A Colorimetric Nanofiber Film Based on Ethyl Cellulose/Gelatin/Purple Sweet Potato Anthocyanins for Monitoring Pork Freshness
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PSPAs
2.3. Characterization of PSPAs
2.4. Preparation of the Nanofiber Films
2.5. Color Response to Violate Ammonia
2.6. Characterizations of Nanofiber Films
2.6.1. Encapsulation Efficiency (EE) of PSPAs
2.6.2. Scanning Electron Microscopy (SEM)
2.6.3. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR)
2.6.4. Water Vapor Permeability (WVP) and Water Contact Angle (WCA)
2.6.5. Antioxidant Capacity of Nanofiber Films
2.7. Application Test
2.8. Data Analysis
3. Results and Discussion
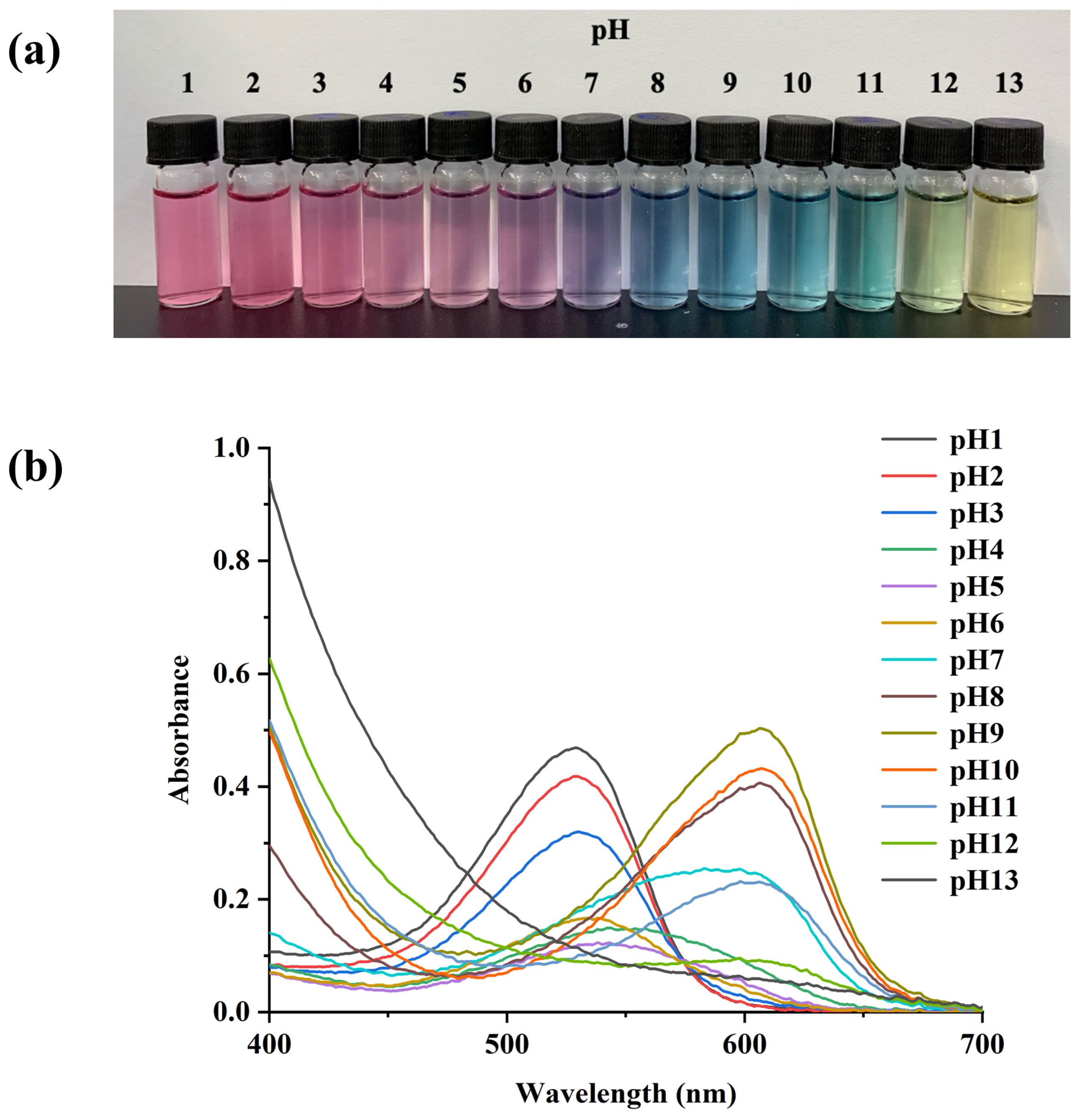
3.1. Color Changes and UV-Vis Spectroscopy of PSPAs
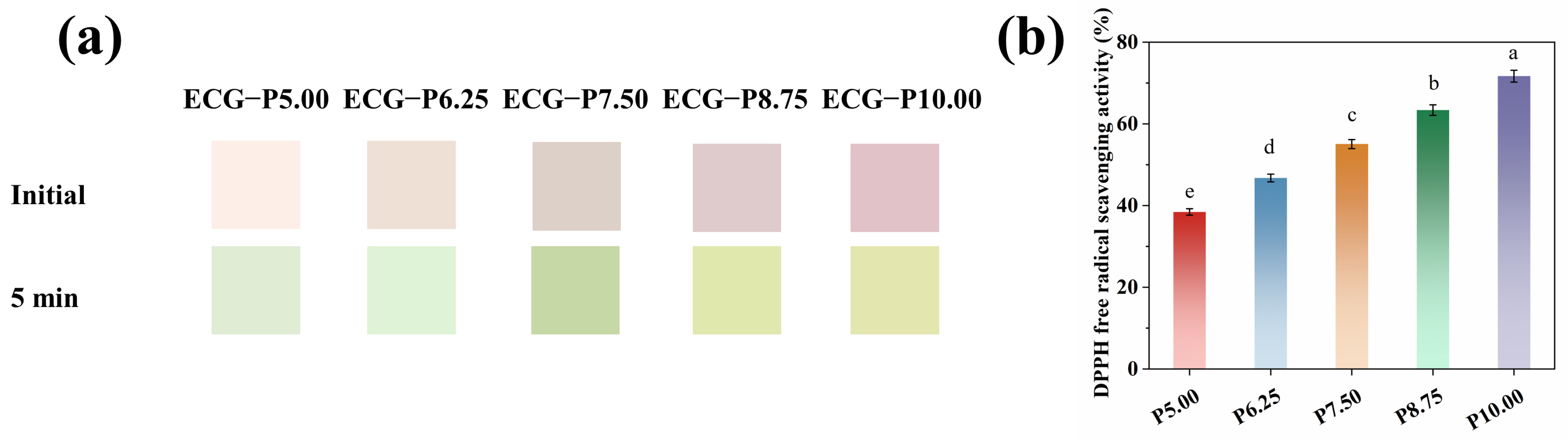
3.2. Colors and Antioxidant Properties of PSPAs
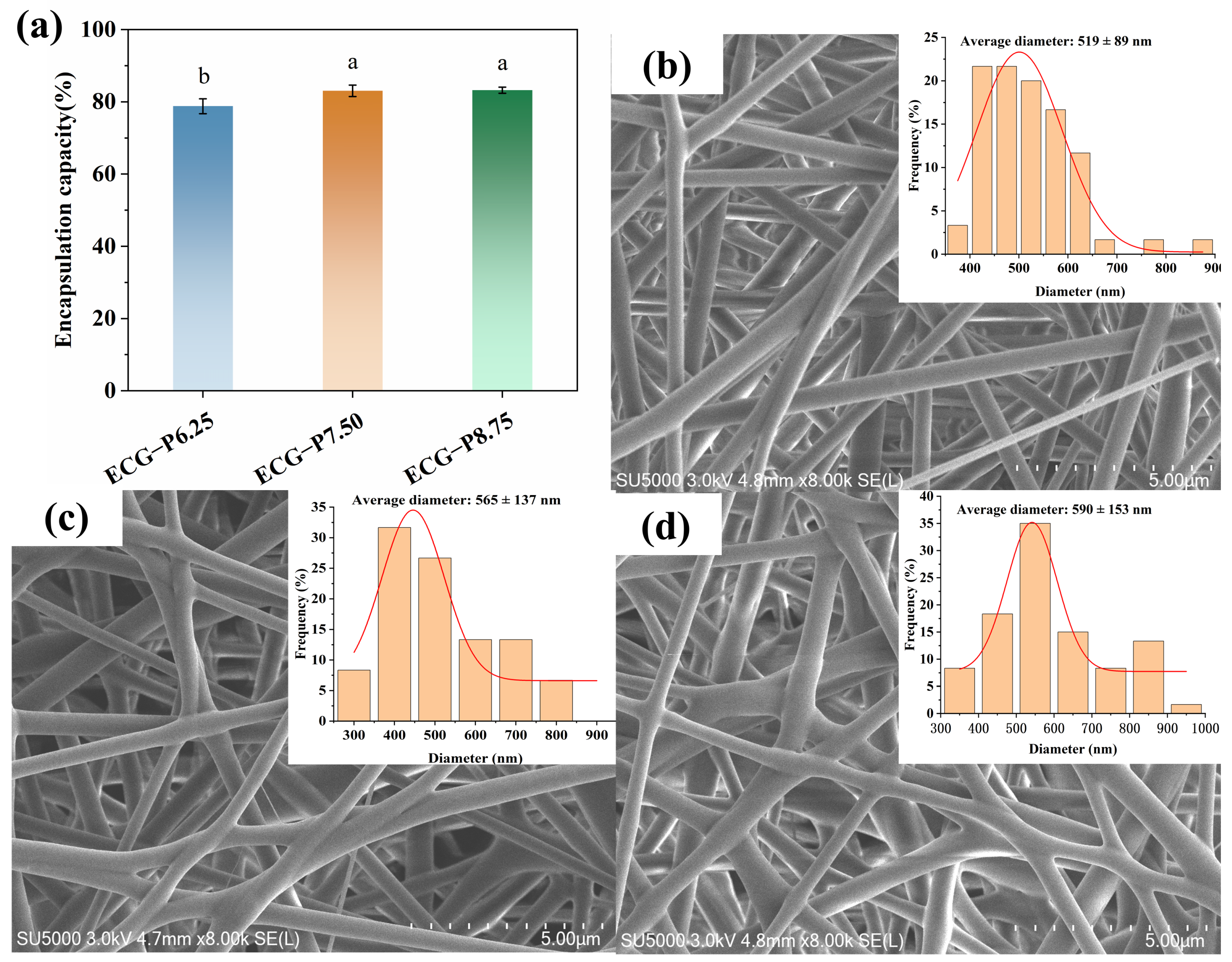
3.3. EE and Morphology Analysis
3.4. FTIR Spectroscopy of the Film
3.5. Physical Properties of the Film
3.6. Antioxidant Capacity of the Film
3.7. Color Response to Violate Ammonia of the Film
3.8. Application of ECG−P7.50 Nanofiber Film for Pork Freshness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Wen, J.; Huang, S.; Sun, Y.; Liu, X.; Chen, L.; Li, H.; Zhao, P. Highly TVB-N sensitive film with CMS as the ‘bridge’ via electrostatic interaction and hydrogen bond self-assembly for monitoring food freshness in intelligent packaging. Talanta 2023, 252, 123881. [Google Scholar] [CrossRef]
- Li, Y.; Ying, Y.; Zhou, Y.; Ge, Y.; Yuan, C.; Wu, C.; Hu, Y. A pH-indicating intelligent packaging composed of chitosan-purple potato extractions strength by surface-deacetylated chitin nanofibers. Int. J. Biol. Macromol. 2019, 127, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ghasemlou, M.; Ariffin, F.; Singh, Z.; Al-Hassan, A.A. Natural anthocyanins: Sources, extraction, characterization, and suitability for smart packaging. Food Packag. Shelf Life 2022, 33, 100872. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, Y.; Lu, D.; Gao, W.; Zhao, Q.; Shi, X. Multifunctional intelligent film integrated with purple sweet potato anthocyanin and quercetin-loaded chitosan nanoparticles for monitoring and maintaining freshness of shrimp. Food Packag. Shelf Life 2023, 35, 101022. [Google Scholar] [CrossRef]
