Dietary Acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts
Abstract
1. Introduction
2. Materials and Methods
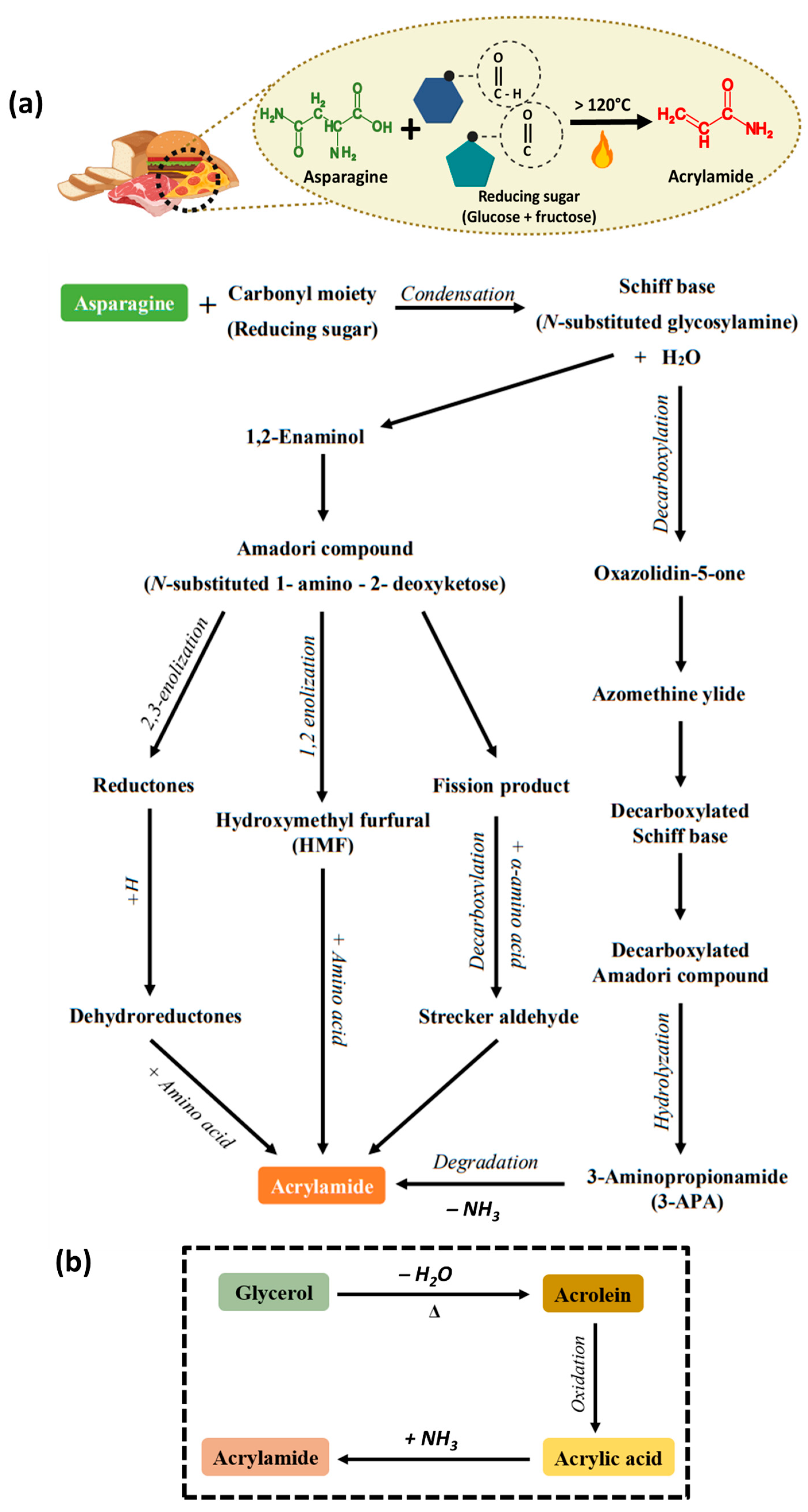
3. Mechanism of Acrylamide Formation in Food
4. Factors Influencing the Formation of AA
4.1. Cooking Temperature and Time
4.2. pH and Surface Area
4.3. Moisture Content
4.4. Food Composition
4.5. Cooking Method
4.6. Use of Fertilizer, Harvesting, and Storage Condition
5. Diseases Related to Acrylamide Exposure
5.1. Genotoxicity and Carcinogenicity
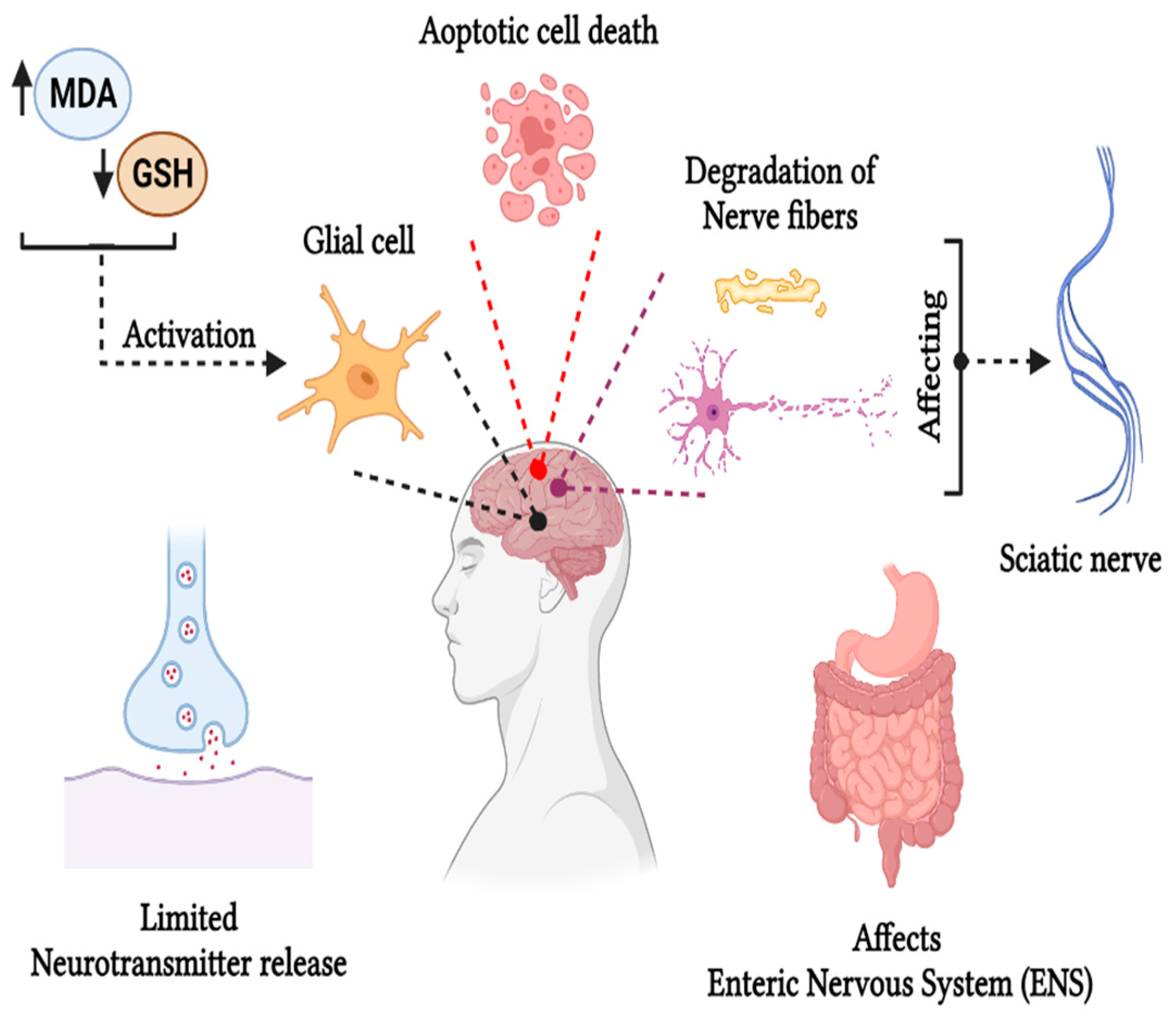
5.2. Neurotoxicity
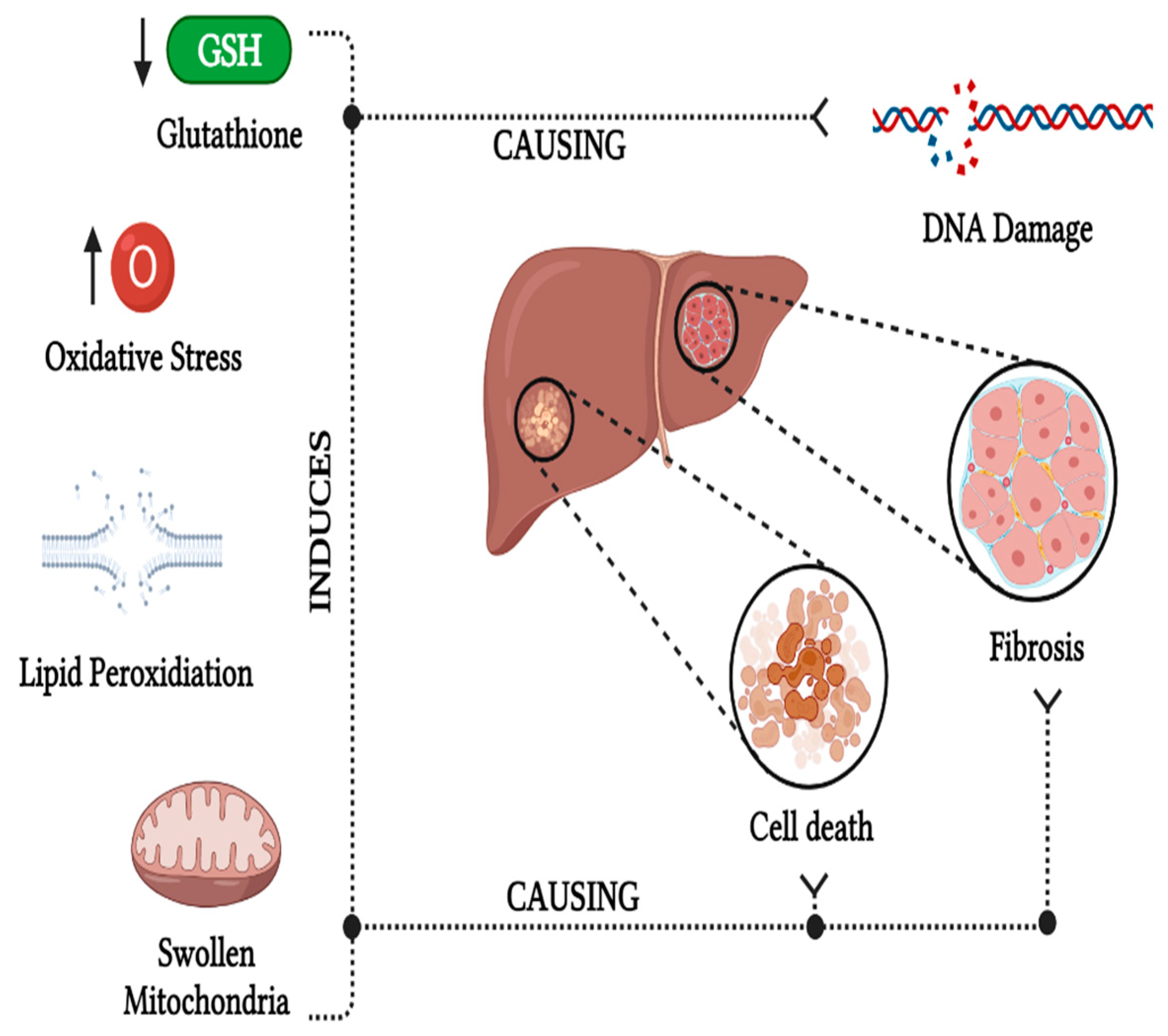
5.3. Hepatoxicity
5.4. Cardiovascular Toxicity
5.5. Reproductive Toxicity and Prenatal and Postnatal Effects of Acrylamide
6. Detection of Acrylamide in Food
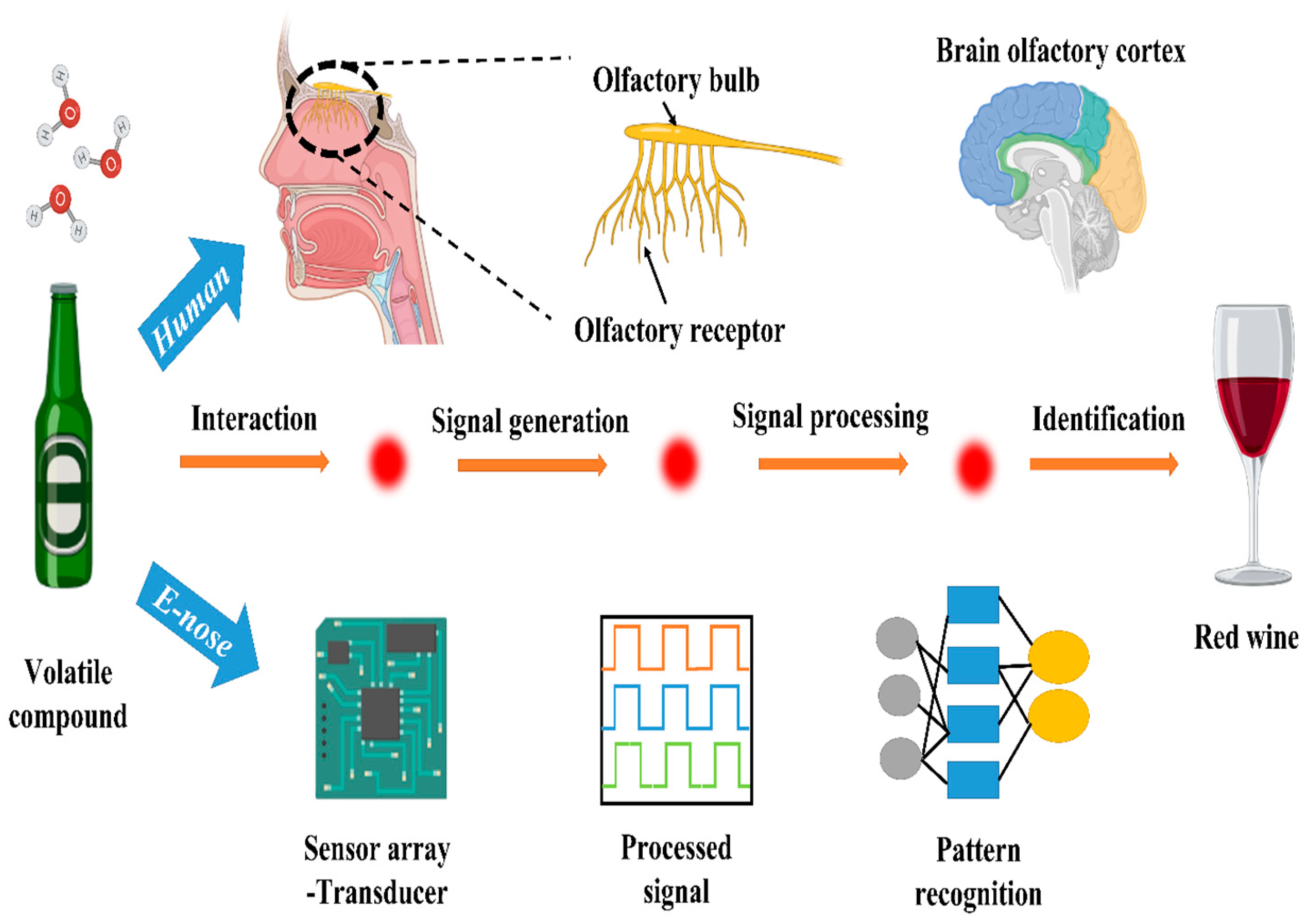
6.1. Electronic Tongue and Nose
6.2. Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
6.3. Gas Chromatography–Mass Spectrometry (GC–MS)
6.4. Hemoglobin Nanoparticles (HbNPs)
6.5. Fluorescence Biosensor
6.6. Surface-Enhanced Raman Spectroscopy (SERS)
7. Mitigation Strategies of Dietary Acrylamide
7.1. Air and Vacuum-Frying
7.2. Blanching
7.3. Addition of Additives
7.4. pH and Water Content
7.5. Fermentation
7.6. Hydrocolloid-Based Coating
7.7. Inhibitory and Inert Baking Atmosphere
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rifai, L.; Saleh, F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef]
- Semla, M.; Goc, Z.; Martiniaková, M.; Omelka, R.; Formicki, G. Acrylamide: A Common Food Toxin Related to Physiological Functions and Health. Physiol. Res. 2017, 66, 205–217. [Google Scholar] [CrossRef]
