Preparation, Structural Identification, and Screening of Egg-Derived Peptides with Facilitating Alcohol Metabolism Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Egg-Derived Peptides with Facilitating Alcohol Metabolism (EPs)
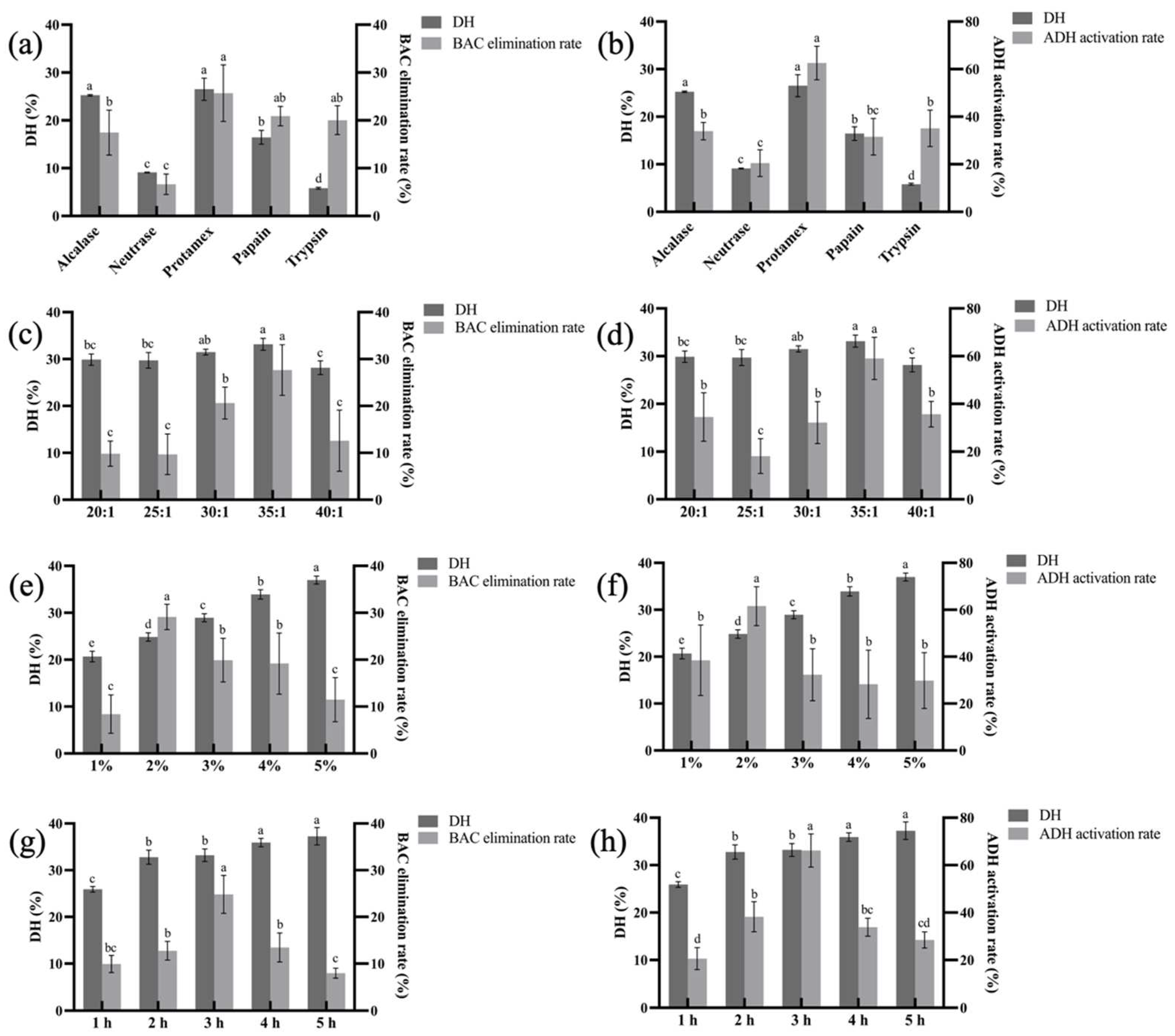
2.3. Optimization of Enzymatic Hydrolysis Conditions
2.4. Determination of the DH
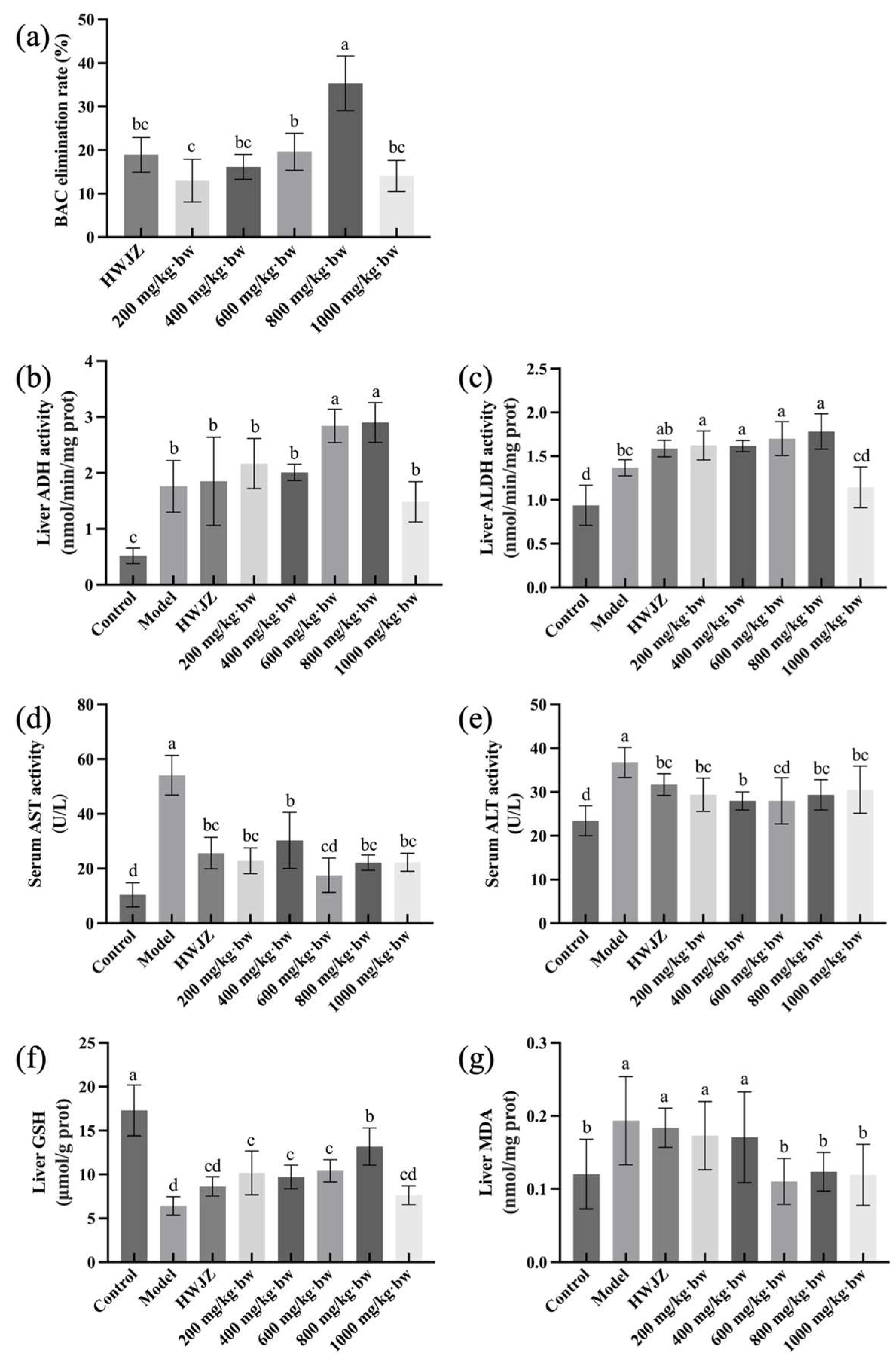
2.5. Experimental Animals and Treatments
2.6. Determination of the BAC by Gas Chromatography (GC)
2.7. Determination of Biochemical Indicators
2.8. Determination of the ADH Activation In Vitro
2.9. Amino Acid Analysis
2.10. Characterizations of the Structures of EPs
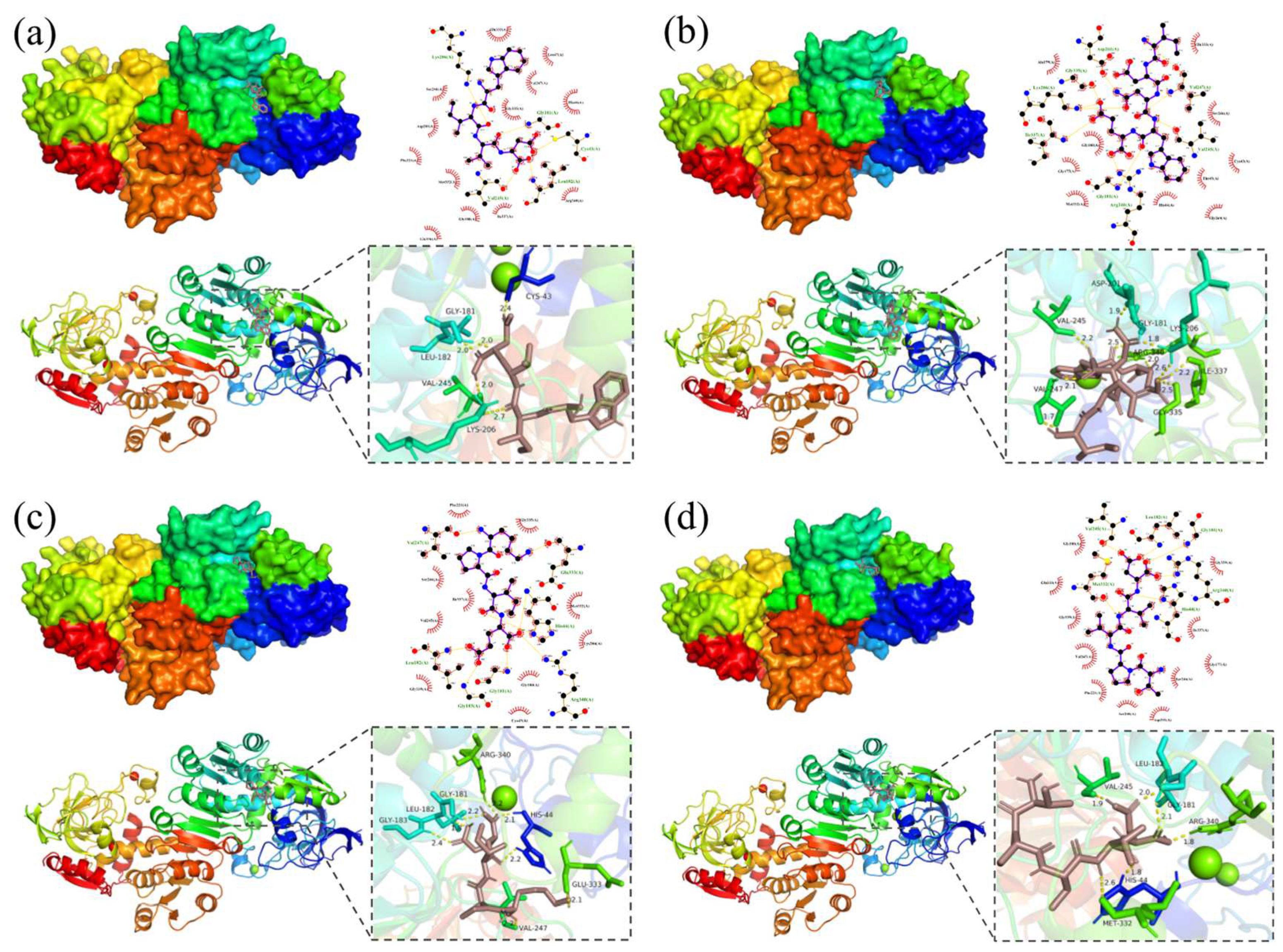
2.11. Molecular Docking and Visual Analysis
2.12. Peptide Synthesis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Enzymatic Hydrolysis Conditions
3.2. Dose–Response Relationship of Eps
3.3. Amino Acid Analysis
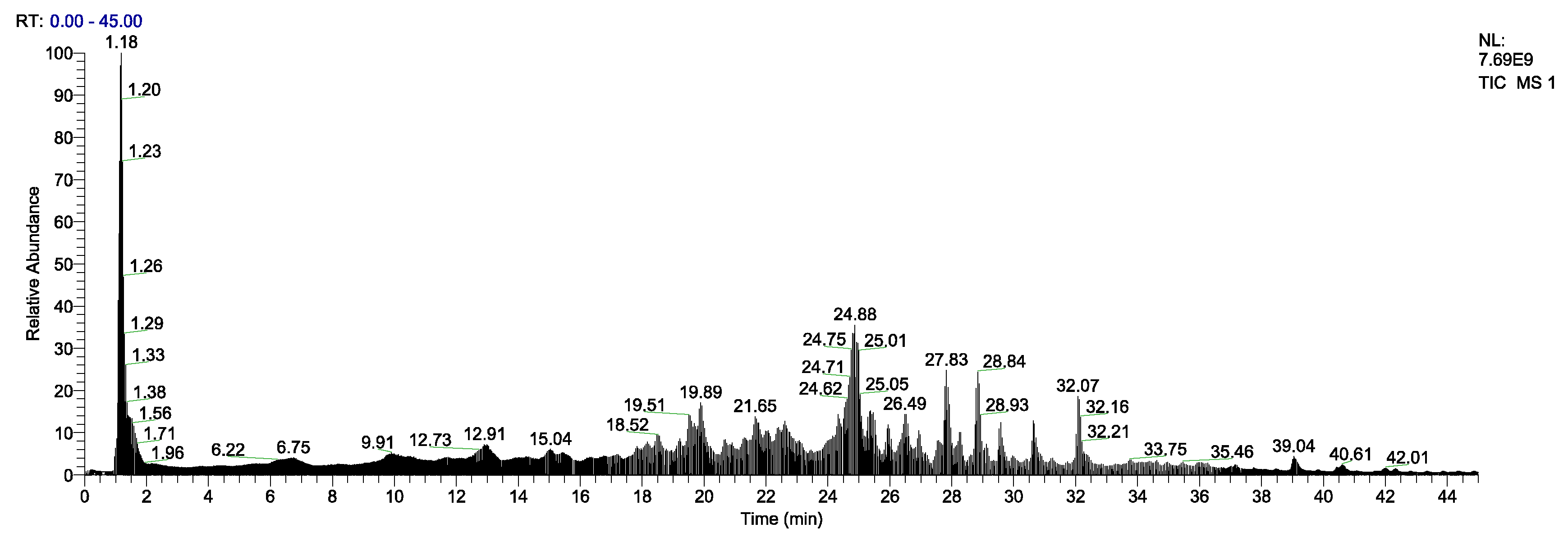
3.4. Identification of EPs by LC-MS/MS
3.5. Molecular Docking and Visual Analysis
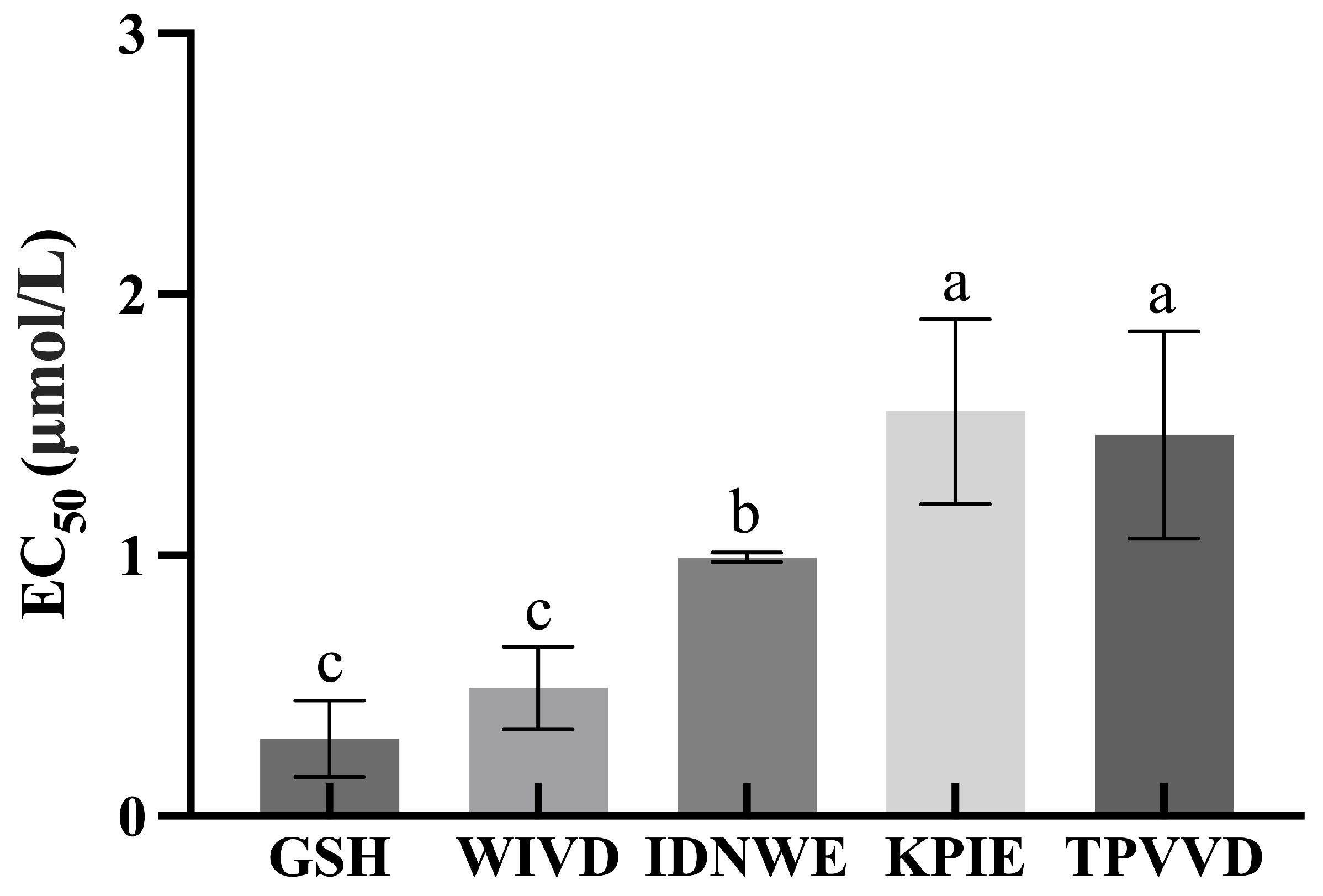
3.6. In Vitro Activity Validation of Peptides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Fisher, J.A.; VoPham, T.; Vasiliou, V.; Jones, R.R. Associations between per- and polyfluoroalkyl substances, liver function, and daily alcohol consumption in a sample of U.S. adults. Environ. Res. 2023, 235, 116651. [Google Scholar] [CrossRef] [PubMed]
- Bryazka, D.; Reitsma, M.B.; Griswold, M.G.; Abate, K.H.; Abbafati, C.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abdoli, A.; Abdollahi, M.; Abdullah, A.Y.M.; et al. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: A systematic analysis for the Global Burden of Disease Study 2020. Lancet 2022, 400, 185–235. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shnitko, T.A.; Hsu, L.-M.; Broadwater, M.A.; Sardinas, M.; Wang, T.-W.W.; Robinson, D.L.; Vetreno, R.P.; Crews, F.T.; Shih, Y.-Y.I. Acute alcohol induces greater dose-dependent increase in the lateral cortical network functional connectivity in adult than adolescent rats. Addict. Neurosci. 2023, 7, 100105. [Google Scholar] [CrossRef]
- McMahan, R.H.; Anton, P.; Coleman, L.G.; Cresci, G.A.M.; Crews, F.T.; Crotty, K.M.; Luck, M.E.; Molina, P.E.; Vachharajani, V.; Weinberg, J.; et al. Alcohol and Immunology: Mechanisms of multi-organ damage. Summary of the 2022 alcohol and Immunology research interest group (AIRIG) meeting. Alcohol 2023, 110, 57–63. [Google Scholar] [CrossRef]
