The Effects of Jet-Milling and Pulsed Electric Fields on the Preservation of Spinach Juice Lutein Contents during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Particle Size Distribution
2.2. Physical Properties of Spinach Powder (SP)
2.3. Scanning Electron Microscope (SEM)
2.4. Spinach Juice (SJ) Preparation and Pasteurization
2.5. Determination of Microbial Activity
2.6. Analysis of Lutein Contents
2.7. Determination of Antioxidant Activities
3. Results and Discussion
3.1. Particle Properties of Differently Sized SP
3.2. Hydration Properties and Oil Holding Capacity (OHC) of Differently Sized SP
3.3. Lutein Contents and Antioxidant Activities of SP
3.4. Microstructure of Differently Sized SP
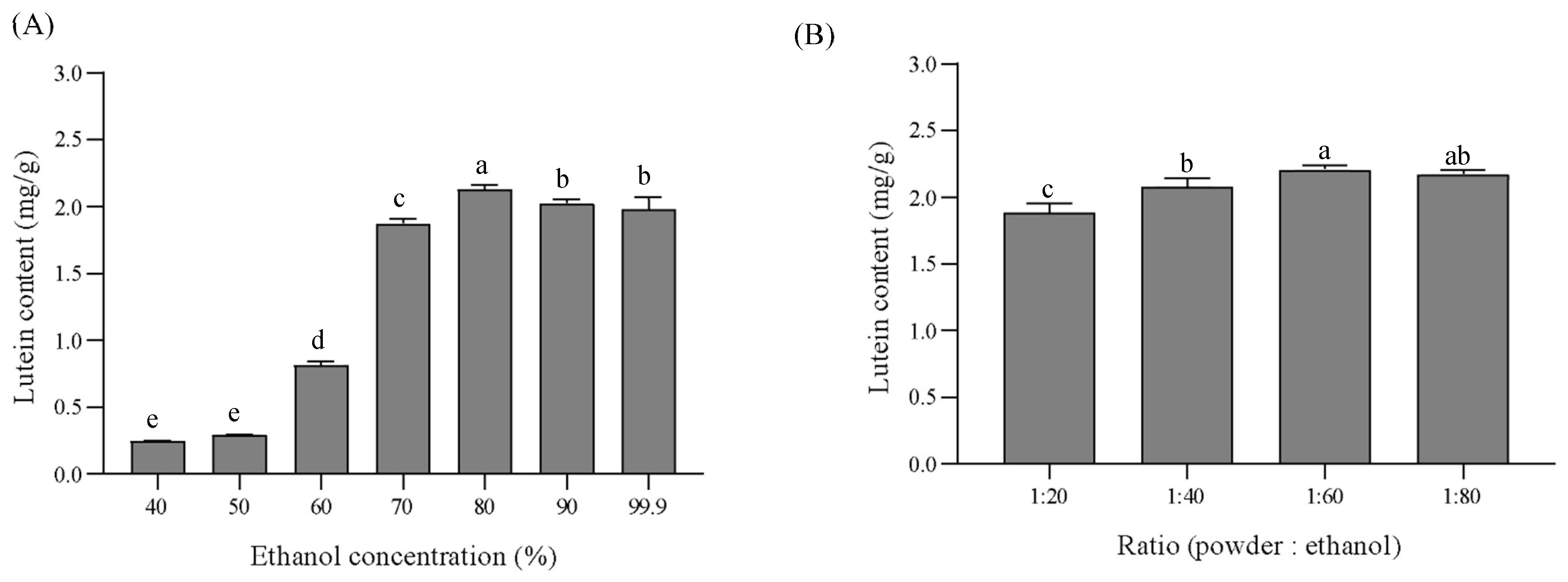
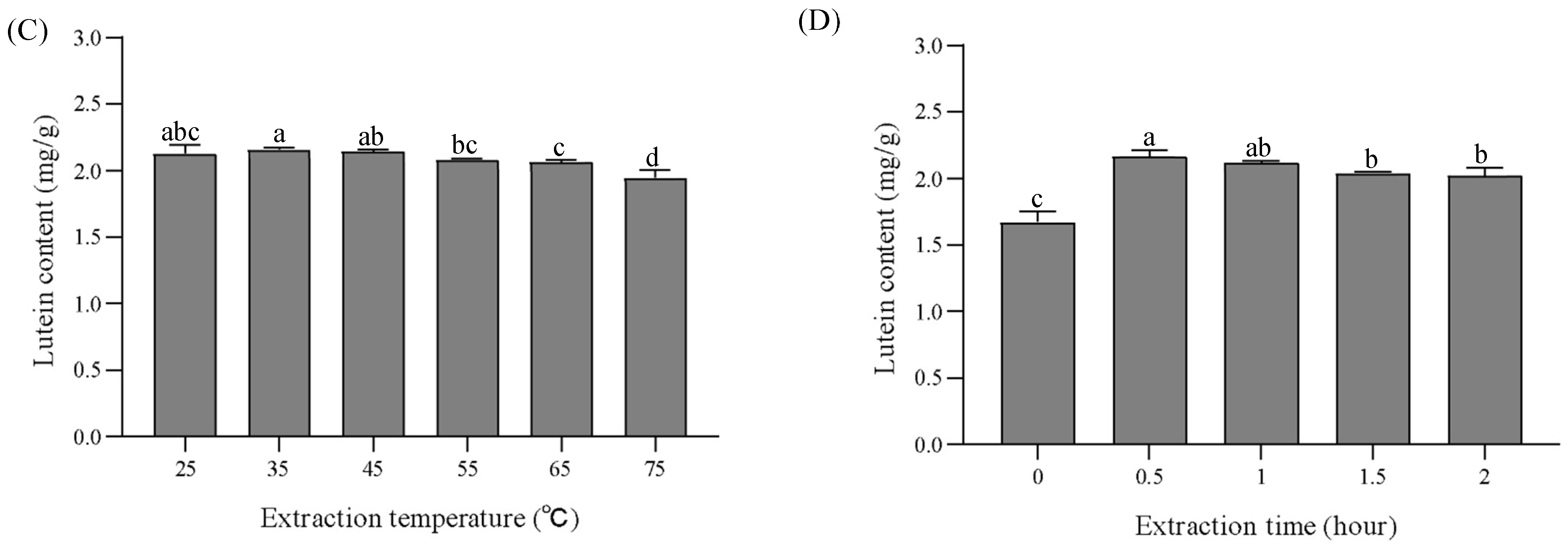
3.5. Optimization of Lutein Extraction Conditions
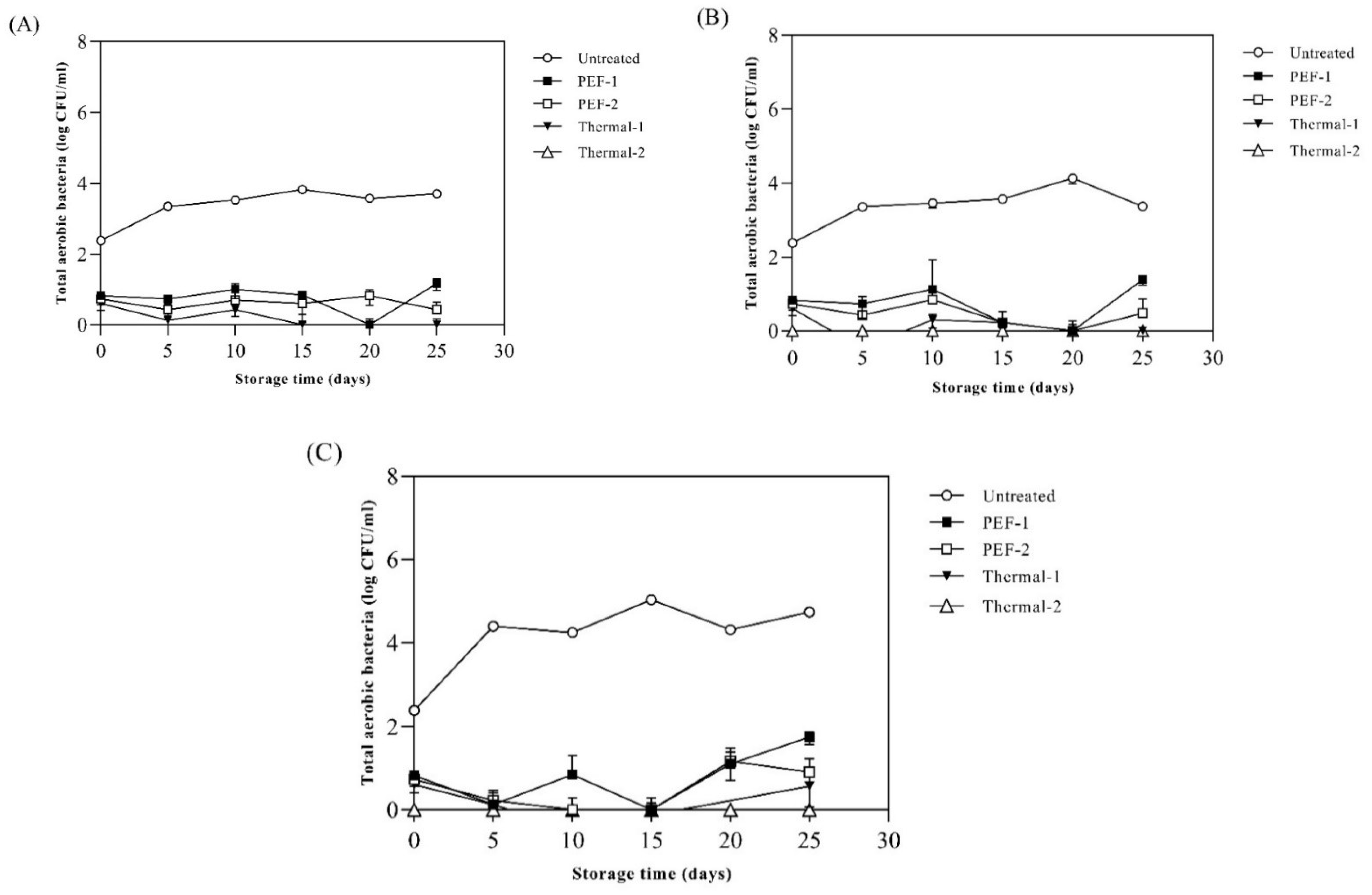
3.6. Different Thermal/Non-Thermal Treatment Effects on Microbial Inactivation of SJ with SP-Superfine
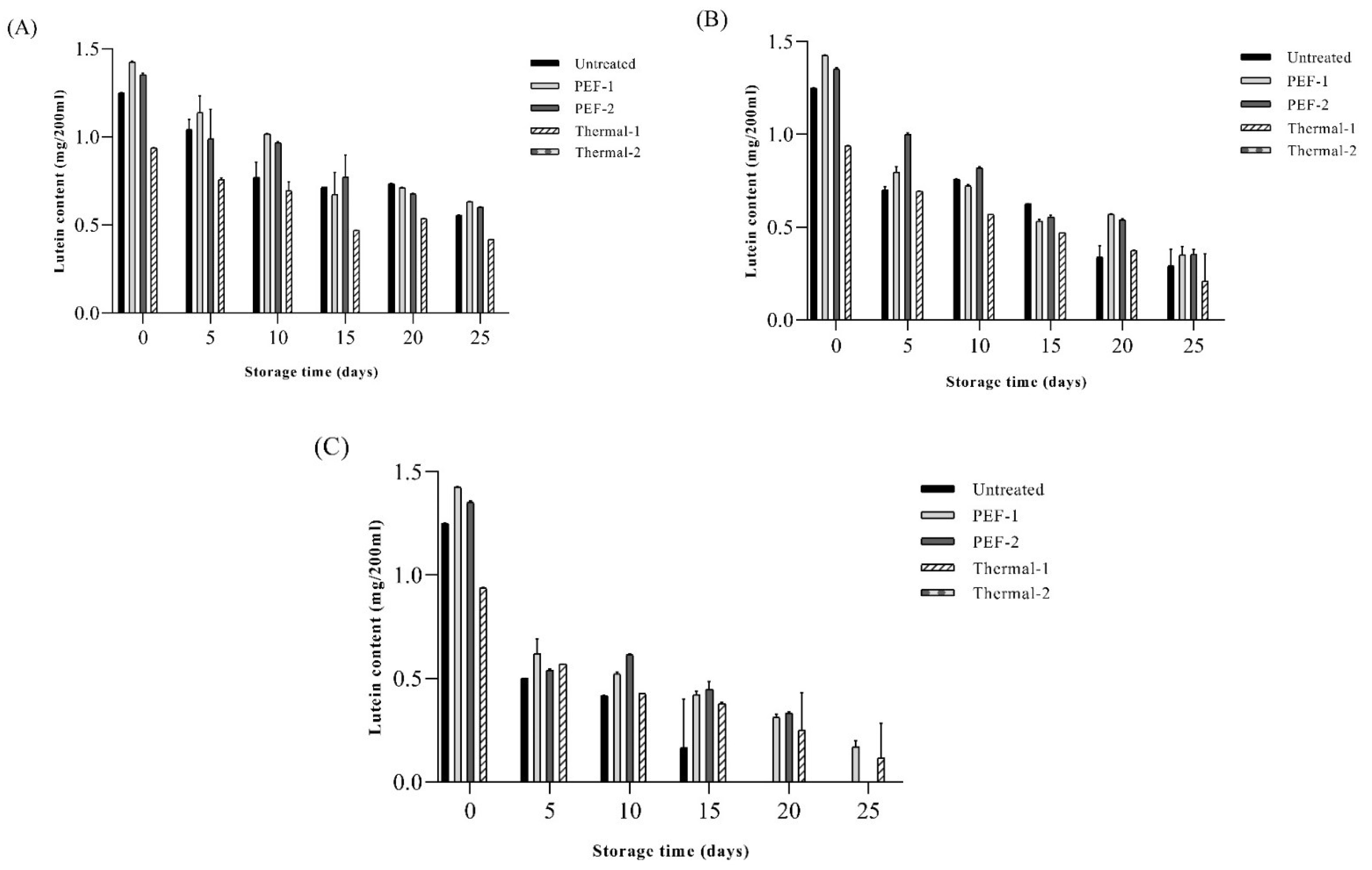
3.7. Lutein Contents and Antioxidant Activity of SJ Ring Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzoor, M.F.; Zeng, X.-A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158617. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Empis, J. Relative stabilities of microalgal carotenoids in microalgal extracts, biomass and fish feed: Effect of storage conditions. Innov. Food Sci. Emerg. Technol. 2003, 4, 227–233. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Díaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Hong, S.-Y.; Choi, H.-S.; Kim, J.-H.; Jeong, S.-H.; Lee, S.-Y.; Kim, S.-H.; Lee, D.