Assessment of Technological and Sensory Properties, Digestibility, and Bioactive Compounds in Polentas from Different Maize Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Material
2.2. Grain Physical Properties
2.3. Maize Milling
2.4. Physico-Chemical Semolina Traits
2.5. Polenta Technological Quality
2.6. Polenta In Vitro Digestion
2.7. Polyphenols Extraction and Quantification
2.8. Antioxidant Capacity
2.9. Protein Digestion
2.10. Polenta Sensory Quality
2.11. Statistical Analysis
3. Results and Discussion
3.1. Grain and Semolina for Polenta Physical-Chemical Traits
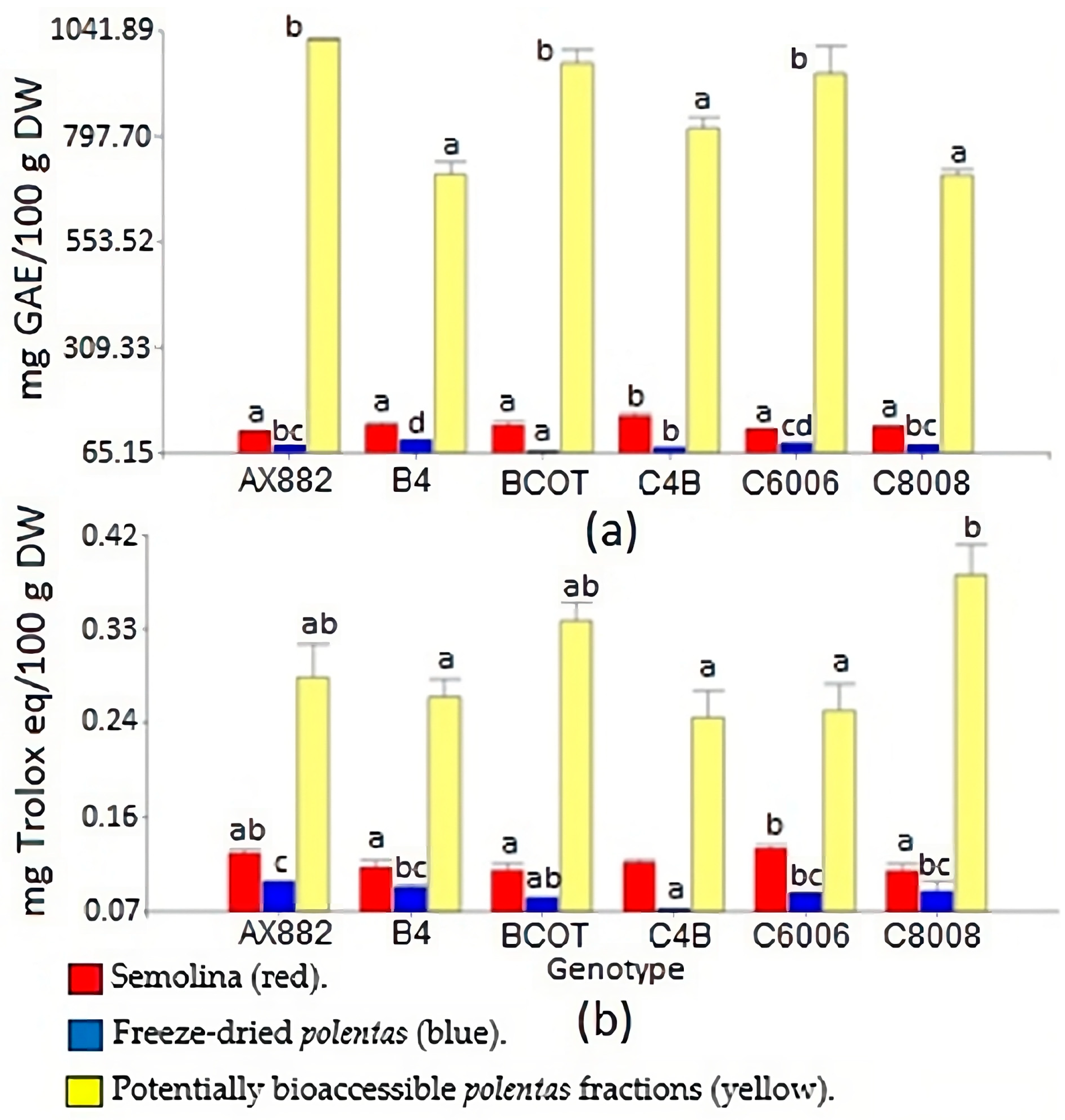
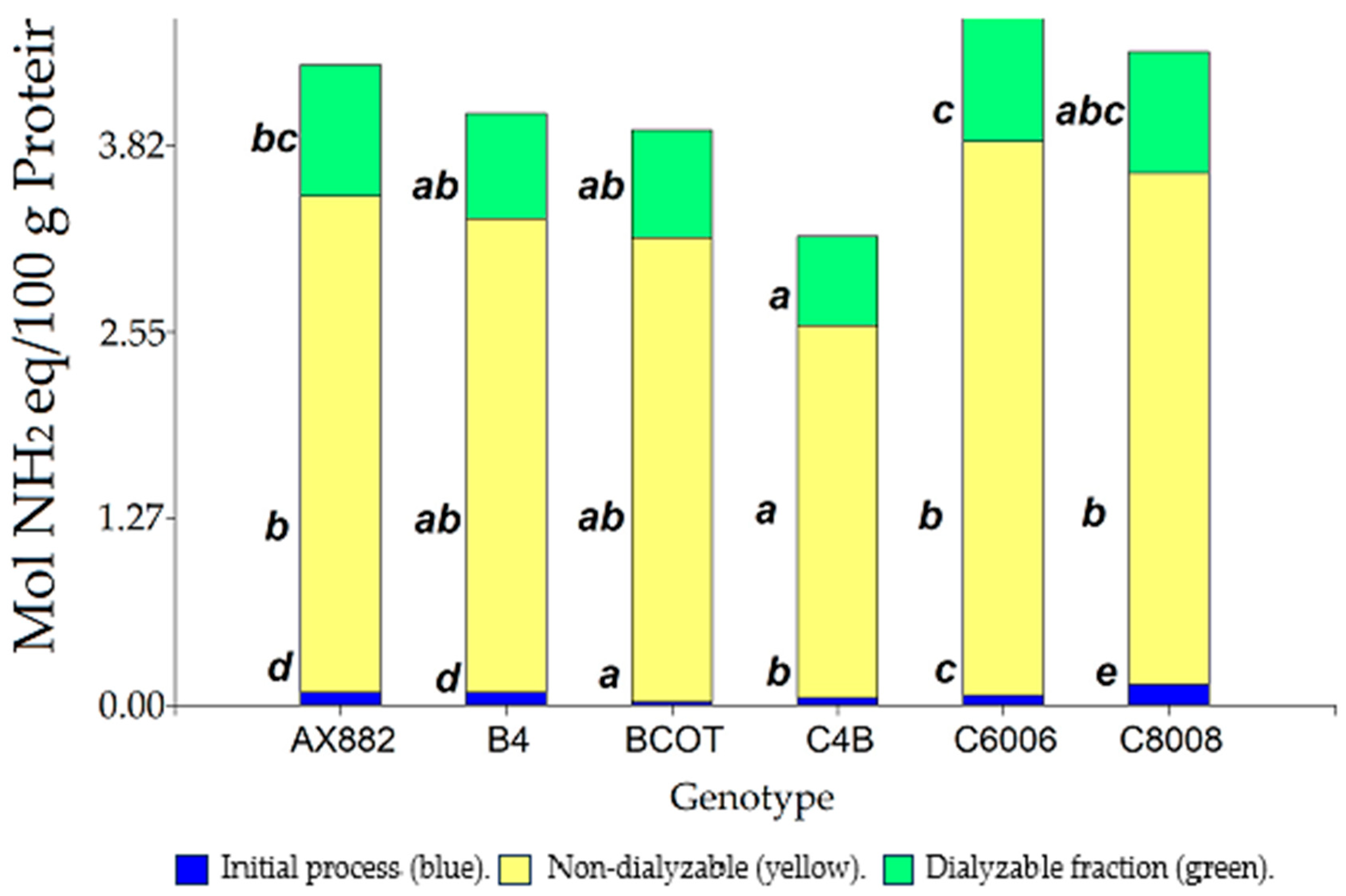
3.2. Static In Vitro Digestion
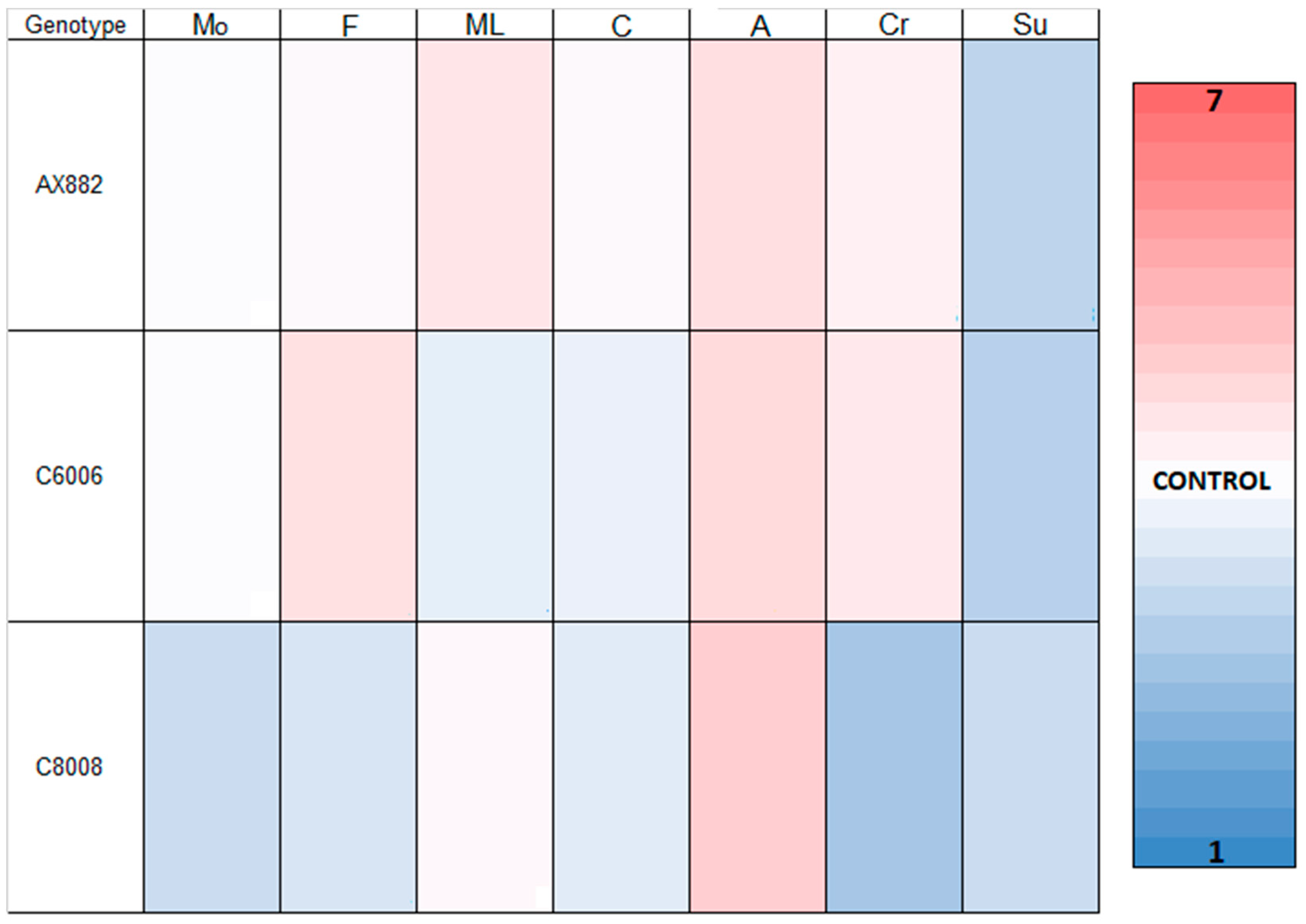
3.3. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, L.; Li, W.; Sun, J.; Qiu, Y.; Wei, X.; Luan, G.; Hu, Y.; Tatsumi, E. Grinding of maize: The effects of fine grinding on compositional, functional and physicochemical properties of maize flour. J. Cereal Sci. 2016, 68, 25–30. [Google Scholar] [CrossRef]
- Miele, N.A.; Di Monaco, R.; Formisano, D.; Masi, P.; Cavella, S. Polenta-based snack development: From maize flour to final product by assessing structural, mechanical and sensory properties. J. Food Sci. Technol. 2018, 55, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Lago, C.; Cassani, E.; Zanzi, C.; Landoni, M.; Trovato, R.; Pilu, R. Development and study of a maize cultivar rich in anthocyanins: Coloured polenta, a new functional food. Plant Breed. 2014, 133, 210–217. [Google Scholar] [CrossRef]
- Stagnati, L.; Martino, M.; Soffritti, G.; Lanubile, A.; Ravasio, A.; Marocco, A.; Rossi, G.; Busconi, M. Microsatellite and morphological characterization of three Rostrato di Val Chiavenna (Sondrio, Italy) maize (Zea mays L.) accessions. Genet. Resour. Crop Evol. 2021, 68, 3025–3038. [Google Scholar] [CrossRef]
