Purification and Structural Characterization of Polysaccharides from Polygonum multiflorum Thunb. and Their Immunostimulatory Activity in RAW264.7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction, Isolation, and Purification of PM Polysaccharides
2.2.1. Extraction of PRMPs
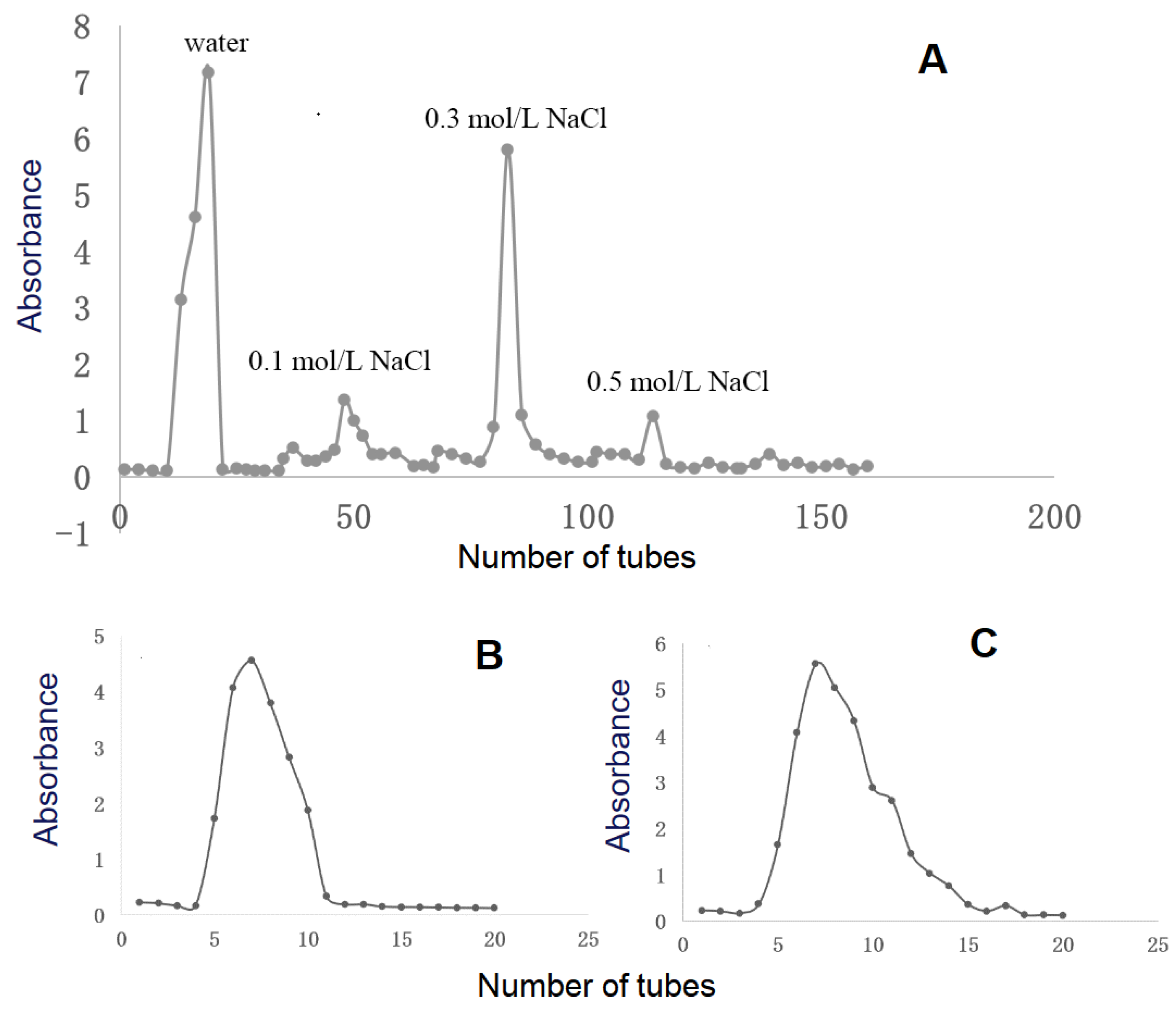
2.2.2. Separation and Purification of RPMPs
2.3. Structural Characterization of Polysaccharides
2.3.1. Analytical Methods
2.3.2. Mw Assessment
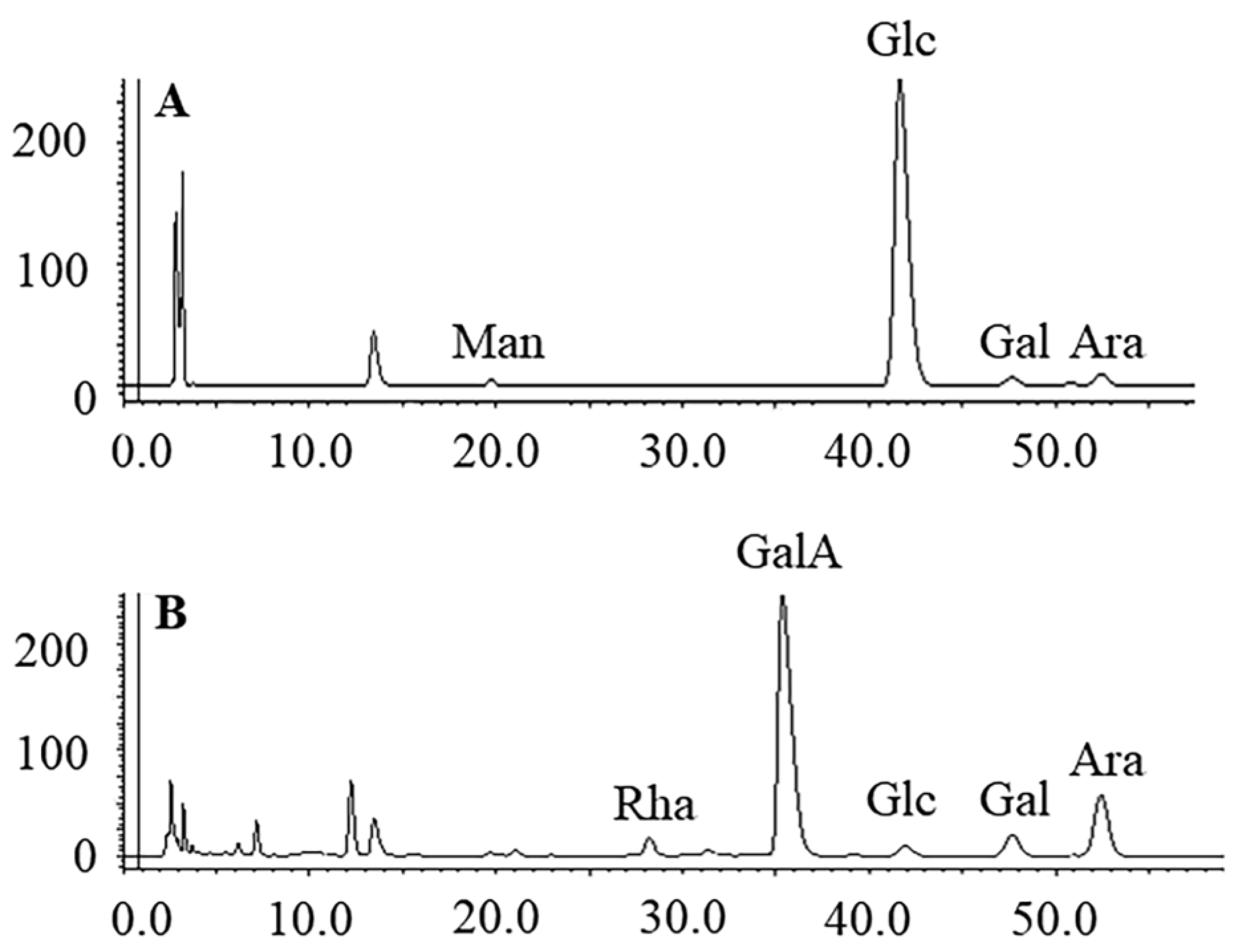
2.3.3. Monosaccharide Compositions
2.3.4. Methylation Analysis
2.3.5. Infrared Spectral Analysis
2.3.6. SEM
2.3.7. NMR Analysis
2.4. Immunoregulatory Activity Test
2.4.1. Cell Viability
2.4.2. Determination of the Levels of NO and Cytokines
2.5. Statistical Analysis
3. Results and Discussion
3.1. Purification and Chemical Composition of Polysaccharides
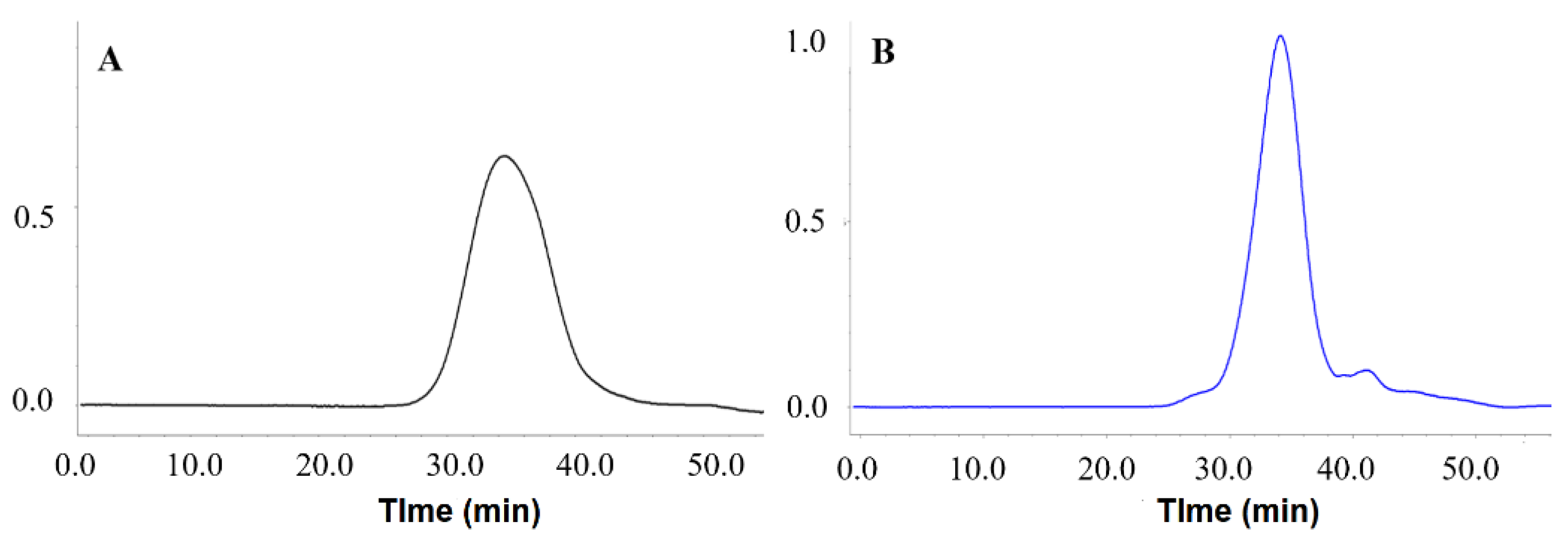
3.2. Molecular Properties of PMPs-1 and PMPs-2
3.3. Methylation Analysis of PMPs-1 and PMPs-2
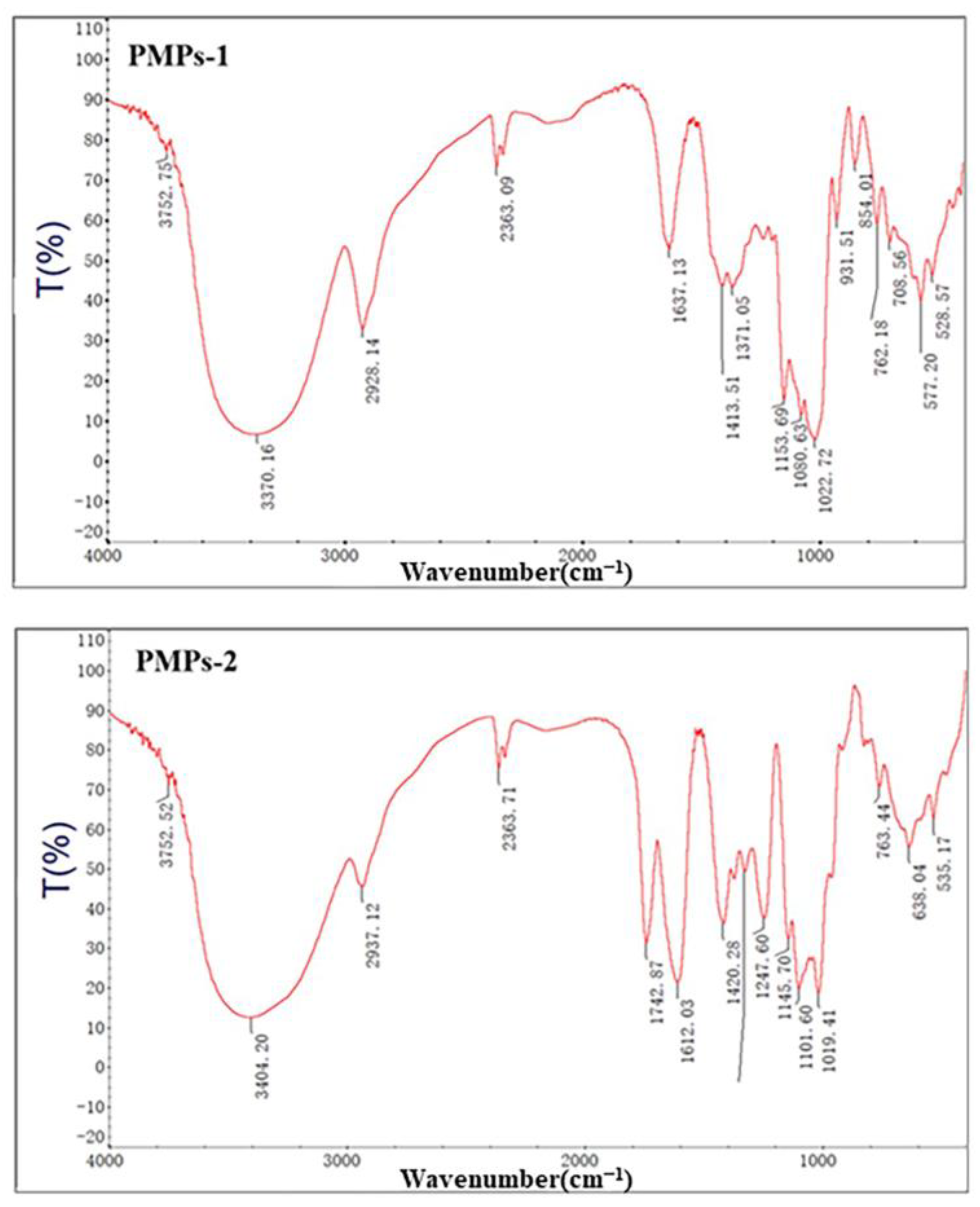
3.4. FT-IR Spectrum Analysis of PMPs-1 and PMPs-2
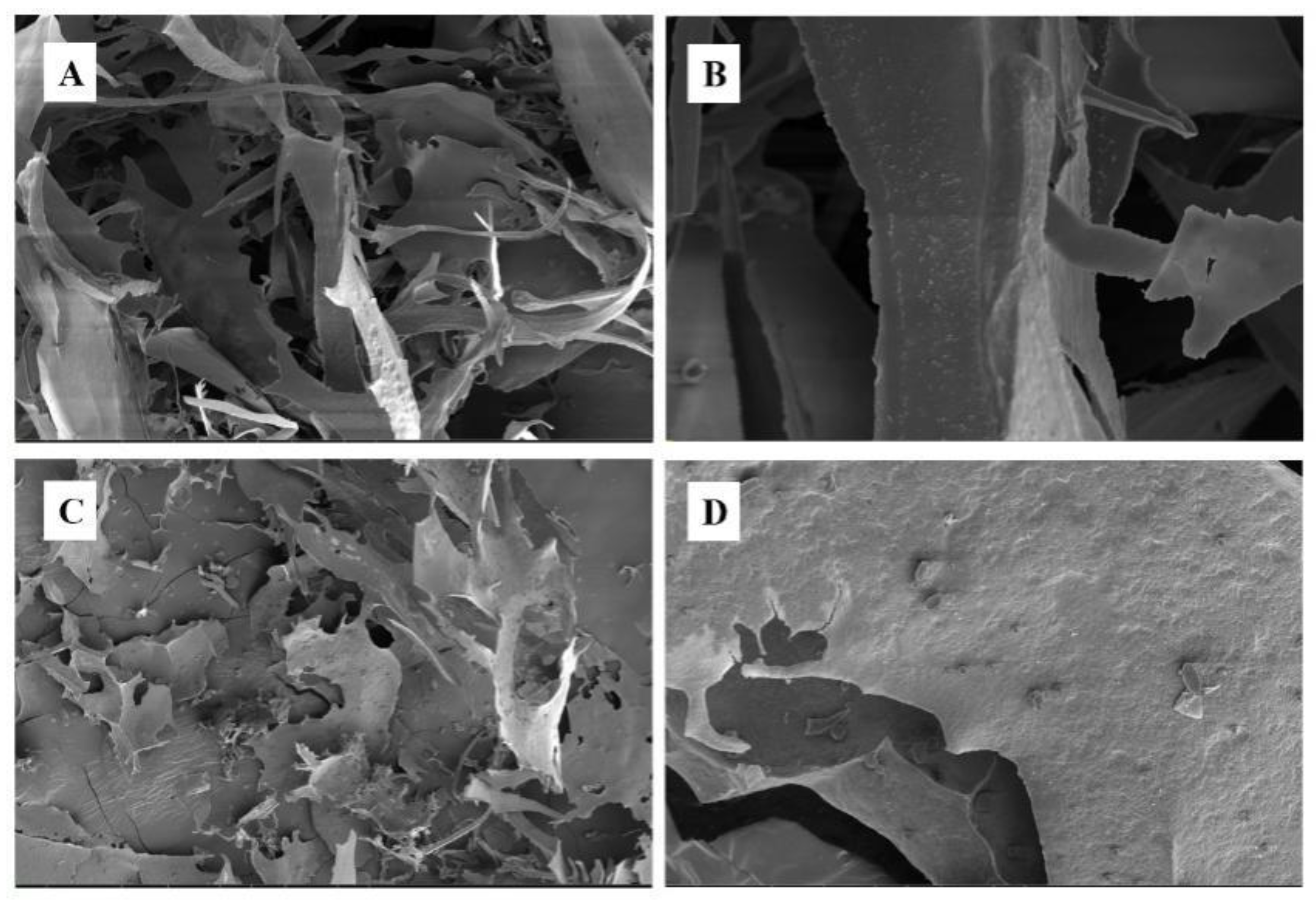
3.5. SEM Analysis of PMPs-1 and PMPs-2
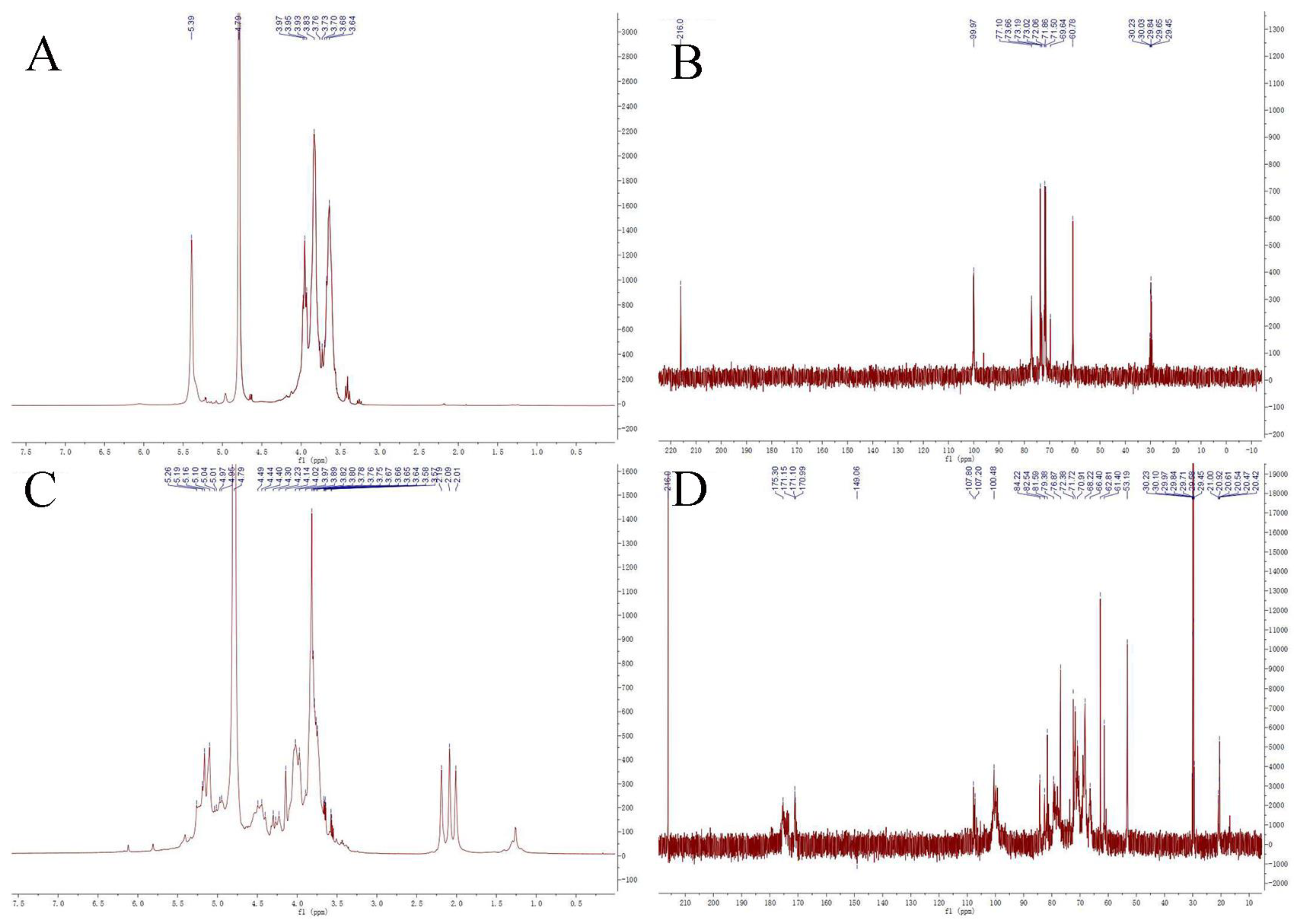
3.6. NMR Analysis of PMPs-1 and PMPs-2
3.7. Immunomodulatory Activities of PMPs-1 and PMPs-2
3.7.1. Effects of PMPs-1 and PMPs-2 on Cell Viability
3.7.2. Effects of PMPs-1 and PMPs-2 on the Contents of Cytokines in RAW264.7 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, A.J.; Yu, J.; Ji, H.Y.; Zhang, H.C.; Zhang, Y.; Liu, H.P. Extraction of a novel cold-watersoluble polysaccharide from Astragalus membranaceus and its antitumor and kmmunological activities. Molecules 2017, 23, 62. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.O.; Assreuy, A.M.; Madeira, J.C.; Chagas, F.D.; Parreiras, L.A.; Santos, G.R.; Mourao, P.A.; Pereira, M.G. Purified polysaccharides of Geoffroea spinosa barks have anticoagulant and antithrombotic activities devoid of hemorrhagic risks. Carbohydr. Polym. 2015, 124, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Anvari, M.; Joyner, H.S.; Behnam, S.; Tabarsa, A. Rheological behavior and antioxidant activity of a highly acidic gum from Althaea officinalis flower. Food Hydrocoll. 2017, 69, 432–439. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F.; Wang, L.; Yang, T.; Shi, L.; Li, X.; Shen, J.; Xu, W.; Guo, T.; Lin, Q. Antihyperlipidemic effect of rice bran polysaccharide and its potential mechanism in high-fat diet mice. Food Funct. 2017, 8, 4028–4041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, B.C.; Wang, Z.Y.; Li, M.J.; Zhao, W. Natural polysaccharides with immunomodulatory activities. Mini-Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef]
- Niu, Y.G. Introduction to the Special Issue: Preparation, Physicochemical Properties and Application of Natural Plant Polysaccharides. Foods 2023, 12, 2457. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Sources, Y.S. Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef]
- Rao, T.; Liu, Y.T.; Zeng, X.C.; Li, C.P.; Ou-Yang, D.S. The hepatotoxicity of Polygonum multiflorum: The emerging role of the immune-mediated liver injury. Acta. Pharmacol. Sin. 2020, 42, 27–35. [Google Scholar] [CrossRef]
- Lin, L.F.; Ni, B.R.; Lin, H.M.; Zhang, M.; Li, X.C.; Yin, X.B.; Qu, C.J.