-U. Superfine Marigold Powder Improves the Quality of Sponge Cake: Lutein Fortification, Texture, and Sensory Properties. Foods 2023, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Chen, S.-C.; Wang, Q.-L.; Liu, C.-Q.; Xiao, J.-H.; Huang, D.-W. Effects of traditional grinding and superfine grinding technologies on the properties and volatile components of Protaetia brevitarsis larvae powder. LWT 2023, 173, 114307. [Google Scholar] [CrossRef]
- Chamayou, A.; Dodds, J.A. Air jet milling. Handb. Powder Technol. 2007, 12, 421–435. [Google Scholar]
- Patel, R.P.; Baria, A.H.; Patel, N.A. An overview of size reduction technologies in the field of pharmaceutical manufacturing. Asian J. Pharm. 2008, 2. [Google Scholar] [CrossRef]
- Drakos, A.; Kyriakakis, G.; Evageliou, V.; Protonotariou, S.; Mandala, I.; Ritzoulis, C. Influence of jet milling and particle size on the composition, physicochemical and mechanical properties of barley and rye flours. Food Chem. 2017, 215, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.-J.; You, Q.; Luo, Y.; Ye, Y.; Lei, L.; Deng, Z.; Rong, H. Effect of superfine grinding Sargassum fusiforme residue powder on sponge cakes properties. LWT 2022, 165, 113735. [Google Scholar] [CrossRef]
- Angelidis, G.; Protonotariou, S.; Mandala, I.; Rosell, C.M. Jet milling effect on wheat flour characteristics and starch hydrolysis. J. Food Sci. Technol. 2016, 53, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Wang, M.-S. Effect of pulsed electric fields (PEFs) on the pigments extracted from spinach (Spinacia oleracea L.). Innov. Food Sci. Emerg. Technol. 2017, 43, 26–34. [Google Scholar] [CrossRef]