- Bongianino, N.F.; Steffolani, M.E.; Morales, C.D.; Biasutti, C.A.; León, A.E. Technological and Sensory Quality of Gluten-Free Pasta Made from Flint Maize Cultivars. Foods 2023, 12, 2780. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, G.; Bertolino, M.; Rolle, L. Quantitative descriptive analysis of Italian polenta produced with different corn cultivars. J. Sci. Food Agric. 2012, 92, 412–417. [Google Scholar] [CrossRef]
- Almeida-Dominguez, H.; Suhendro, E.; Rooney, L. Factors Affecting Rapid Visco Analyser Curves for the Determination of Maize Kernel Hardness. J. Cereal Sci. 1997, 25, 93–102. [Google Scholar] [CrossRef]
- Sharifi, S.; Majzoobi, M.; Farahnaky, A. Effects of particle size and moisture content of maize grits on physical properties of expanded snacks. J. Texture Stud. 2021, 52, 110–123. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Zhang, B.; Fu, X.; Li, L.; Huang, Q. Structure, physicochemical and in vitro digestion properties of ternary blends containing swollen maize starch, maize oil and zein protein. Food Hydrocoll. 2018, 76, 88–95. [Google Scholar] [CrossRef]
- Bustos, M.C.; Vignola, M.B.; Pérez, G.T.; León, A.E. In vitro digestion kinetics and bioaccessibility of starch in cereal food products. J. Cereal Sci. 2017, 77, 243–250. [Google Scholar] [CrossRef]
- Caballero-Rothar, N.N.; Borrás, L.; Gerde, J.A. Physical and chemical kernel traits affect starch digestibility and glycemic index of cooked maize flours. Food Chem. 2022, 369, 130953. [Google Scholar] [CrossRef]
- Elisa, D.-H.; Marcela, G.-M.; Alejandra, G.-U.J.; Elena, D.-H.M. The nutraceutical value of maize (Zea mays L.) landraces and the determinants of its variability: A review. J. Cereal Sci. 2022, 103, 103399. [Google Scholar] [CrossRef]
- Wang, M.; Mao, H.; Ke, Z.; Huang, R.; Chen, J.; Qi, L.; Wang, J. Effect of proanthocyanidins from different sources on the digestibility, physicochemical properties and structure of gelatinized maize starch. Int. J. Biol. Macromol. 2023, 248, 125935. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Félix-Medina, J.V.; Sepúlveda-Haro, A.G.; Quintero-Soto, M.F. Stability of antioxidant and hypoglycemic activities of peptide fractions of Maize (Zea mays L.) under different processes. J. Food Meas. Charact. 2023, 17, 362–370. [Google Scholar] [CrossRef]