- Monk, R.L.; Qureshi, A.W.; Knibb, G.; McGale, L.; Nair, L.; Kelly, J.; Collins, H.; Heim, D. In people who drink more, facets of theory of mind may be impaired by alcohol stimuli. Drug Alcohol Depend. 2023, 245, 109811. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.-E. Impact of drinking alcohol on gut microbiota: Recent perspectives on ethanol and alcoholic beverage. Curr. Opin. Food Sci. 2021, 37, 91–97. [Google Scholar] [CrossRef]
- Provan, L.; Forrest, E.H. Alcohol and the liver. Medicine 2023, 51, 331–335. [Google Scholar] [CrossRef]
- Caballería, J. Current concepts in alcohol metabolism. Ann. Hepatol. 2003, 2, 60–68. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Laniewska-Dunaj, M.; Jelski, W.; Orywal, K.; Kochanowicz, J.; Rutkowski, R.; Szmitkowski, M. The Activity of Class I, II, III and IV of Alcohol Dehydrogenase (ADH) Isoenzymes and Aldehyde Dehydrogenase (ALDH) in Brain Cancer. Neurochem. Res. 2013, 38, 1517–1521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugimoto, K.; Takei, Y. Pathogenesis of alcoholic liver disease. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2017, 47, 70–79. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Carr, R. Alcohol effects on hepatic lipid metabolism. J. Lipid Res. 2020, 61, 470–479. [Google Scholar] [CrossRef]
- Li, H.; Xia, X.; Cheng, S.; Zang, J.; Wang, Z.; Du, M. Oyster (Crassostrea gigas) ferritin relieves lead-induced liver oxidative damage via regulating the mitophagy. Int. J. Biol. Macromol. 2023, 253, 126965. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jia, S.; Suo, K.; Kang, Q.; Hao, L.; Lu, L.; Liu, X.; Huang, J.; Lu, J. Positive effect of ethanol-induced Lactococcus lactis on alcohol metabolism in mice. Food Sci. Hum. Wellness 2022, 11, 1183–1190. [Google Scholar] [CrossRef]
- Zhao, R.-J.; Huo, C.-Y.; Qian, Y.; Ren, D.-F.; Lu, J. Ultra-high-pressure processing improves proteolysis and release of bioactive peptides with activation activities on alcohol metabolic enzymes in vitro from mushroom foot protein. Food Chem. 2017, 231, 25–32. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, F.; Wu, Y.; Dai, L.; Feng, Y.; Chen, S.; Wang, G.; Ma, H.; Li, X.; Dai, C. Identification of a novel peptide that activates alcohol dehydrogenase from crucian carp swim bladder and how it protects against acute alcohol-induced liver injury in mice. J. Pharm. Biomed. Anal. 2022, 207, 114426. [Google Scholar] [CrossRef]