- Noci, F.; Riener, J.; Walkling-Ribeiro, M.; Cronin, D.; Morgan, D.; Lyng, J. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. J. Food Eng. 2008, 85, 141–146. [Google Scholar] [CrossRef]
- Yeom, H.W.; Streaker, C.B.; Zhang, Q.H.; Min, D.B. Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. J. Agric. Food Chem. 2000, 48, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.; Rodrigo, M. Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT-Food Sci. Technol. 2006, 39, 1163–1170. [Google Scholar] [CrossRef]
- Timmermans, R.; Mastwijk, H.; Berendsen, L.; Nederhoff, A.; Matser, A.; Van Boekel, M.; Groot, M.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019, 298, 63–73. [Google Scholar] [CrossRef]
- Muttakin, S.; Kim, M.S.; Lee, D.-U. Tailoring physicochemical and sensorial properties of defatted soybean flour using jet-milling technology. Food Chem. 2015, 187, 106–111. [Google Scholar] [CrossRef]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech 2008, 9, 250–258. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, H.; Peng, Z.; Luo, Q.; Ming, J.; Zhao, G. Characterization of stipe and cap powders of mushroom (Lentinus edodes) prepared by different grinding methods. J. Food Eng. 2012, 109, 406–413. [Google Scholar] [CrossRef]
- Choi, S.-M.; Ma, C.-Y. Extraction, purification and characterization of globulin from common buckwheat (Fagopyrum esculentum Moench) seeds. Food Res. Int. 2006, 39, 974–981. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a sustainable purification protocol for lutein and chlorophyll from spinach by-products by a saponification procedure using Box Behnken design and desirability function. Food Bioprod. Process. 2019, 116, 54–62. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Nykamp, G.; Carstensen, U.; Müller, B. Jet milling—A new technique for microparticle preparation. Int. J. Pharm. 2002, 242, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Guerin, E.; Tchoreloff, P.; Leclerc, B.; Tanguy, D.; Deleuil, M.; Couarraze, G. Rheological characterization of pharmaceutical powders using tap testing, shear cell and mercury porosimeter. Int. J. Pharm. 1999, 189, 91–103. [Google Scholar] [CrossRef]
- Hao, T. Understanding empirical powder flowability criteria scaled by Hausner ratio or Carr index with the analogous viscosity concept. RSC Adv. 2015, 5, 57212–57215. [Google Scholar] [CrossRef]
- Lv, G.; Zhang, Z.; Pan, H.; Fan, L. Effect of physical modification of mushroom (A. chaxingu) powders on their physical and chemical properties. Food Sci. Technol. Res. 2014, 20, 731–738. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Zhang, N.; Xiong, S.; Zhang, L.; Wang, J. Effect of superfine grinding on the physicochemical properties of straw mushroom (Volvariella volvacea) powders rich in vitamin D2. J. Food Process. Preserv. 2022, 46, e17192. [Google Scholar] [CrossRef]
- Gong, P.; Huang, Z.; Guo, Y.; Wang, X.; Yue, S.; Yang, W.; Chen, F.; Chang, X.; Chen, L. The effect of superfine grinding on physicochemical properties of three kinds of mushroom powder. J. Food Sci. 2022, 87, 3528–3541. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Chen, L.; Hong, H.; Li, J. Effect of superfine grinding on the physico-chemical, morphological and thermogravimetric properties of Lentinus edodes mushroom powders. J. Sci. Food Agric. 2015, 95, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Phat, C.; Li, H.; Lee, D.-U.; Moon, B.; Yoo, Y.-B.; Lee, C. Characterization of Hericium erinaceum powders prepared by conventional roll milling and jet milling. J. Food Eng. 2015, 145, 19–24. [Google Scholar] [CrossRef]