- Ferrer-Aguirre, A.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Simple and Fast Determination of Acrylamide and Metabolites in Potato Chips and Grilled Asparagus by Liquid Chromatography Coupled to Mass Spectrometry. Food Anal. Methods 2016, 9, 1237–1245. [Google Scholar] [CrossRef]
- Timmermann, C.; Mølck, S.; Kadawathagedara, M.; Bjerregaard, A.; Törnqvist, M.; Brantsæter, A.; Pedersen, M. A Review of Dietary Intake of Acrylamide in Humans. Toxics 2021, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Seung, D. Amylose in Starch: Towards an Understanding of Biosynthesis, Structure and Function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Jayanty, S.S.; Diganta, K.; Raven, B. Effects of Cooking Methods on Nutritional Content in Potato Tubers. Am. J. Potato Res. 2019, 96, 183–194. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Hong, L.; Xie, X.; Wang, S. Review of Research into the Determination of Acrylamide in Foods. Foods 2020, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, B.; Johansson, Y.; Andreassen, M.; Husøy, T.; Dirven, H.; Hofer, T.; Knutsen, H.K.; Caspersen, I.H.; Vejrup, K.; Paulsen, R.E.; et al. Does the Food Processing Contaminant Acrylamide Cause Developmental Neurotoxicity? A Review and Identification of Knowledge Gaps. Reprod. Toxicol. 2021, 101, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.N.; Hewavitharana, G.G.; Navaratne, S.B. Comprehensive Study on the Acrylamide Content of High Thermally Processed Foods. Biomed Res. Int. 2021, 2021, 6258508. [Google Scholar] [CrossRef] [PubMed]