- Xiao, C.; Zhou, L.; Gao, J.; Jia, R.; Zheng, Y.; Zhao, S.; Zhao, M.; Toldrá, F. Musculus senhousei as a promising source of bioactive peptides protecting against alcohol-induced liver injury. Food Chem. Toxicol. 2023, 174, 113652. [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Wang, Q.Y.; Hussain, M.A.; Wang, C.Y.; Hou, J.C.; Jiang, Z.M. Protective Effect of Polyphenols, Protein, Peptides, and Polysaccharides on Alcoholic Liver Disease: A Review of Research Status and Molecular Mechanisms. J. Agric. Food Chem. 2023, 71, 5861–5883. [Google Scholar] [CrossRef]
- Ren, J.; Li, S.; Song, C.; Sun, X.; Liu, X. Black soybean-derived peptides exerted protective effect against alcohol-induced liver injury in mice. J. Funct. Foods 2021, 87, 104828. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, C.; Qin, X.; Cao, W.; Chen, J.; Li, Y.; Zheng, H.; Lin, H.; Chen, Z. Hepatoprotective effect of clam (Corbicula fluminea) protein hydrolysate on alcohol-induced liver injury in mice and partial identification of a hepatoprotective peptide from the hydrolysate. Food Sci. Technol. 2022, 42, e61522. [Google Scholar] [CrossRef]
- Lu, J.; He, H.; Fan, Y.; Wang, Z.; Nie, Z.; Ma, Z. Anti-Alcohol Function of Corn Peptides Produced by Continuous Enzymatic Membrane Reaction. Food Sci. 2012, 33, 241–245. [Google Scholar]
- Razi, S.M.; Fahim, H.; Amirabadi, S.; Rashidinejad, A. An overview of the functional properties of egg white proteins and their application in the food industry. Food Hydrocoll. 2023, 135, 108183. [Google Scholar] [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Miguel, M. Egg-derived peptides and hydrolysates: A new bioactive treasure for cardiometabolic diseases. Trends Food Sci. Technol. 2020, 104, 208–218. [Google Scholar] [CrossRef]
- Meng, Y.; Qiu, N.; Guyonnet, V.; Mine, Y. Unveiling and application of the chicken egg proteome: An overview on a two-decade achievement. Food Chem. 2022, 393, 133403. [Google Scholar] [CrossRef]
- Zheng, J.; Bu, T.; Liu, L.; He, G.; Li, S.; Wu, J. Naturally occurring low molecular peptides identified in egg white show antioxidant activity. Food Res. Int. 2020, 138, 109766. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, M.; Liu, J.; Zhao, W. Underlying antihypertensive mechanism of egg white-derived peptide QIGLF using renal metabolomics analysis. Food Res. Int. 2022, 157, 111457. [Google Scholar] [CrossRef]