- Fei, P.; Zeng, F.; Zheng, S.; Chen, Q.; Hu, Y.; Cai, J. Acylation of blueberry anthocyanins with maleic acid: Improvement of the stability and its application potential in intelligent color indicator packing materials. Dye. Pigments 2021, 184, 108852. [Google Scholar] [CrossRef]
- Zhao, M.; Nuerjiang, M.; Bai, X.; Feng, J.; Kong, B.; Sun, F.; Li, Y.; Xia, X. Monitoring dynamic changes in chicken freshness at 4 °C and 25 °C using pH-sensitive intelligent films based on sodium alginate and purple sweet potato peel extracts. Int. J. Biol. Macromol. 2022, 216, 361–373. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zhang, J.; Liu, L.; Shi, J.; Muhammad, A.; Zhai, X.; Zou, X.; Xiao, J.; Li, Z.; et al. Development of nanofiber indicator with high sensitivity for pork preservation and freshness monitoring. Food Chem. 2022, 381, 132224. [Google Scholar] [CrossRef]
- Huang, H.; Song, Y.; Zhang, Y.; Li, Y.; Li, J.; Lu, X.; Wang, C. Electrospun Nanofibers: Current Progress and Applications in Food Systems. J. Agric. Food Chem. 2022, 70, 1391–1409. [Google Scholar] [CrossRef]
- Kang, D.H.; Kang, H.W. Surface energy characteristics of zeolite embedded PVDF nanofiber films with electrospinning process. Appl. Surf. Sci. 2016, 387, 82–88. [Google Scholar] [CrossRef]
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation of carotenoids extracted from tomato peels into zein fibers by electrospinning. J. Sci. Food Agric. 2019, 99, 759–766. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H. Design and testing of an electrospun nanofiber mat as a pH biosensor and monitor the pH associated quality in fresh date fruit (Rutab). Polym. Test. 2019, 75, 76–84. [Google Scholar] [CrossRef]
- Zhang, M.; Ahmed, A.; Xu, L. Electrospun Nanofibers for Functional Food Packaging Application. Materials 2023, 16, 5937. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Development of polyvinyl alcohol/chitosan bio-nanocomposite films reinforced with cellulose nanocrystals isolated from rice straw. Appl. Surf. Sci. 2018, 449, 591–602. [Google Scholar] [CrossRef]