- Zhong, C.; Zu, Y.; Zhao, X.; Li, Y.; Ge, Y.; Wu, W.; Zhang, Y.; Li, Y.; Guo, D. Effect of superfine grinding on physicochemical and antioxidant properties of pomegranate peel. Int. J. Food Sci. Technol. 2016, 51, 212–221. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Ni, D. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT-Food Sci. Technol. 2012, 45, 8–12. [Google Scholar] [CrossRef]
- Li, G.; Guo, W.; Gao, X.; Wang, Y.; Sun, S. Effect of superfine grinding on physicochemical and antioxidant properties of soybean residue powder. Food Sci. Nutr. 2020, 8, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of superfine grinding on the physicochemical properties and antioxidant activity of red grape pomace powders. Powder Technol. 2015, 286, 838–844. [Google Scholar] [CrossRef]
- Latasa, M.; Van Lenning, K.; Garrido, J.; Scharek, R.; Estrada, M.; Rodríguez, F.; Zapata, M. Losses of chlorophylls and carotenoids in aqueous acetone and methanol extracts prepared for RPHPLC analysis of pigments. Chromatographia 2001, 53, 385–391. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT-Food Sci. Technol. 2017, 79, 170–177. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Isolation of chlorophylls from stinging nettle (Urtica dioica L.). Sep. Purif. Technol. 2007, 57, 37–46. [Google Scholar] [CrossRef]
- XuJie, H.; Na, Z.; SuYing, X.; ShuGang, L.; BaoQiu, Y. Extraction of BaChu mushroom polysaccharides and preparation of a compound beverage. Carbohydr. Polym. 2008, 73, 289–294. [Google Scholar] [CrossRef]