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Yang, J.B.; Guo, X.H.; Liu, W.X.; Ma, S.C.; Li, S.P. Polygonum multiflorum Thunb.: A Review on Chemical Analysis, Processing Mechanism, Quality Evaluation, and Hepatotoxicity. Front. Pharmacol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Teka, T.; Wang, L.M.; Gao, J.; Mou, J.J.; Pan, G.X.; Yu, H.Y.; Gao, X.M.; Han, L.F. Polygonum multiflorum: Recent updates on newly isolated compounds, potential hepatotoxic compounds and their mechanisms. J. Ethnopharmacol. 2021, 271, 113864. [Google Scholar] [CrossRef]
- Yang, J.B.; Wang, Q.; Gao, H.Y.; Wang, X.T.; Song, Y.F.; Wang, Y.; Cheng, X.L.; Wei, F.; Jin, H.T.; Ma, S.C.; et al. Dianthrones of Polygoni multiflori radix and Polygoni multiflora caulis: A review. Mod. Chin. Med. 2022, 24, 1431–1436. [Google Scholar]
- Zhang, Q.; Xu, Y.; Lv, J.; Cheng, M.; Wu, Y.; Cao, K.; Zhang, X.F.; Mou, X.; Fan, Q. Structure characterization of two functional polysaccharides from Polygonum multiflorum and its immunomodulatory. Int. J. Biol. Macromol. 2018, 113, 195–204. [Google Scholar] [CrossRef]
- Gu, D.L.; Wang, Y.; Jin, H.Y.; Kang, S.; Liu, Y.; Zan, K.; Fan, J.; Wei, F.; Ma, S.C. Changes of Physicochemical Properties and Immunomodulatory Activity of Polysaccharides during Processing of Polygonum multiflorum Thunb. Front Pharmacol. 2020, 13, 934710. [Google Scholar] [CrossRef] [PubMed]
- Michel, D.B.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; AsboeHansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.B.; Jin, H.Y.; Gu, D.L.; Wang, Q.; Liu, Y.; Zan, K.; Fan, J.; Wei, F.; Ma, S.C.; et al. Comparisons of physicochemical features and hepatoprotective potentials of unprocessed and processed polysaccharides from Polygonum multiflorum Thunb. Int. J. Biol. Macromol. 2023, 235, 123901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, H.Y.; Dong, X.X.; Yang, S.; Ma, S.C.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivities. Chin. Med. 2019, 14, 49. [Google Scholar] [CrossRef]