- Williams, P.J.; Kucheryavskiy, S. Classification of maize kernels using NIR hyperspectral imaging. Food Chem. 2016, 209, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, M.R.; Gely, M.C.; Pagano, A.M. Estudio de Las Propiedades Físicas y de La Cinética de Secado de Granos de Maíz Colorado Duro. ACI 2012, 3, 153–171. [Google Scholar]
- Palacios Rojas, N. Calidad Nutricional e Industrial de Maíz: Laboratorio de Calidad Nutricional de Maíz “Evangelina Villegas”: Protocolos; International Maize and Wheat Improvement Center (CIMMYT): Mexico City, México, 2018. [Google Scholar]
- Bongianino, N.F.; Steffolani, M.E.; Biasutti, C.A.; León, A.E. Suitability of Argentinian Maize Hybrids for Polenta Production. Int. J. Food Sci. Technol. 2022, 57, 4859–4867. [Google Scholar] [CrossRef]
- Ribotta, P.D.; Pérez, G.T.; Añón, M.C.; León, A.E. Optimization of Additive Combination for Improved Soy–Wheat Bread Quality. Food Bioprocess Technol. 2010, 3, 395–405. [Google Scholar] [CrossRef]
- Mansilla, P.S.; Nazar, M.C.; Pérez, G.T. Flour functional properties of purple maize (Zea mays L.) from Argentina. Influence of environmental growing conditions. Int. J. Biol. Macromol. 2020, 146, 311–319. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Duarte, A.P.; Mason, S.C.; Jackson, D.S.; Kiehl, J.d.C. Grain Quality of Brazilian Maize Genotypes as Influenced by Nitrogen Level. Crop Sci. 2005, 45, 1958–1964. [Google Scholar] [CrossRef]
- Mayer, L.I.; Cirilo, A.G.; Maddonni, G.A. Kernel Hardness-Related Traits in Response to Heat Stress during the Grain-Filling Period of Maize Crops. Crop Sci. 2019, 59, 318–332. [Google Scholar] [CrossRef]
- Caballero-Rothar, N.N.; Abdala, L.J.; Borrás, L.; Gerde, J.A. Role of yield genetic progress on the biochemical determinants of maize kernel hardness. J. Cereal Sci. 2019, 87, 301–310. [Google Scholar] [CrossRef]