- Jin, Z.T.; Zhang, Q.H. Pulsed electric field inactivation of microorganisms and preservation of quality of cranberry juice. J. Food Process. Preserv. 1999, 23, 481–497. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nabi, B.G.; Arshad, R.N.; Roobab, U.; Yaseen, B.; Ranjha, M.M.A.N.; Aadil, R.M.; Ibrahim, S.A. Potential impact of ultrasound, pulsed electric field, high-pressure processing, microfludization against thermal treatments preservation regarding sugarcane juice (Saccharum officinarum). Ultrason. Sonochem. 2022, 90, 106194. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, R.; Mastwijk, H.; Knol, J.; Quataert, M.; Vervoort, L.; Van der Plancken, I.; Hendrickx, M.; Matser, A. Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice. Part I: Impact on overall quality attributes. Innov. Food Sci. Emerg. Technol. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Morales-de la Peña, M.; Welti-Chanes, J.; Guerrero-Beltrán, J.Á. Pulsed electric field processing of a pomegranate (Punica granatum L.) fermented beverage. Innov. Food Sci. Emerg. Technol. 2022, 79, 103045. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Rico-Alderete, I.A.; Rostro-Alanís, M.D.J.; Welti-Chanes, J.; Escobedo-Avellaneda, Z.J.; Soto-Caballero, M.C. Effect of High Hydrostatic Pressure and Pulsed Electric Fields Processes on Microbial Safety and Quality of Black/Red Raspberry Juice. Foods 2022, 11, 2342. [Google Scholar] [CrossRef] [PubMed]
- Jalali-Jivan, M.; Abbasi, S.; Fathi-Achachlouei, B. Lutein extraction by microemulsion technique: Evaluation of stability versus thermal processing and environmental stresses. LWT 2021, 149, 111839. [Google Scholar] [CrossRef]