- Lee, S.J.; In, G.; Han, S.T.; Lee, M.H.; Lee, J.W.; Shin, K.S. Structural characteristics of a red ginseng acidic polysaccharide rhamnogalacturonan I with immunostimulating activity from red ginseng. J. Ginseng Res. 2020, 44, 570–579. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hu, D.J.; Zhu, H.; Zhang, Y.Y.; Qin, J.; Wang, F.; Zhang, Z.D.; Lv, G.P. Preparation, characterization and immunoregulatory activity of derivatives of polysaccharide from Atractylodes lancea (Thunb.) DC. Int. J. Biol. Macromol. 2022, 216, 225–234. [Google Scholar] [CrossRef]
- Lv, L.S.; Cheng, Y.H.; Zheng, T.S.; Li, X.M.; Zhai, R. Purification, antioxidant activity and antiglycation of polysaccharides from Polygonum multiflorum Thunb. Carbohydr. Polym. 2014, 99, 765–773. [Google Scholar] [CrossRef]
- Wu, D.T.; Lam, S.C.; Cheong, K.L.; Wei, F.; Lin, P.C.; Long, Z.; Lv, X.J.; Zhao, J.; Ma, S.C.; Li, S.P. Long Simultaneous determination of molecular weights and contents of water-soluble polysaccharides and their fractions from Lycium barbarum collected in China. J. Pharm. Biomed. Anal. 2016, 129, 210–218. [Google Scholar] [CrossRef]
- He, K.; Mergens, B.; Yatcilla, M.; Zheng, Q.Y. Molecular Weight Determination of Aloe Polysaccharides Using Size Exclusion Chromatography Coupled with Multi-Angle Laser Light Scattering and Refractive Index Detectors. J. AOAC Int. 2018, 101, 1729–1740. [Google Scholar] [CrossRef]
- Chi, Y.; Li, Y.; Zhang, G.; Gao, Y.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydr. Polym. 2018, 181, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhu, Z.Y.; Liu, Y.; Sun, H.Q.; Song, Q.Y.; Zhang, Y.M. The chemical structure and anti-aging bioactivity of an acid polysaccharide obtained from rose buds. Food Funct. 2018, 9, 2300–2312. [Google Scholar] [CrossRef]