- Ma, B.; Guo, Y.; Fu, X.; Jin, Y. Identification and antimicrobial mechanisms of a novel peptide derived from egg white ovotransferrin hydrolysates. LWT 2020, 131, 109720. [Google Scholar] [CrossRef]
- Ge, H.; Cai, Z.; Chai, J.; Liu, J.; Liu, B.; Yu, Y.; Liu, J.; Zhang, T. Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 2021, 360, 129981. [Google Scholar] [CrossRef] [PubMed]
- Kan, R.; Yu, Z.; Zhao, W. Identification and molecular action mechanism of novel TAS2R14 blocking peptides from egg white proteins. LWT 2023, 180, 114716. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, D.; Yu, Z.; Ding, L.; Liu, J. Novel membrane peptidase inhibitory peptides with activity against angiotensin converting enzyme and dipeptidyl peptidase IV identified from hen eggs. J. Funct. Foods 2020, 64, 103649. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Limited enzymic degradation of proteins: A new approach in the industrial application of hydrolases. J. Chem. Technol. Biotechnol. 1982, 32, 138–156. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T. Egg yolk protein modification by controlled enzymatic hydrolysis for improved functionalities. Int. J. Food Sci. Technol. 2010, 44, 763–769. [Google Scholar] [CrossRef]
- Ma, Z.L.; Zhang, W.J.; Yu, G.C.; He, H.; Zhang, Y. The primary structure identification of a corn peptide facilitating alcohol metabolism by HPLC-MS/MS. Peptides 2012, 37, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ruan, B.L.; Hu, G.Y. Anti-intoxication and protective effects of a recombinant serine protease inhibitor from Lentinula edodes against acute alcohol-induced liver injury in mice. Appl. Microbiol. Biotechnol. 2020, 104, 4985–4993. [Google Scholar] [CrossRef]
- Xiao, C.; Toldrá, F.; Zhou, F.; Mora, L.; Luo, L.; Zheng, L.; Luo, D.; Zhao, M. Chicken-derived tripeptide KPC (Lys-Pro-Cys) stabilizes alcohol dehydrogenase (ADH) through peptide-enzyme interaction. LWT 2022, 161, 113376. [Google Scholar] [CrossRef]