- Tovar, J.; Sáyago-Ayerdi, S.G.; Peñalver, C.; Paredes-López, O.; Bello-Pérez, L.A. In Vitro Starch Hydrolysis Index and Predicted Glycemic Index of Corn Tortilla, Black Beans (Phaseolus vulgaris L.), and Mexican “taco”. Cereal Chem. 2003, 80, 533–535. [Google Scholar] [CrossRef]
- Rocchetti, G.; Giuberti, G.; Gallo, A.; Bernardi, J.; Marocco, A.; Lucini, L. Effect of dietary polyphenols on the in vitro starch digestibility of pigmented maize varieties under cooking conditions. Food Res. Int. 2018, 108, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L.A. Functional Study of Raw and Cooked Blue Maize Flour: Starch Digestibility, Total Phenolic Content and Antioxidant Activity. J. Cereal Sci. 2017, 76, 179–185. [Google Scholar] [CrossRef]
- Chiremba, C.; Taylor, J.R.; Rooney, L.W.; Beta, T. Phenolic acid content of sorghum and maize cultivars varying in hardness. Food Chem. 2012, 134, 81–88. [Google Scholar] [CrossRef]
- Trehan, S.; Singh, N.; Kaur, A. Diversity and relationship among grain, flour and starch characteristics of Indian Himalayan colored corn accessions. J. Food Sci. Technol. 2020, 57, 3801–3813. [Google Scholar] [CrossRef]
- Peralta-Veran, L.; Espinosa-Leal, C.; Escalante-Aburto, A.; Preciado-Ortiz, R.E.; Puente-Garza, C.A.; Serna-Saldivar, S.O.; García-Lara, S. Effects of pozole broth production on phenolic acids and antioxidant activity of specialty maize landraces. J. Cereal Sci. 2022, 107, 103543. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Menchaca-Armenta, M.; Frutos, M.J.; Ramírez-Wong, B.; Valero-Cases, E.; Muelas-Domingo, R.; Quintero-Ramos, A.; Torres-Chávez, P.I.; Carbonell-Barrachina, Á.A.; Ledesma-Osuna, A.I.; Campas-Baypoli, O.N. Changes in phytochemical content, bioaccesibility and antioxidant capacity of corn tortillas during simulated in vitro gastrointestinal digestion. Food Chem. 2023, 405, 134223. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, V.; Salinas-Moreno, Y.; Ramírez-Díaz, J.L.; Vázquez-Carrillo, G.; Domínguez-López, A.; Ramírez-Romero, A.G. Color, phenolic composition and antioxidant activity of blue tortillas from Mexican maize races. CyTA-J. Food 2016, 14, 473–481. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.-H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT-Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Hwang, T.; Ndolo, V.U.; Katundu, M.; Nyirenda, B.; Bezner-Kerr, R.; Arntfield, S.; Beta, T. Provitamin A Potential of Landrace Orange Maize Variety (Zea mays L.) Grown in Different Geographical Locations of Central Malawi. Food Chem. 2016, 196, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Saenz, E.; Borrás, L.; Gerde, J.A. Carotenoid profiles in maize genotypes with contrasting kernel hardness. J. Cereal Sci. 2021, 99, 103206. [Google Scholar] [CrossRef]
- Muleya, M.; Li, D.; Chiutsi-Phiri, G.; Botoman, L.; Brameld, J.M.; Salter, A.M. In vitro determination of the protein quality of maize varieties cultivated in Malawi using the INFOGEST digestion method. Heliyon 2023, 9, e19797. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Alvarez-Ramirez, J.; Rodríguez-Huezo, E.; Meraz-Rodríguez, M.; Vernon-Carter, E.; Lobato-Calleros, C. Effect of the preparation method and storage time on the in vitro protein digestibility of maize tortillas. J. Cereal Sci. 2018, 84, 7–12. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Angkustsiri, K.; German, J.B. Dietary Considerations in Autism Spectrum Disorders: The Potential Role of Protein Digestion and Microbial Putrefaction in the Gut-Brain Axis. Front. Nutr. 2018, 5, 40. [Google Scholar] [CrossRef]
- Onyango, C.; Noetzold, H.; Bley, T.; Henle, T. Proximate composition and digestibility of fermented and extruded uji from maize–finger millet blend. LWT-Food Sci. Technol. 2004, 37, 827–832. [Google Scholar] [CrossRef]
- Scholten, E. Composite foods: From structure to sensory perception. Food Funct. 2017, 8, 481–497. [Google Scholar] [CrossRef]
- Appelqvist, I.A.; Cochet-Broch, M.; Poelman, A.A.; Day, L. Morphologies, volume fraction and viscosity of cell wall particle dispersions particle related to sensory perception. Food Hydrocoll. 2015, 44, 198–207. [Google Scholar] [CrossRef]
