] [CrossRef]
- Perez-Santaescolastica, C.; Carballo, J.; Fulladosa, E.; Garcia-Perez Jose, V.; Benedito, J.; Lorenzo, J.M. Application of temperature and ultrasound as corrective measures to decrease the adhesiveness in dry-cured ham. Influence on free amino acid and volatile compound profile. Food Res. Int. 2018, 114, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, X.; Chen, D.; Yu, B.; Yin, J.; Huang, Z. Effects of dietary apple polyphenol supplementation on carcass traits, meat quality, muscle amino acid and fatty acid composition in finishing pigs. Food Funct. 2019, 10, 7426–7434. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Wu, Z.; Xu, J.; Yu, Y.; Tang, Y.; Xie, X.; Chen, J.; Wang, Z.; Zhang, D.; Tang, J.; et al. Effects of replacement partial sodium chloride on characteristic flavor substances of bacon during storage based on GCxGC-MS and non-targeted metabolomics analyses. Food Chem. 2023, 428, 136805. [Google Scholar] [CrossRef]
- Lara, M.S.; Gutierrez, J.I.; Timon, M.; Andres, A.I. Evaluation of two natural extracts (Rosmarinus officinalis L. and Melissa officinalis L.) as antioxidants in cooked pork patties packed in MAP. Meat Sci. 2011, 88, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Luo, J.; Quan, W.; Lou, A.; Shen, Q. Antioxidant Activity and Sensory Quality of Bacon. Foods 2022, 11, 236. [Google Scholar] [CrossRef]
- Sun, W.; Cui, C.; Zhao, M.; Zhao, Q.; Yang, B. Effects of composition and oxidation of proteins on their solubility, aggregation and proteolytic susceptibility during processing of Cantonese sausage. Food Chem. 2011, 124, 336–341. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Shand, P.; Dugan, M.E.R.; Gariepy, C.; Aalhus, J.L.; Estevez, M.; Juarez, M. Influence of cooking methods and storage time on lipid and protein oxidation and heterocyclic aromatic amines production in bacon. Food Res. Int. 2017, 99, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3564–3578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Toldra, F.; Zhang, W. Insight into Ultrasound-Induced Modifications of the Proteome and Flavor-Related Proteins of Unsmoked Bacon by Applying Label-Free Quantitation Technology. J. Agric. Food Chem. 2022, 70, 10259–10270. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhu, X.; Sun, R.; Jiang, W.; Zhang, D.; Liu, H.; Sun, B. Sensory attributes and characterization of aroma profiles of fermented sausages based on fibrous-like meat substitute from soybean protein and Coprinus comatus. Food Chem. 2022, 373, 131537. [Google Scholar] [CrossRef]
| Item | Hunan Bacon Low-Temperature Liquid Smoking Time (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |
| L* | 48.3 ± 1.12 e | 59.5 ± 2.05 bc | 63.9 ± 1.36 a | 61.0 ± 2.14 ab | 54.7 ± 1.67 d | 57.0 ± 2.28 cd |