- Lieber, C.S. Metabolism of Alcohol. Clin. Liver Dis. 1998, 9, 1–35. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, N.; He, L.; Peng, G.; Xue, X.; Wu, L.; Cao, W. Antioxidant and hepatoprotective effects of A. cerana honey against acute alcohol-induced liver damage in mice. Food Res. Int. 2017, 101, 35–44. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Lai, Q.; Yang, Q.; Dong, Y.; Liu, X.; Wang, W.; Zhang, J.; Jia, L. Antioxidant and hepatoprotective activities of modified polysaccharides from Coprinus comatus in mice with alcohol-induced liver injury. Int. J. Biol. Macromol. Struct. Funct. Interact. 2019, 127, 476–485. [Google Scholar] [CrossRef]
- Fu, Y.; Zeng, Z.; Feng, S.; Chen, Y.; Ding, Q.; Shi, Y. JBB attenuates acute and chronic alcoholic liver injury by regulating the NFκB, KEAP1, and HIF1 pathways. J. Funct. Foods 2023, 109, 105782. [Google Scholar] [CrossRef]
- King, A.J. Multidisciplinary uses of the chicken egg. Avian Biol. Res. 2009, 2, 107–119. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Nishikiori, F.; Ito, M.; Furukawa, Y. The Effects of Corn Peptide Ingestion on Facilitating Alcohol Metabolism in Healthy Men. Biosci. Biotechnol. Biochem. 1997, 61I, 1474–1481. [Google Scholar] [CrossRef]
- Murakami, H.; Ito, M.; Furukawa, Y.; Komai, M. Leucine accelerates blood ethanol oxidation by enhancing the activity of ethanol metabolic enzymes in the livers of SHRSP rats. Amino Acids 2012, 43, 2545–2551. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takada, M.; Nozaki, O.; Ito, M.; Furukawa, Y. Preparation of corn peptide from corn gluten meal and its administration effect on alcohol metabolism in stroke-prone spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 1996, 42, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, F.; Zhao, M.; Su, G.; Sun, B. Chicken breast muscle hydrolysates ameliorate acute alcohol-induced liver injury in mice through alcohol dehydrogenase (ADH) activation and oxidative stress reduction. Food Funct. 2018, 9, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Benedé, S.; Molina, E. Chicken Egg Proteins and Derived Peptides with Antioxidant Properties. Foods 2020, 9, 735. [Google Scholar] [CrossRef]
- Yuan, J.; Zheng, Y.; Wu, Y.; Chen, H.B.; Tong, P.; Gao, J.Y. Double enzyme hydrolysis for producing antioxidant peptide from egg white: Optimization, evaluation, and potential allergenicity. J. Food Biochem. 2020, 44, e13113. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dickman, M.B. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 2005, 102, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Haseba, T.; Tomita, Y.; Kurosu, M.; Ohno, Y. Dose and time changes in liver alcohol dehydrogenase (ADH) activity during acute alcohol intoxication involve not only class I but also class III ADH and govern elimination rate of blood ethanol. Leg. Med. 2003, 5, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Xuan-Yu, M.; Hong-Xing, Z.; Mihaly, M.; Meng, C. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput.-Aided Drug Des. 2011, 7, 146–157. [Google Scholar]
- He, S.; Zhang, Y.; Sun, H.; Du, M.; Qiu, J.; Tang, M.; Sun, X.; Zhu, B. Antioxidative Peptides from Proteolytic Hydrolysates of False Abalone (Volutharpa ampullacea perryi): Characterization, Identification, and Molecular Docking. Mar. Drugs 2019, 17, 116. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of antioxidative peptides from Atlantic sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef] [PubMed]
- Feig, M.; Onufriev, A.; Lee, M.S.; Im, W.; Case, D.A.; Brooks, C.L. Performance comparison of generalized born and Poisson methods in the calculation of electrostatic solvation energies for protein structures. J. Comput. Chem. 2004, 25, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, X.; Chen, Q.; Wu, Q.; He, Q. Screening and mechanisms of novel angiotensin-I-converting enzyme inhibitory peptides from rabbit meat proteins: A combined in silico and in vitro study. Food Chem. 2022, 370, 131070. [Google Scholar] [CrossRef]
- Herzenberg, L.A.; DeRosa, S.C.; Dubs, J.G.; Roederer, M.; Anderson, M.T.; Ela, S.W.; Deresinski, S.C.; Herzenberg, L.A. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA 1997, 94, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Manna, S.K.; Golla, S.; Krausz, K.W.; Cai, Y.; Garcia-Milian, R.; Chakraborty, T.; Chakraborty, J.; Chatterjee, R.; Thompson, D.C.; et al. Glutathione deficiency-elicited reprogramming of hepatic metabolism protects against alcohol-induced steatosis. Free Radic. Biol. Med. 2019, 143, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zan, R.; Zhu, L.; Wu, G.; Zhang, H. Identification of Novel Peptides with Alcohol Dehydrogenase (ADH) Activating Ability in Chickpea Protein Hydrolysates. Foods 2023, 12, 1574. [Google Scholar] [CrossRef]
| Amino Acid | g/100 g of Sample |
|---|---|
| essential amino acid | |
| Ile * | 2.94 ± 0.09 |
| Leu * | 5.26 ± 0.16 |
| Lys | 4.31 ± 0.13 |
| Met * | 1.88 ± 0.07 |
| Val * | 3.78 ± 0.11 |
| Thr | 2.74 ± 0.08 |
| His | 1.34 ± 0.04 |
| Phe * | 3.24 ± 0.11 |
| Trp n.d. | |
| non-essential amino acid | |