- Abdala, L.J.; Gambin, B.L.; Borrás, L. Sowing date and maize grain quality for dry milling. Eur. J. Agron. 2018, 92, 1–8. [Google Scholar] [CrossRef]
| Genotype | AX882 | B4 | BCOT | C4B | C6006 | C8008 |
|---|---|---|---|---|---|---|
| W100 (g) | 35.27 ± 4.79 bc | 36.89 ± 0.17 bc | 31.53 ± 0.30 b | 23.72 ± 0.39 a | 32.95 ± 1.41 bc | 38.24 ± 3.56 c |
| TW (Kg/HL) | 85.00 ± 2.26 a | 88.90 ± 1.84 bc | 93.40 ± 0.28 de | 95.50 ± 0.14 e | 92.00 ± 0.00 cd | 86.10 ± 0.71 ab |
| FI (%) | 90.50 ± 3.42 e | 47.50 ± 10.12 c | 14.50 ± 4.43 ab | 5.75 ± 4.03 a | 21.50 ± 5.51 b | 64.00 ± 8.00 d |
| D90 (µm) | 1135.95 ± 46.21 a | 1366.08 ± 56.85 b | 1367.08 ± 188.91 b | 1233.81 ± 197.11 ab | 1256.00 ± 97.90 ab | 1256.00 ± 97.90 ab |
| Span | 0.79 ± 0.04 a | 0.84 ± 0.04 ab | 0.90 ± 0.09 ab | 0.93 ± 0.14 b | 0.82 ± 0.06 ab | 0.82 ± 0.06 ab |
| Protein (%) | 7.84 ± 0.32 a | 11.48 ± 0.84 cd | 10.82 ± 0.73 c | 11.67 ± 0.21 d | 10.02 ± 0.35 b | 9.58 ± 0.27 b |
| Ash (%) | 1.12 ± 0.03 bc | 0.97 ± 0.04 a | 1.31 ± 0.06 d | 1.15 ± 0.02 c | 1.14 ± 0.03 c | 1.07 ± 0.04 b |
| Oil (%) | 2.26 ± 0.31 b | 1.66 ± 0.23 a | 2.87 ± 0.51 c | 2.59 ± 0.19 bc | 2.61 ± 0.26 bc | 2.69 ± 0.18 bc |
| Total starch (%) | 81.08 ± 0.28 b | 77.82 ± 1.47 b | 71.45 ± 3.60 a | 79.74 ± 0.21 b | 80.85 ± 2.52 b | 76.73 ± 0.84 ab |
| Peak viscosity (cP) | 5832.67 ± 281.48 c | 3921.50 ± 130.46 a | 3834.50 ± 239.24 a | 3817.17 ± 398.32 a | 3934.50 ± 322.71 a | 4498.50 ± 160.54 b |
| Pt (°C) | 73.38 ± 0.52 a | 83.38 ± 0.84 c | 88.89 ± 0.39 d | 87.78 ± 1.59 d | 87.26 ± 0.28 d | 79.15 ± 6.56 b |
| Cn (gf) | 4301.99 ± 441.02 c | 3502.95 ± 262.77 b | 3033.92 ± 346.81 a | 3044.41 ± 207.56 a | 3426.56 ± 221.47 b | 3452.29 ± 166.44 b |
| L* | 64.04 ± 0.66 a | 64.74 ± 0.39 a | 70.04 ± 0.73 c | 70.37 ± 0.94 c | 66.2 ± 0.36 b | 70.74 ± 0.54 c |
| a* | 4.59 ± 0.55 d | 5.57 ± 0.32 e | 2.05 ± 0.6 b | 2.73 ± 0.41 c | 7.55 ± 0.39 f | −1.5 ± 0.12 a |