- Wang, W.; Zou, Y.; Li, Q.; Mao, R.W.; Shao, X.J.; Jin, D.; Zheng, D.H.; Zhao, T.; Zhu, H.F.; Zhang, L.; et al. Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages. Process Biochem. 2016, 51, 542–553. [Google Scholar] [CrossRef]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Tian, W.; Dai, L.W.; Lu, S.M.; Luo, Z.F.; Qiu, Z.Y.; Li, J.J.; Li, P.; Du, B. Effect of Bacillus sp. DU-106 fermentation on Dendrobium officinale polysaccharide: Structure and immunoregulatory activities. Int. J. Biol. Macromol. 2019, 135, 1034–1042. [Google Scholar] [CrossRef]
- Hu, X.; Pan, X.; Wang, P.; Chen, M. Isolation and characterization of an antioxidant exopolysaccharide produced by Bacillus sp. S-1 from Sichuan pickles. Carbohydr. Polym. 2019, 204, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.S.; Li, Y.X.; Jiang, S.L.; Song, A.N.; Fu, Z.; Dong, C.X.; Yao, Z.; Qiao, W. Isolation, purification, and structural characterization of polysaccharidesfrom Atractylodis Macrocephalae Rhizoma and their immunostimulatory activity in RAW264.7 cells. Int. J. Biol. Macromol. 2020, 163, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.J.; Yang, L.; Hui, L.B.; Lin, Z.C. A polysaccharide purified from Radix Adenophorae promotes cell activation and pro-inflammatory cytokine production in murine RAW264.7 macrophages. Chin. J. Nat. Med. 2016, 14, 370–376. [Google Scholar]
- Zhou, Y.; Wang, S.C.; Feng, W.S.; Zhang, Z.L.; Li, H.W. Structural characterization and immunomodulatory activities of two polysaccharides from Rehmanniae Radix Praeparata. Int. J. Biol. Macromol. 2021, 186, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef]
- Franken, L.; Schiwon, M.; Kurts, C. Macrophages: Sentinels and regulators of the immune system. Cell. Microbiol. 2016, 18, 475–487. [Google Scholar] [CrossRef]