- Porras-Saavedra, J.; Ricaurte, L.; Pérez-Pérez, N.C.; Quintanilla-Carvajal, M.X. Development and characterization of Sechium edule starch and polyvinyl alcohol nanofibers obtained by electrospinning. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129456. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Zhang, C.; Chen, K.; Feng, F.; Zhang, H. Comparison of ethyl cellulose–gelatin composite films fabricated by electrospinning versus solvent casting. J. Appl. Polym. Sci. 2018, 135, 46824. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Zhang, C.; Feng, F.; Zhang, H. Tunable Physical Properties of Ethylcellulose/Gelatin Composite Nanofibers by Electrospinning. J. Agric. Food Chem. 2018, 66, 1907–1915. [Google Scholar] [CrossRef]
- Chen, S.; Wu, M.; Lu, P.; Gao, L.; Yan, S.; Wang, S. Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil. Int. J. Biol. Macromol. 2020, 149, 271–280. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.M.; Sánchez, M.Á.A.; Labidi, J. Halochromic and antioxidant capacity of smart films of chitosan/chitin nanocrystals with curcuma oil and anthocyanins. Food Hydrocoll. 2022, 123, 107119. [Google Scholar] [CrossRef]
- Lin, X.; Li, N.; Xiao, Q.; Guo, Y.; Wei, J.; Jiao, T.; Chen, Q.; Chen, Q.; Chen, X. Polyvinyl alcohol/starch-based film incorporated with grape skin anthocyanins and metal-organic framework crystals for colorimetric monitoring of pork freshness. Food Chem. 2022, 395, 133613. [Google Scholar] [CrossRef]
- Ma, L.; Xiong, Y.L. Textural attributes and oxidative stability of pork longissimus muscle injected with marbling-like emulsified lipids. Meat Sci. 2011, 89, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, H.; Qi, D.; Xia, L.; Li, L.; Li, X.; Jiang, S. Multifunctional colorimetric cellulose acetate membrane incorporated with Perilla frutescens (L.) Britt. anthocyanins and chamomile essential oil. Carbohydr. Polym. 2022, 278, 118914. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.; Sun, Z.; Liu, F.; Wang, D. A fast-response visual indicator film based on polyvinyl alcohol/methylcellulose/black wolfberry anthocyanin for monitoring chicken and shrimp freshness. Food Packag. Shelf Life 2022, 34, 100939. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Tang, H.; Hao, W.; Wu, J.; Sun, Y.; Sun, J.; Liu, Y.; Jiang, L. Development, characterization and application of intelligent/active packaging of chitosan/chitin nanofibers films containing eggplant anthocyanins. Food Hydrocoll. 2023, 139, 108496. [Google Scholar] [CrossRef]