- Chung, R.W.; Leanderson, P.; Gustafsson, N.; Jonasson, L. Liberation of lutein from spinach: Effects of heating time, microwave-reheating and liquefaction. Food Chem. 2019, 277, 573–578. [Google Scholar] [CrossRef]
- Vicaş, S.I.; Bandici, L.; Teuşdea, A.C.; Turcin, V.; Popa, D.; Bandici, G.E. The bioactive compounds, antioxidant capacity, and color intensity in must and wines derived from grapes processed by pulsed electric field. CYTA-J. Food 2017, 15, 553–562. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Geveke, D.J.; Fan, X.; Sites, J.E.; Wang, L. Evaluation of microbial stability, bioactive compounds, physicochemical properties, and consumer acceptance of pomegranate juice processed in a commercial scale pulsed electric field system. Food Bioprocess Technol. 2014, 7, 2112–2120. [Google Scholar] [CrossRef]
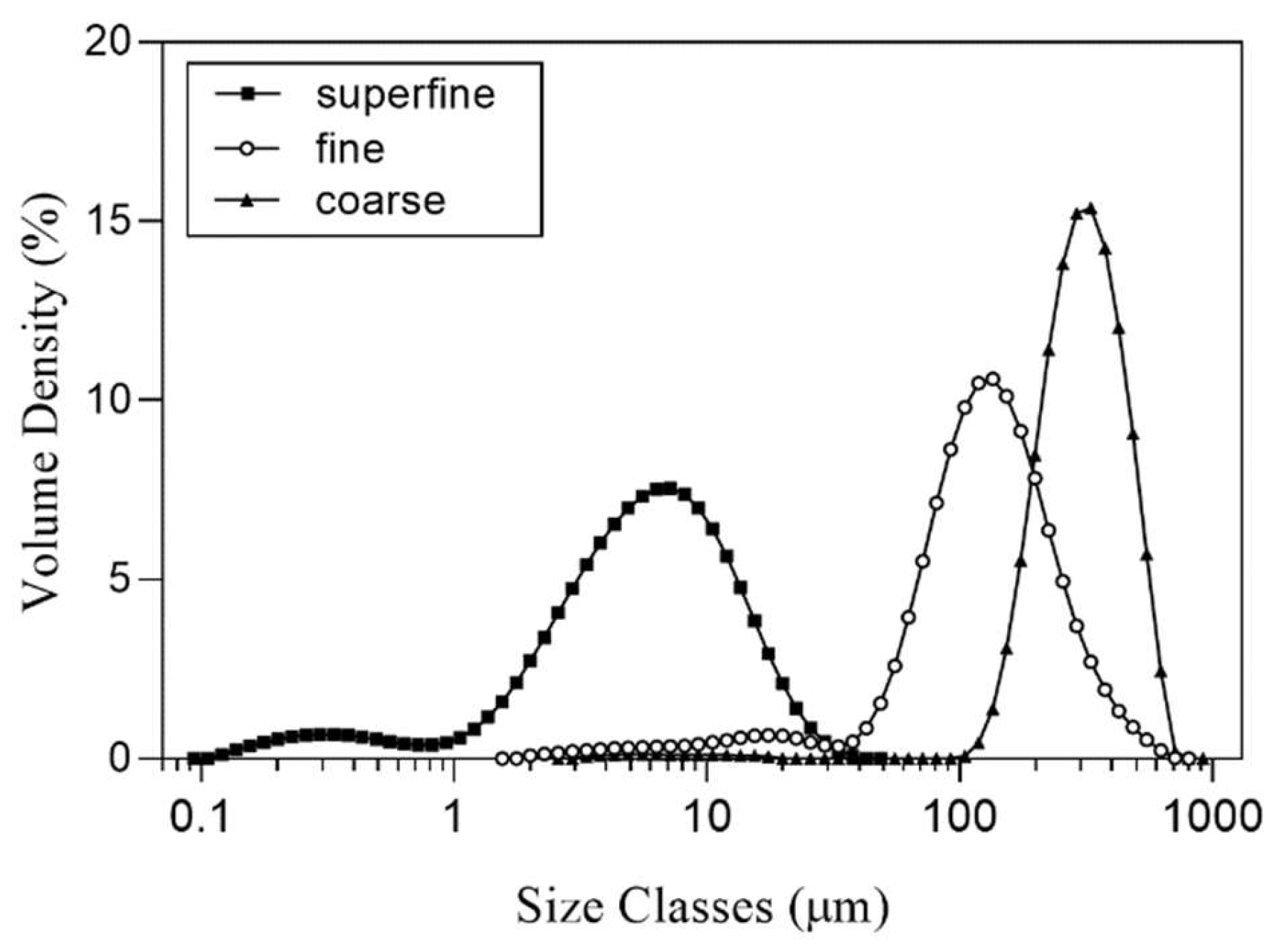

| Samples | Particle-Size Distribution | Powder Properties Flowability | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dv10 (μm) | Dv50 (μm) | Dv90 (μm) | D[4,3] (μm) | Span | Specific Surface Area (m2/kg) | Bulk Density (g/mL) | Tap Density (g/mL) | Carr Index (%) | |
| SP-coarse (1) | 191.60 ± 5.41 a,(2),(3) | 315.20 ± 3.37 a | 495.00 ± 5.15 a | 329.00 ± 6.44 a | 0.96 ± 0.03 c | 97.19 ± 4.11 b | 0.44 ± 0.00 a | 0.51 ± 0.00 b | 13.65 ± 0.28 c |
| SP-fine | 53.22 ± 2.84 b | 125.20 ± 1.30 b | 259.60 ± 4.45 b | 143.6 ± 2.07 b | 1.65 ± 0.04 b | 334.26 ± 11.33 b | 0.44 ± 0.00 a | 0.58 ± 0.00 a | 24.27 ± 0.59 b |
| SP-superfine | 1.59 ± 0.08 c | 5.59 ± 0.24 c | 14.16 ± 0.35 c | 6.93 ± 0.24 c | 2.24 ± 0.05 a | 8671.20 ± 414.26 a | 0.22 ± 0.00 b | 0.39 ± 0.00 c | 43.47 ± 0.49 a |