| Asx | 6.06 ± 0.19 |
| Glx | 7.75 ± 0.27 |
| Ser | 4.31 ± 0.13 |
| Gly | 2.04 ± 0.06 |
| Ala * | 3.25 ± 0.09 |
| Tyr | 2.21 ± 0.08 |
| Pro * | 2.11 ± 0.07 |
| Arg | 3.67 ± 0.13 |
| Cys n.d. | |
| TAA | 56.85 ± 1.82 |
| EAA | 25.46 ± 0.80 |
| HAA | 22.43 ± 0.71 |
| EAA/TAA | 44.78% ± 0.03% |
| HAA/TAA | 39.44% ± 0.02% |
| Annotated Sequence | Top Apex RT [min] | Theo. MH+ [Da] |
|---|---|---|
| KDALN | 1.2 | 560 |
| TASEE | 1.2 | 550 |
| VSSEE | 1.2 | 550 |
| VSSDE | 1.2 | 550 |
| VLQAACRQE | 1.2 | 1000 |
| VNMDE | 1.2 | 620 |
| QEIE | 1.2 | 520 |
| QELD | 1.2 | 520 |
| QDIE | 1.2 | 520 |
| QDID | 1.2 | 520 |
| NEIE | 1.2 | 520 |
| CTASE | 1.2 | 520 |
| VGADD | 1.5 | 490 |
| LAAD | 1.8 | 400 |
| IAAE | 1.8 | 400 |
| AGDLD | 5.3 | 520 |
| GADLE | 5.3 | 520 |
| GADID | 5.3 | 520 |
| AGDIE | 5.3 | 520 |
| VAVE | 5.4 | 420 |
| VAVD | 5.4 | 420 |
| LGVE | 5.4 | 420 |
| IGVD | 5.4 | 420 |
| VSVE | 7.4 | 430 |
| VSVD | 7.4 | 430 |
| SSAME | 7.4 | 520 |
| SSAMD | 7.4 | 520 |
| IINE | 12 | 490 |
| IIND | 12 | 490 |
| IIGGD | 12 | 490 |
| SVGLF | 14 | 500 |
| VSGLE | 14 | 500 |
| VSGLD | 14 | 500 |
| ATGIE | 14 | 500 |
| KPKIE | 16 | 630 |
| KNNLE | 16 | 620 |
| SVGEVE | 17 | 630 |
| ILGEE | 17 | 560 |
| ILGED | 17 | 560 |
| ILGDE | 17 | 560 |
| IIGDD | 17 | 560 |
| SVDADID | 18 | 730 |
| IENL | 18 | 490 |
| VAALD | 18 | 500 |
| VAALE | 18 | 500 |
| VQIE | 18 | 500 |
| GIGLE | 18 | 500 |
| GIGID | 18 | 500 |
| KPID | 18 | 500 |
| AIGIE | 18 | 500 |
| ALGLD | 18 | 500 |
| KPIE | 18 | 500 |
| IAGLE | 18 | 500 |
| LAGLD | 18 | 500 |
| QVSELE | 19 | 700 |
| VSNELE | 19 | 700 |
| GVETDID | 20 | 760 |
| LSEDGIE | 20 | 760 |
| LDASDIE | 20 | 760 |
| VLQIE | 22 | 600 |
| VLNLE | 22 | 600 |
| VIAGIE | 22 | 600 |
| SVDIIE | 22 | 680 |
| VSDLLD | 22 | 680 |
| ATDLIE | 22 | 680 |
| QLGI | 22 | 430 |
| IIIDE | 23 | 600 |
| LIIDD | 23 | 600 |
| DLNWE | 24 | 700 |
| IDNWE | 24 | 700 |
| VAATL | 25 | 490 |
| WIVD | 25 | 550 |
| WIVE | 25 | 550 |
| VDVVD | 25 | 560 |
| TPVVE | 26 | 570 |
| TPVVD | 26 | 570 |
| TVIID | 26 | 570 |
| TVILE | 26 | 570 |
| LSLID | 26 | 570 |
| DIITVD | 26 | 690 |
| AVGGLVD | 26 | 640 |
| ISGDVL | 26 | 620 |
| IGVDVE | 27 | 650 |
| ASTIINME | 29 | 900 |
| Sequence | Binding Energy (kcal/mol) | Sequence | Binding Energy (kcal/mol) |
|---|---|---|---|
| WIVD | −7.16 | QDID | −4.29 |
| IDNWE | −6.77 | IIGGD | −4.25 |
| KPIE | −6.76 | IENL | −4.25 |