- Mollakhalili-Meybodi, N.; Khorshidian, N.; Nematollahi, A.; Arab, M. Acrylamide in Bread: A Review on Formation, Health Risk Assessment, and Determination by Analytical Techniques. Environ. Sci. Pollut. Res. 2021, 28, 15627–15645. [Google Scholar] [CrossRef] [PubMed]
- Raffan, S.; Oddy, J.; Halford, N.G. The Sulphur Response in Wheat Grain and Its Implications for Acrylamide Formation and Food Safety. Int. J. Mol. Sci. 2020, 21, 3876. [Google Scholar] [CrossRef]
- Xing, H.; Yaylayan, V. Insight into the Mechanochemistry of the Maillard Reaction: Degradation of Schiff Bases via 5-Oxazolidinone Intermediate. Eur. Food Res. Technol. 2021, 247, 1095–1106. [Google Scholar] [CrossRef]
- Aarabi, F.; Seyedain Ardebili, M. The Effect of Sugar Type and Baking Condition on Formation of Acrylamide in Industrial Rotary Moulded Biscuit. J. Food Meas. Charact. 2020, 14, 2230–2239. [Google Scholar] [CrossRef]
- Aalaei, K.; Rayner, M.; Sjöholm, I. Chemical Methods and Techniques to Monitor Early Maillard Reaction in Milk Products; A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1829–1839. [Google Scholar] [CrossRef]
- Maan, A.A.; Anjum, M.A.; Khan, M.K.I.; Nazir, A.; Saeed, F.; Afzaal, M.; Aadil, R.M. Acrylamide Formation and Different Mitigation Strategies during Food Processing—A Review. Food Rev. Int. 2022, 38, 70–87. [Google Scholar] [CrossRef]
- Zhang, J.; Sturla, S.; Lacroix, C.; Schwab, C. Gut Microbial Glycerol Metabolism as an Endogenous Acrolein Source. mBio 2018, 9, e01947-17. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, S.; Li, H.; Xu, L.; Hu, X.; Barati, B.; Zheng, A. Insight into the Mechanism of Glycerol Dehydration and Subsequent Pyridine Synthesis. ACS Sustain. Chem. Eng. 2021, 9, 3095–3103. [Google Scholar] [CrossRef]