- Duan, M.; Yu, S.; Sun, J.; Jiang, H.; Zhao, J.; Tong, C.; Hu, Y.; Pang, J.; Wu, C. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int. J. Biol. Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef]
- Shan, P.; Wang, K.; Yu, F.; Yi, L.; Sun, L.; Li, H. Gelatin/sodium alginate multilayer composite film crosslinked with green tea extract for active food packaging application. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131013. [Google Scholar] [CrossRef]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Li, W.; Yang, L.; Han, L.; Yu, Q.-l. Active-intelligent film based on pectin from watermelon peel containing beetroot extract to monitor the freshness of packaged chilled beef. Food Hydrocoll. 2021, 119, 106751. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Prietto, L.; Pinto, V.Z.; El Halal, S.L.M.; de Morais, M.G.; Costa, J.A.V.; Lim, L.-T.; Dias, A.R.G.; Zavareze, E.d.R. Ultrafine fibers of zein and anthocyanins as natural pH indicator. J. Sci. Food Agric. 2018, 98, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.-W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, F.; Xie, X.; Xie, C.; Li, A.; Xia, N.; Gong, X.; Zhang, H. Development and characterization of chitosan/guar gum active packaging containing walnut green husk extract and its application on fresh-cut apple preservation. Int. J. Biol. Macromol. 2022, 209, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jiang, H.; Zhao, J.; Humayun, M.; Wu, S.; Wang, C.; Zhi, Z.; Pang, J. A novel strategy to formulate edible active-intelligent packaging films for achieving dynamic visualization of product freshness. Food Hydrocoll. 2022, 133, 107998. [Google Scholar] [CrossRef]
- Hu, D.; Liu, X.; Qin, Y.; Yan, J.; Yang, Q. A novel intelligent film with high stability based on chitosan/sodium alginate and coffee peel anthocyanin for monitoring minced beef freshness. Int. J. Food Sci. Technol. 2022, 57, 4673–4686. [Google Scholar] [CrossRef]
- Guo, Z.; Zuo, H.; Ling, H.; Yu, Q.; Gou, Q.; Yang, L. A novel colorimetric indicator film based on watermelon peel pectin and anthocyanins from purple cabbage for monitoring mutton freshness. Food Chem. 2022, 383, 131915. [Google Scholar] [CrossRef]