| Samples | Hydration Properties | Antioxidant Activities | ||||
|---|---|---|---|---|---|---|
| WHC (g/g) | SC (mL/g) | OHC (g/g) | Lutein Content (mg/g) | ABTS (%) | DPPH (%) | |
| SP-coarse (1) | 4.55 ± 0.47 a,(2),(3) | 1.97 ± 0.06 a | 2.86 ± 0.01 c | 1.59 ± 0.02 b | 56.56 ± 2.01 b | 39.36 ± 1.94 a |
| SP-fine | 3.87 ± 0.08 b | 1.60 ± 0.10 b | 2.51 ± 0.03 b | 1.70 ± 0.10 b | 55.31 ± 0.31 b | 32.67 ± 1.36 b |
| SP-superfine | 3.26 ± 0.23 c | 1.33 ± 0.29 b | 2.30 ± 0.04 a | 2.09 ± 0.13 a | 65.01 ± 1.25 a | 36.82 ± 1.65 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Lee, Y.-G.; Ju, H.-I.; Jeon, J.-H.; Jeong, S.-H.; Lee, D.-U. The Effects of Jet-Milling and Pulsed Electric Fields on the Preservation of Spinach Juice Lutein Contents during Storage. Foods 2024, 13, 834. https://doi.org/10.3390/foods13060834
Kim S-Y, Lee Y-G, Ju H-I, Jeon J-H, Jeong S-H, Lee D-U. The Effects of Jet-Milling and Pulsed Electric Fields on the Preservation of Spinach Juice Lutein Contents during Storage. Foods. 2024; 13(6):834. https://doi.org/10.3390/foods13060834
Chicago/Turabian StyleKim, Si-Yeon, Yeong-Geol Lee, Hye-In Ju, Ji-Hee Jeon, Se-Ho Jeong, and Dong-Un Lee. 2024. "The Effects of Jet-Milling and Pulsed Electric Fields on the Preservation of Spinach Juice Lutein Contents during Storage" Foods 13, no. 6: 834. https://doi.org/10.3390/foods13060834
APA StyleKim, S.-Y., Lee, Y.-G., Ju, H.-I., Jeon, J.-H., Jeong, S.-H., & Lee, D.-U. (2024). The Effects of Jet-Milling and Pulsed Electric Fields on the Preservation of Spinach Juice Lutein Contents during Storage. Foods, 13(6), 834. https://doi.org/10.3390/foods13060834