- Rayappa, M.K.; Viswanathan, P.A.; Rattu, G.; Krishna, P.M. Nanomaterials Enabled and Bio/Chemical Analytical Sensors for Acrylamide Detection in Thermally Processed Foods: Advances and Outlook. J. Agric. Food Chem. 2021, 69, 4578–4603. [Google Scholar] [CrossRef]
- Udomkun, P.; Swennen, R.; Masso, C.; Innawong, B.; Fotso Kuate, A.; Alakonya, A.; Vanlauwe, B. Influence of Bunch Maturation and Chemical Precursors on Acrylamide Formation in Starchy Banana Chips. Int. J. Food Sci. Technol. 2021, 56, 5417–5431. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Shadnoush, M.; Siadat, S.D.; Mohammadi, M.; Mortazavian, A.M. Using Probiotics for Mitigation of Acrylamide in Food Products: A Mini Review. Curr. Opin. Food Sci. 2020, 32, 67–75. [Google Scholar] [CrossRef]
- Lee, J.-S.; Han, J.-W.; Jung, M.; Lee, K.-W.; Chung, M.-S. Effects of Thawing and Frying Methods on the Formation of Acrylamide and Polycyclic Aromatic Hydrocarbons in Chicken Meat. Foods 2020, 9, 573. [Google Scholar] [CrossRef]
- Michalak, J.; Czarnowska-Kujawska, M.; Klepacka, J.; Gujska, E. Effect of Microwave Heating on the Acrylamide Formation in Foods. Molecules 2020, 25, 4140. [Google Scholar] [CrossRef]
- Žilić, S.; Aktağ, I.G.; Dodig, D.; Filipović, M.; Gökmen, V. Acrylamide Formation in Biscuits Made of Different Wholegrain Flours Depending on Their Free Asparagine Content and Baking Conditions. Food Res. Int. 2020, 132, 109109. [Google Scholar] [CrossRef]