| a* | 4.91 ± 0.76 abc | 3.51 ± 1.38 cd | 3.10 ± 0.45 d | 5.73 ± 0.75 a | 3.55 ± 0.13 bcd | 5.08 ± 0.59 ab |
| b* | 5.28 ± 0.47 b | 5.33 ± 0.47 b | 7.39 ± 1.37 b | 10.84 ± 1.97 a | 6.77 ± 1.29 b | 10.3 ± 1.35 a |
| Hardness | 235 ± 75.4 c | 693 ± 135 b | 362 ± 135 bc | 675 ± 284 b | 1193 ± 283 a | 1219 ± 160 a |
| Elasticity | 0.86 ± 0.08 | 0.85 ± 0.02 | 0.81 ± 0.04 | 0.88 ± 0.08 | 0.95 ± 0.06 | 0.91 ± 0.08 |
| Adhesiveness | 211 ± 82.1 c | 682 ± 31.9 b | 351 ± 103 c | 364 ± 69.9 c | 958 ± 172 a | 959 ± 32.7 a |
| Chewiness | 157 ± 52.0 d | 584 ± 79.6 b | 344 ± 106 c | 241 ± 38.9 cd | 888 ± 162 a | 836 ± 41.2 a |
| Resilience | 0.19 ± 0.08 b | 0.20 ± 0.02 b | 0.16 ± 0.03 b | 0.21 ± 0.03 b | 0.31 ± 0.04 a | 0.32 ± 0.05 a |
| Moisture | 70.0 ± 1.56 a | 60.5 ± 1.73 b | 43.1 ± 0.39 d | 41.2 ± 1.67 de | 49.4 ± 1.59 c | 39.2 ± 1.75 e |
| Volatile Components (µg/kg) | VIP | CAS | Hunan Bacon Low-Temperature Liquid Smoking Time (Days) | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |||
| 2′,4′-dihydroxyacetophenone | 1.02201 | 89-84-9 | 0 | 0 | 0.01 ± 0.01 c | 108 ± 10.6 a | 69.1 ± 26.3 b | 108 ± 14.2 a |
| 4-methyl-2H-furan-5-one | 1.03731 | 22122-36-7 | 0 | 0 | 0.01 ± 0.02 b | 193 ± 27.6 a | 181 ± 11.9 a | 200 ± 29.4 a |
| Nonanal | 1.07986 | 124-19-6 | 6.58 ± 4.93 a | 34.2 ± 8.54 ab | 58.9 ± 5.55 b | 137 ± 41.5 a | 111 ± 12.5 a | 133 ± 24.9 a |
| 2-Acetyl-5-methylfuran | 1.12646 | 1193-79-9 | 0.11 ± 0.05 b | 5.02 ± 0.79 b | 35.9 ± 5.15 b | 254 ± 16.1 a | 242 ± 34.1 a | 244 ± 35.8 a |
| 2-Furanone,2,5-dihydro-3,5-dimethyl | 1.26189 | 0.02 ± 0.03 b | 18.9 ± 2.70 b | 48.1 ± 3.75 b | 353 ± 48.6 a | 342 ± 24.6 a | 338 ± 64.2 a | |
| N,N-Dibutylformamide | 1.39613 | 761-65-9 | 0 | 22.6 ± 3.91 cd | 18.3 ± 1.86 d | 33.1 ± 5.48 bc | 37.1 ± 6.01 b | 59.5 ± 10.9 a |
| Hexadecanal | 1.66915 | 629-80-1 | 1.63 ± 0.65 c | 30.6 ± 3.16 c | 98.2 ± 21.19 b | 241 ± 34.8 a | 107 ± 6.75 b | 245 ± 32.3 a |
| 3-methylcyclopent-2-en-1-one | 1.6924 | 2758-18-1 | 0 | 0.30 ± 0.23 b | 72.0 ± 21.7 b | 537 ± 91.7 a | 513 ± 34.5 a | 511 ± 100 a |
| 2,3-Dimethyl-2-cyclopentene-1-one | 1.7476 | 1121-05-7 | 0 | 51.5 ± 2.27 b | 109 ± 7.54 b | 561 ± 43.3 a | 570 ± 76.9 a | 546 ± 105 a |
| Furfural | 2.372 | 98-01-1 | 0.62 ± 0.41 c | 1.18 ± 0.70 c | 0.46 ± 0.37 c | 735 ± 189 a | 672 ± 33.5 a | 337 ± 115 b |
| 3-Methyl-1,2-cyclopentanedione | 2.88217 | 765-70-8 | 38.6 ± 1.26 b | 62.3 ± 3.70 b | 63.6 ± 4.27 b | 1553 ± 95.9 a | 1569 ± 289 a | 1592 ± 217 a |
| FAA (mg/100 g) | Hunan Bacon Low-Temperature Liquid Smoking Time (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |
| Asp | 0.84 ± 0.54 cd | 0.76 ± 0.05 d | 1.35 ± 0.36 c | 2.54 ± 0.07 b | 2.61 ± 0.26 b | 3.21 ± 0.12 a |