- Du, H.; Chen, J.; Tian, S.; Gu, H.; Li, N.; Sun, Y.; Ru, J.J.; Wang, J.R. Extraction optimization, preliminary characterization and immunological activities in vitro of polysaccharides from Elaeagnus angustifolia L. Pulp. Carbohydr. Polym. 2016, 151, 348–357. [Google Scholar] [CrossRef]
- Gong, G.; Dang, T.; Deng, Y.; Han, J.; Zou, Z.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.J.; Wang, Z.F. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Bogdan, C.; Ollinghoff, M.R.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Habijanic, J.; Berovic, M.; Boh, B.; Plankl, M.; Wraber, B. Submerged cultivation of Ganoderma lucidum and the effects of its polysaccharides on the production of human cytokines TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. New Biotechnol. 2015, 32, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Tian, Y.Q.; Shao, J.J.; Shu, X.; Jia, J.X.; Ren, X.J.; Guan, Y. Macrophage immunomodulatory activity of the polysaccharide isolated from Collybia radicata mushroom. Int. J. Biol. Macromol. 2018, 108, 300–306. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Shi, H.; Li, C.; Huang, W.; Zhang, M.; Luo, Y.Y.; Song, L.Y.; Yu, R.M.; Zhu, J.H. Structural characterization and immunoregulatory activity of a novel acidic polysaccharide from Scapharca subcrenata. Int. J. Biol. Macromol. 2022, 210, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, K.; Xiao, L.; Lei, Z.; Zhang, Z. Characterization of polysaccharide from Helicteres angustifolia L. and its immunomodulatory activities on macrophages RAW264.7. Biomed. Pharmacother. 2019, 109, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Al-Banna, N.A.; Cyprian, F.; Albert, M.J. Cytokine responses in campylobacteriosis: Linking pathogenesis to immunity. Cytokine Growth Factor Rev. 2018, 41, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.M.; Xu, S.S.; Li, L.; Pan, T.M.; Shi, C.L.; Liu, H.; Cao, M.J.; Su, W.J.; Liu, G.M. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Jokar, B.N.; Mehdi, T.; Guan, Y.S.; Masoud, R. Effects of extraction methods on molecular characteristics, antioxidant properties and immunomodulation of alginates from Sargassum angustifolium. Int. J. Biol. Macromol. 2017, 101, 703–711. [Google Scholar] [CrossRef]
- Cao, R.A.; Lee, S.H.; You, S. Structural effects of sulfated-glycoproteins from Stichopus japonicus on the nitric oxide secretion ability of RAW 264.7 cells. Prev. Nutr. Food Sci. 2014, 19, 307–313. [Google Scholar] [CrossRef][Green Version]
- Rostami, Z.; Tabarsa, M.; You, S.; Rezaei, M. Relationship between molecular weights and biological properties of alginates extracted under different methods from Colpomenia peregrina. Process Biochem. 2017, 58, 289–297. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, S.Z.; Ying, H.Z.; Dai, X.Y.; Ye, H.C. Chemical characterization and immunostimulatory effects of a polysaccharide from Polygoni Multiflori Radix Praeparata in cyclophosphamide-induced anemic mice. Carbohydr. Polym. 2012, 88, 1476–1482. [Google Scholar] [CrossRef]

| Sample | Retention Time (min) | Linkage Pattern | Mass Fragments (m/z) | Peak Area Ratio (%) |
|---|---|---|---|---|
| PMPs-1 | 13.840 | T-Glcp | 87, 101, 102, 118, 129, 145, 161, 162, 205 | 8.03 |
| 18.014 | 1,6-Linked-Glcp | 102, 113, 118, 129, 173, 233 | 2.81 | |
| 18.295 | 1,4-Linked-Glcp | 87, 99, 102, 113, 118, 129, 173, 233 | 80.54 | |
| 21.707 | 1,4,6-Linked-Glcp | 118, 261 | 8.62 | |
| PMPs-2 | 10.560 | T-Rhap | 89, 102, 118, 131, 162, 175 | 1.62 |
| 10.950 | T-Araf | 87, 102, 118, 129, 145, 161, 162 | 5.41 | |