- Gallego, M.; Arnal, M.; Talens, P.; Toldrá, F.; Mora, L. Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat. Polymers 2020, 12, 1032. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, J.; Guo, X.; Sun, M.; Li, H.; Bai, H.; Cui, H.; Shi, L. pH-responsive chitosan-based film containing oregano essential oil and black rice bran anthocyanin for preserving pork and monitoring freshness. Food Chem. 2023, 403, 134393. [Google Scholar] [CrossRef]
- Eskandarabadi, S.M.; Mahmoudian, M.; Farah, K.R.; Abdali, A.; Nozad, E.; Enayati, M. Active intelligent packaging film based on ethylene vinyl acetate nanocomposite containing extracted anthocyanin, rosemary extract and ZnO/Fe-MMT nanoparticles. Food Packag. Shelf Life 2019, 22, 100389. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, P.; Quan, S.; Zhang, H.; Wang, K.; Liu, J. Preparation, characterization and application of smart packaging films based on locust bean gum/polyvinyl alcohol blend and betacyanins from cockscomb (Celosia cristata L.) flower. Int. J. Biol. Macromol. 2021, 191, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Zhou, W.; Tu, Z.; Yang, S.; Xia, T.; Chen, Z.; Du, Y. Intelligent films based on highland barley β-glucan/highland barley prolamin incorporated with black rice bran anthocyanins. Food Packag. Shelf Life 2023, 39, 101146. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. pH-responsive color indicator films based on methylcellulose/chitosan nanofiber and barberry anthocyanins for real-time monitoring of meat freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, X.; Zou, X.; Shi, J.; Zhai, X.; Liu, L.; Li, Z.; Holmes, M.; Gong, Y.; Povey, M.; et al. A visual indicator based on curcumin with high stability for monitoring the freshness of freshwater shrimp, Macrobrachium rosenbergii. J. Food Eng. 2021, 292, 110290. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, W.; Xia, M.; Zeng, Q.; Cai, Z. Intelligent colorimetric film incorporated with anthocyanins-loaded ovalbumin-propylene glycol alginate nanocomplexes as a stable pH indicator of monitoring pork freshness. Food Chem. 2022, 368, 130825. [Google Scholar] [CrossRef]