- Suman, M.; Generotti, S.; Cirlini, M.; Dall’Asta, C. Acrylamide Reduction Strategy in Combination with Deoxynivalenol Mitigation in Industrial Biscuits Production. Toxins 2019, 11, 499. [Google Scholar] [CrossRef]
- Shakeri, F.; Shakeri, S.; Ghasemi, S.; Troise, A.D.; Fiore, A. Effects of Formulation and Baking Process on Acrylamide Formation in Kolompeh, a Traditional Cookie in Iran. J. Chem. 2019, 2019, 1425098. [Google Scholar] [CrossRef]
- Sung, W.-C.; Chang, Y.-W.; Chou, Y.-H.; Hsiao, H.-I. The Functional Properties of Chitosan-Glucose-Asparagine Maillard Reaction Products and Mitigation of Acrylamide Formation by Chitosans. Food Chem. 2018, 243, 141–144. [Google Scholar] [CrossRef]
- Ofosu, I.W.; Ankar-Brewoo, G.M.; Lutterodt, H.E.; Benefo, E.O.; Menyah, C.A. Estimated Daily Intake and Risk of Prevailing Acrylamide Content of Alkalized Roasted Cocoa Beans. Sci. Afr. 2019, 6, e00176. [Google Scholar] [CrossRef]
- Aiswarya, R.; Baskar, G. Enzymatic Mitigation of Acrylamide in Fried Potato Chips Using Asparaginase from Aspergillus Terreus. Int. J. Food Sci. Technol. 2018, 53, 491–498. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Naviglio, D.; Giosafatto, C.; Mariniello, L. Hydrocolloid-Based Coatings Are Effective at Reducing Acrylamide and Oil Content of French Fries. Coatings 2018, 8, 147. [Google Scholar] [CrossRef]
- Sansano, M.; Heredia, A.; Peinado, I.; Andrés, A. Dietary Acrylamide: What Happens during Digestion. Food Chem. 2017, 237, 58–64. [Google Scholar] [CrossRef]