| 13.485 | 1,2-Linked-Rhap | 89, 100, 115, 130, 131, 190 | 3.06 | |
| 13.830 | 1,3-Linked-Araf | 87, 101, 102, 118, 129, 145, 161, 162, 205 | 5.30 | |
| 14.680 | T-GalpA | 87, 102, 118, 129, 161, 162, 205 | 4.89 | |
| 15.125 | 1,5-Linked-Araf | 87, 102, 118, 129, 189 | 3.38 | |
| 17.752 | 1,3,5-Linked-Araf | 85, 99, 118, 127 | 5.47 | |
| 17.785 | 1,4-Linked-Galp(A) | 87, 99, 102, 113, 118, 131, 175 | 63.78 | |
| 18.285 | 1,4-Linked-Glcp | 87, 99, 102, 113, 118, 129, 233 | 7.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, Y.; Gu, D.; Fan, J.; Yang, J.; Zan, K.; Liu, J.; Jin, H.; Wang, Y.; Wei, F.; Ma, S. Purification and Structural Characterization of Polysaccharides from Polygonum multiflorum Thunb. and Their Immunostimulatory Activity in RAW264.7 Cells. Foods 2024, 13, 932. https://doi.org/10.3390/foods13060932
Gou Y, Gu D, Fan J, Yang J, Zan K, Liu J, Jin H, Wang Y, Wei F, Ma S. Purification and Structural Characterization of Polysaccharides from Polygonum multiflorum Thunb. and Their Immunostimulatory Activity in RAW264.7 Cells. Foods. 2024; 13(6):932. https://doi.org/10.3390/foods13060932
Chicago/Turabian StyleGou, Yan, Donglin Gu, Jing Fan, Jianbo Yang, Ke Zan, Jingjing Liu, Hongyu Jin, Ying Wang, Feng Wei, and Shuangcheng Ma. 2024. "Purification and Structural Characterization of Polysaccharides from Polygonum multiflorum Thunb. and Their Immunostimulatory Activity in RAW264.7 Cells" Foods 13, no. 6: 932. https://doi.org/10.3390/foods13060932
APA StyleGou, Y., Gu, D., Fan, J., Yang, J., Zan, K., Liu, J., Jin, H., Wang, Y., Wei, F., & Ma, S. (2024). Purification and Structural Characterization of Polysaccharides from Polygonum multiflorum Thunb. and Their Immunostimulatory Activity in RAW264.7 Cells. Foods, 13(6), 932. https://doi.org/10.3390/foods13060932