| b* | 27.1 ± 1.79 c | 31.94 ± 1.73 e | 22.69 ± 2.85 b | 22.29 ± 1.58 b | 29.69 ± 1.2 d | 6.78 ± 0.51 a |
| Genotype | RDS (%) | SDS (%) | RS (%) | AUC Index |
|---|---|---|---|---|
| AX882 | 70.45 ± 0.49 c | 13.97 ± 2.13 c | 15.58 ± 1.64 b | 108.97 ± 6.29 d |
| B4 | 65.74 ± 1.25 b | 4.45 ± 1.35 ab | 29.81 ± 2.60 c | 86.36 ± 2.23 a |
| BCOT | 79.49 ± 1.43 d | 5.68 ± 4.44 ab | 14.83 ± 3.01 b | 101.27 ± 3.99 bc |
| C4B | 55.69 ± 0.34 a | 29.73 ± 0.94 d | 14.58 ± 0.60 b | 107.18 ± 3.43 bcd |
| C6006 | 70.56 ± 2.22 c | 1.92 ± 0.08 a | 27.52 ± 2.31 c | 108.21 ± 4.63 cd |
| C8008 | 72.09 ± 4.04 c | 18.31 ± 4.40 c | 9.60 ± 0.36 a | 104.45 ± 3.10 bcd |
| Bread | 78.88 ± 0.63 d | 8.58 ± 0.74 b | 12.54 ± 0.11 ab | 100.00 ± 0.20 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongianino, N.F.; Steffolani, M.E.; Rodríguez, M.D.; Bustos, M.C.; Biasutti, C.A.; León, A.E. Assessment of Technological and Sensory Properties, Digestibility, and Bioactive Compounds in Polentas from Different Maize Genotypes. Foods 2024, 13, 590. https://doi.org/10.3390/foods13040590
Bongianino NF, Steffolani ME, Rodríguez MD, Bustos MC, Biasutti CA, León AE. Assessment of Technological and Sensory Properties, Digestibility, and Bioactive Compounds in Polentas from Different Maize Genotypes. Foods. 2024; 13(4):590. https://doi.org/10.3390/foods13040590
Chicago/Turabian StyleBongianino, Nicolás Francisco, María Eugenia Steffolani, Marianela Desiree Rodríguez, Mariela Cecilia Bustos, Carlos Alberto Biasutti, and Alberto Edel León. 2024. "Assessment of Technological and Sensory Properties, Digestibility, and Bioactive Compounds in Polentas from Different Maize Genotypes" Foods 13, no. 4: 590. https://doi.org/10.3390/foods13040590
APA StyleBongianino, N. F., Steffolani, M. E., Rodríguez, M. D., Bustos, M. C., Biasutti, C. A., & León, A. E. (2024). Assessment of Technological and Sensory Properties, Digestibility, and Bioactive Compounds in Polentas from Different Maize Genotypes. Foods, 13(4), 590. https://doi.org/10.3390/foods13040590