- Seilani, F.; Shariatifar, N.; Nazmara, S.; Khaniki, G.J.; Sadighara, P.; Arabameri, M. The Analysis and Probabilistic Health Risk Assessment of Acrylamide Level in Commercial Nuggets Samples Marketed in Iran: Effect of Two Different Cooking Methods. J. Environ. Health Sci. Eng. 2021, 19, 465–473. [Google Scholar] [CrossRef]
- Sun, N.; Rosen, C.J.; Thompson, A.L. Acrylamide Formation in Processed Potatoes as Affected by Cultivar, Nitrogen Fertilization and Storage Time. Am. J. Potato Res. 2018, 95, 473–486. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.; Gupta, S.K.; Rosen, C.J. Potato Tuber Chemical Properties in Storage as Affected by Cultivar and Nitrogen Rate: Implications for Acrylamide Formation. Foods 2020, 9, 352. [Google Scholar] [CrossRef]
- Eisenbrand, G. Revisiting the Evidence for Genotoxicity of Acrylamide (AA), Key to Risk Assessment of Dietary AA Exposure. Arch. Toxicol. 2020, 94, 2939–2950. [Google Scholar] [CrossRef]
- Benford, D.; Bignami, M.; Chipman, J.K.; Ramos Bordajandi, L. Assessment of the Genotoxicity of Acrylamide. EFSA J. 2022, 20, e07293. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Acrylamide. Available online: https://www.efsa.europa.eu/en/topics/topic/acrylamide#:~:text=EFSA’s%20experts%20have%20considered%20possible,current%20levels%20of%20dietary%20exposure (accessed on 8 February 2024).
- Hansen, S.H.; Pawlowicz, A.J.; Kronberg, L.; Gützkow, K.B.; Olsen, A.-K.; Brunborg, G. Using the Comet Assay and Lysis Conditions to Characterize DNA Lesions from the Acrylamide Metabolite Glycidamide. Mutagenesis 2018, 33, 31–39. [Google Scholar] [CrossRef]