| Glu | 9.60 ± 0.78 e | 22.9 ± 2.11 d | 28.7 ± 2.11 c | 40.8 ± 0.99 a | 35.9 ± 3.38 b | 41.3 ± 0.72 a |
| Asn | 2.76 ± 0.27 d | 5.15 ± 0.39 c | 7.79 ± 0.57 a | 6.20 ± 0.49 b | 7.54 ± 0.69 a | 8.22 ± 0.16 a |
| Ser | 5.86 ± 0.46 e | 12.0 ± 0.98 d | 16.9 ± 1.24 c | 19.2 ± 0.77 b | 19.6 ± 0.97 b | 21.9 ± 0.40 a |
| Gln | 28.5 ± 1.90 a | 29.4 ± 2.81 a | 19.8 ± 1.46 b | 14.0 ± 0.34 c | 14.9 ± 1.51 c | 7.92 ± 0.44 d |
| His | 2.12 ± 0.27 e | 4.23 ± 0.48 d | 5.67 ± 0.44 cd | 6.33 ± 0.28 bc | 7.45 ± 1.12 ab | 8.13 ± 1.54 a |
| Gly | 8.98 ± 0.45 e | 14.7 ± 1.29 d | 17.9 ± 1.37 c | 21.1 ± 0.81 b | 23.3 ± 0.93 ab | 25.0 ± 1.22 a |
| Thr | 5.48 ± 0.73 e | 9.99 ± 1.12 d | 13.4 ± 0.93 c | 16.2 ± 0.79 b | 16.6 ± 1.18 b | 18.9 ± 1.29 a |
| Cit | 1.77 ± 0.81 c | 2.27 ± 0.70 c | 3.55 ± 0.22 c | 8.61 ± 0.93 b | 7.94 ± 3.75 b | 13.6 ± 1.88 a |
| Arg | 5.48 ± 0.59 d | 11.1 ± 1.55 c | 16.9 ± 1.29 b | 18.9 ± 0.80 b | 19.5 ± 2.50 b | 23.4 ± 1.88 a |
| Ala | 168 ± 13.0 d | 201 ± 18.7 c | 214 ± 17.7 bc | 208 ± 5.64 bc | 256 ± 11.1 a | 227 ± 4.20 b |
| Tyr | 3.73 ± 0.37 c | 7.79 ± 0.89 b | 10.31 ± 0.82 a | 10.9 ± 0.45 a | 11.0 ± 0.73 a | 10.9 C ± 0.15 a |
| Cys | 1.90 ± 1.44 | 1.72 ± 2.32 | 0.49 ± 0.05 | 0.51 ± 0.02 | 3.69 ± 5.50 | 0.49 ± 0.02 |
| Val | 5.42 ± 0.33 e | 11.0 ± 0.60 d | 14.9 ± 1.14 c | 17.6 ± 0.49 b | 18.1 ± 1.21 b | 21.1 ± 0.34 a |
| Met | 4.86 ± 0.30 c | 7.84 ± 0.62 b | 9.73 ± 0.75 a | 9.62 ± 0.28 a | 10.5 ± 0.48 a | 10.4 ± 0.26 a |
| Nva | 69.8 ± 4.73 d | 77.0 ± 5.64 cd | 83.2 ± 5.65 bc | 77.3 ± 2.64 cd | 111 ± 6.72 a | 89.5 ± 0.61 b |
| Trp | 1.34 ± 0.14 b | 2.19 ± 0.28 a | 2.65 ± 0.24 a | 2.79 ± 0.14 a | 2.27 ± 0.97 a | 2.84 ± 0.05 a |
| Phe | 8.07 ± 0.60 d | 13.6 ± 1.10 c | 19.1 ± 1.45 b | 19.4 ± 0.61 b | 20.8 ± 1.27 ab | 22.3 ± 0.44 a |
| Ile | 4.80 ± 0.34 e | 9.95 ± 0.82 d | 13.9 ± 1.02 c | 15.3 ± 0.40 bc | 15.7 ± 0.96 b | 17.3 ± 0.28 a |
| Leu | 7.44 ± 0.58 e | 15.2 ± 1.40 d | 25.5 ± 1.88 c | 27.9 ± 0.66 bc | 29.4 ± 1.56 b | 32.1 ± 0.58 a |
| Lys | 6.79 ± 0.56 e | 18.2 ± 1.76 d | 26.2 ± 2.00 c | 31.7 ± 0.76 b | 32.7 ± 1.86 b | 38.5 ± 0.75 a |
| Hyp | 40.7 ± 3.00 d | 48.9 ± 3.93 c | 54.1 ± 4.33 bc | 53.4 ± 1.78 bc | 56.5 ± 1.39 b | 58.2 ± 1.93 ab |
| Sar | 9.69 ± 0.65 d | 12.1 ± 1.21 c | 15.0 ± 1.00 b | 15.4 ± 0.59 b | 17.4 ± 0.76 a | 17.3 ± 0.39 a |
| Pro | 2.48 ± 0.78 e | 6.91 ± 2.03 d | 9.79 ± 1.16 cd | 13.6 ± 0.88 ab | 12.1 ± 1.26 bc | 16.8 ± 2.05 a |
| FAA | 407 ± 27.9 d | 546 ± 47.1 c | 630 ± 45.8 b | 657 ± 17.6 b | 752 ± 5.67 a | 737 ± 19.0 a |
| Gene Name | Accession | Protein |
|---|---|---|
| PRMT5 | A0A5G2QRI8 | Protein arginine N-methyltransferase 5 |
| DPP3 | F1RU52 | Dipeptidyl peptidase 3 |
| VCPKMT | A0A287B739 | Valosin-containing protein lysine methyltransferase |