| TPVVD | −6.76 | VDVVD | −4.22 |
| LAAD | −6.72 | ALGLD | −4.18 |
| VAVE | −6.69 | GADID | −4.17 |
| WIVE | −6.61 | SVDIIE | −4.13 |
| QELD | −6.52 | AGDIE | −4.10 |
| VAATL | −6.46 | CTASE | −4.06 |
| ATGIE | −6.41 | VLQIE | −4.06 |
| VIAGIE | −6.23 | AVGGLVD | −3.96 |
| LGVE | −6.16 | NEIE | −3.93 |
| LIIDD | −6.03 | IIGDD | −3.93 |
| SVGLF | −5.84 | TASEE | −3.91 |
| TVIID | −5.83 | KDALN | −3.85 |
| QLGI | −5.75 | LDASDIE | −3.85 |
| IIIDE | −5.71 | ILGDE | −3.79 |
| IIND | −5.56 | VSGLE | −3.69 |
| KPID | −5.41 | SSAME | −3.58 |
| AIGIE | −5.30 | SSAMD | −3.58 |
| IAAE | −5.24 | IINE | −3.53 |
| LAGLD | −5.21 | ILGEE | −3.53 |
| VAVD | −5.19 | VSGLD | −3.43 |
| LSLID | −5.10 | GADLE | −3.40 |
| VSDLLD | −5.07 | QVSELE | −3.36 |
| VSVE | −5.06 | VNMDE | −3.33 |
| IGVD | −5.01 | IGVDVE | −3.33 |
| QEIE | −5.00 | SVGEVE | −3.07 |
| VSVD | −5.00 | IAGLE | −3.02 |
| VQIE | −4.94 | VLNLE | −2.97 |
| VAALD | −4.73 | AGDLD | −2.94 |
| VSSDE | −4.66 | ILGED | −2.89 |
| VAALE | −4.63 | VSNELE | −2.57 |
| TVILE | −4.57 | DIITVD | −2.08 |
| TPVVE | −4.52 | DLNWE | −1.94 |
| ATDLIE | −4.51 | KNNLE | −1.78 |
| ISGDVL | −4.48 | SVDADID | −1.65 |
| KPKIE | −4.43 | GVETDID | −1.31 |
| GIGID | −4.41 | VSSEE | −1.25 |
| VGADD | −4.38 | LSEDGIE | −0.24 |
| GIGLE | −4.38 | VLQAACRQE | - |
| QDIE | −4.37 | ASTIINME | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Wang, Y.; Wan, Y.; Liang, Y.; Liu, Q.; Wei, M.; Hou, T. Preparation, Structural Identification, and Screening of Egg-Derived Peptides with Facilitating Alcohol Metabolism Activity. Foods 2024, 13, 745. https://doi.org/10.3390/foods13050745
Tan Y, Wang Y, Wan Y, Liang Y, Liu Q, Wei M, Hou T. Preparation, Structural Identification, and Screening of Egg-Derived Peptides with Facilitating Alcohol Metabolism Activity. Foods. 2024; 13(5):745. https://doi.org/10.3390/foods13050745
Chicago/Turabian StyleTan, Yali, Yulin Wang, Yuan Wan, Yu Liang, Qiaocui Liu, Mengya Wei, and Tao Hou. 2024. "Preparation, Structural Identification, and Screening of Egg-Derived Peptides with Facilitating Alcohol Metabolism Activity" Foods 13, no. 5: 745. https://doi.org/10.3390/foods13050745
APA StyleTan, Y., Wang, Y., Wan, Y., Liang, Y., Liu, Q., Wei, M., & Hou, T. (2024). Preparation, Structural Identification, and Screening of Egg-Derived Peptides with Facilitating Alcohol Metabolism Activity. Foods, 13(5), 745. https://doi.org/10.3390/foods13050745