| Time (min) | Sample | L* | a* | b* | ΔE | |
|---|---|---|---|---|---|---|
| Nanofiber films | 0 |  | 84.44 ± 0.68 a | 2.99 ± 0.28 a | 5.37 ± 0.28 d | - |
| 1 |  | 85.29 ± 1.38 a | −10.82 ± 1.00 b | 12.52 ± 1.28 c | 15.58 ± 1.54 c | |
| 5 |  | 83.93 ± 1.66 a | −14.56 ± 1.40 c | 23.79 ± 1.22 b | 25.45 ± 1.84 b | |
| 10 |  | 85.77 ± 1.94 a | −11.67 ± 0.06 b | 29.76 ± 1.19 a | 28.49 ± 1.00 a | |
| Casting films | 0 |  | 59.17 ± 1.76 b | 12.71 ± 0.70 a | 1.02 ± 0.73 d | - |
| 1 |  | 62.17 ± 0.49 a | 7.52 ± 0.52 b | 7.50 ± 0.82 c | 8.84 ± 1.06 c | |
| 5 |  | 62.38 ± 1.39 a | 1.98 ± 0.26 c | 16.00 ± 0.16 b | 18.72 ± 0.29 b | |
| 10 |  | 62.75 ± 0.88 a | −2.01 ± 0.51 d | 23.15 ± 1.33 a | 26.83 ± 0.67 a |
| Time | TBARS | L* | a* | b* | ∆E | Color |
|---|---|---|---|---|---|---|
| 0 | 0.22 ± 0.012 a | 80.11 ± 1.50 bc | 7.63 ± 0.95 d | 6.65 ± 0.31 a | — |  |
| 2 | 0.27 ± 0.003 b | 80.32 ± 0.53 bc | 3.45 ± 1.13 c | 6.85 ± 0.75 ab | 4.26 ± 1.12 e |  |
| 4 | 0.34 ± 0.011 c | 82.47 ± 0.42 c | 0.95 ± 0.28 b | 8.07 ± 0.78 bc | 7.25 ± 0.45 d |  |
| 6 | 0.48 ± 0.028 d | 79.20 ± 1.51 b | −0.84 ± 1.10 a | 8.90 ± 0.37 c | 8.91 ± 1.03 cd |  |
| 8 | 0.57 ± 0.036 e | 75.63 ± 2.01 a | −0.31 ± 0.73 ab | 12.94± 0.99 d | 11.24 ± 0.13 b |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, P.; Wu, J.; Wu, J.; Wang, H.; Wu, H. A Colorimetric Nanofiber Film Based on Ethyl Cellulose/Gelatin/Purple Sweet Potato Anthocyanins for Monitoring Pork Freshness. Foods 2024, 13, 717. https://doi.org/10.3390/foods13050717
Wen P, Wu J, Wu J, Wang H, Wu H. A Colorimetric Nanofiber Film Based on Ethyl Cellulose/Gelatin/Purple Sweet Potato Anthocyanins for Monitoring Pork Freshness. Foods. 2024; 13(5):717. https://doi.org/10.3390/foods13050717
Chicago/Turabian StyleWen, Peng, Jinling Wu, Jiahui Wu, Hong Wang, and Hong Wu. 2024. "A Colorimetric Nanofiber Film Based on Ethyl Cellulose/Gelatin/Purple Sweet Potato Anthocyanins for Monitoring Pork Freshness" Foods 13, no. 5: 717. https://doi.org/10.3390/foods13050717
APA StyleWen, P., Wu, J., Wu, J., Wang, H., & Wu, H. (2024). A Colorimetric Nanofiber Film Based on Ethyl Cellulose/Gelatin/Purple Sweet Potato Anthocyanins for Monitoring Pork Freshness. Foods, 13(5), 717. https://doi.org/10.3390/foods13050717