| ALDH5A1 | F1RUE3 | Succinate-semialdehyde dehydrogenase |
| LHPP | A0A480STU2 | Phospholysine phosphohistidine inorganic pyrophosphate phosphatase |
| PPP1CC | Q2EHH7 | Serine/threonine-protein phosphatase |
| AKR1B1 | P80276 | Aldo-keto reductase family 1 member B1 |
| CZIB | F1S765 | CXXC motif-containing zinc-binding protein |
| ANKRD2 | A0A5G2QKX2 | Ankyrin repeat domain 2 |
| PREP | P23687 | Prolyl endopeptidase |
| MOBKL3 | F2Z5T8 | MOB-like protein phocein |
| HIBCH | A0A5G2QLF4 | 3-hydroxyisobutyryl-CoA hydrolase |
| AIMP1 | A0A287AKA2 | Aminoacyl tRNA synthetase complex interacting multifunctional protein 1 |
| STRAP | A0A4X1VAU7 | Serine-threonine kinase receptor-associated protein |
| NAP1L4 | A0A481CQ30 | Nucleosome assembly protein 1-like 4 |
| QDPR | A0A5G2QZN1 | Quinoid dihydropteridine reductase |
| PA2G4 | A0A8D0X235 | Peptidase M24 domain-containing protein |
| MAPK1 | A0A8W4FG96 | Mitogen-activated protein kinase |
| PEPD | A0A5G2QM19 | Peptidase D |
| CMPK1 | Q29561 | UMP-CMP kinase |
| SUCLG1 | O19069 | Succinate—CoA ligase [ADP/GDP-forming] subunit alpha, mitochondrial |
| MPI | A0A287BNZ5 | Mannose-6-phosphate isomerase |
| ELOC | A0A8D0P6A0 | Elongin-C |
| TXNDC17 | A0A286ZZM7 | Thioredoxin domain-containing protein 17 |
| PIR | K7GKW6 | Pirin |
| LOC100157017 | A0A287A5X0 | Glyoxylate reductase/hydroxypyruvate reductase |
| SGTA | A0A287A1V6 | Small glutamine-rich tetratricopeptide repeat co-chaperone alpha |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Deng, C.; Li, J.; Lou, A.; Liu, Y.; Luo, J.; Shen, Q.; Quan, W. Quantitative Proteomics Reveals the Relationship between Protein Changes and Volatile Flavor Formation in Hunan Bacon during Low-Temperature Smoking. Foods 2024, 13, 1360. https://doi.org/10.3390/foods13091360
Zou H, Deng C, Li J, Lou A, Liu Y, Luo J, Shen Q, Quan W. Quantitative Proteomics Reveals the Relationship between Protein Changes and Volatile Flavor Formation in Hunan Bacon during Low-Temperature Smoking. Foods. 2024; 13(9):1360. https://doi.org/10.3390/foods13091360
Chicago/Turabian StyleZou, Huiyu, Chuangye Deng, Junnian Li, Aihua Lou, Yan Liu, Jie Luo, Qingwu Shen, and Wei Quan. 2024. "Quantitative Proteomics Reveals the Relationship between Protein Changes and Volatile Flavor Formation in Hunan Bacon during Low-Temperature Smoking" Foods 13, no. 9: 1360. https://doi.org/10.3390/foods13091360
APA StyleZou, H., Deng, C., Li, J., Lou, A., Liu, Y., Luo, J., Shen, Q., & Quan, W. (2024). Quantitative Proteomics Reveals the Relationship between Protein Changes and Volatile Flavor Formation in Hunan Bacon during Low-Temperature Smoking. Foods, 13(9), 1360. https://doi.org/10.3390/foods13091360