- Hölzl-Armstrong, L.; Kucab, J.E.; Moody, S.; Zwart, E.P.; Loutkotová, L.; Duffy, V.; Luijten, M.; Gamboa da Costa, G.; Stratton, M.R.; Phillips, D.H.; et al. Mutagenicity of Acrylamide and Glycidamide in Human TP53 Knock-in (Hupki) Mouse Embryo Fibroblasts. Arch. Toxicol. 2020, 94, 4173–4196. [Google Scholar] [CrossRef]
- Algarni, A.A. Genotoxic Effects of Acrylamide in Mouse Bone Marrow Cells. Caryologia 2018, 71, 160–165. [Google Scholar] [CrossRef]
- Sarion, C.; Codină, G.G.; Dabija, A. Acrylamide in Bakery Products: A Review on Health Risks, Legal Regulations and Strategies to Reduce Its Formation. Int. J. Environ. Res. Public Health 2021, 18, 4332. [Google Scholar] [CrossRef]
- Nowak, A.; Zakłos-Szyda, M.; Żyżelewicz, D.; Koszucka, A.; Motyl, I. Acrylamide Decreases Cell Viability, and Provides Oxidative Stress, DNA Damage, and Apoptosis in Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 368. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhang, G.; Jia, W.; Ren, Y.; Wu, Y. Biomarker Analysis of Hemoglobin Adducts of Acrylamide and Glycidamide Enantiomers for Mid-Term Internal Exposure Assessment by Isotope Dilution Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Talanta 2018, 178, 825–833. [Google Scholar] [CrossRef]
- Birkett, N.; Al-Zoughool, M.; Bird, M.; Baan, R.A.; Zielinski, J.; Krewski, D. Overview of Biological Mechanisms of Human Carcinogens. J. Toxicol. Environ. Health Part B 2019, 22, 288–359. [Google Scholar] [CrossRef]
- Hogervorst, J.G.F.; van den Brandt, P.A.; Godschalk, R.W.L.; van Schooten, F.-J.; Schouten, L.J. Interaction between Dietary Acrylamide Intake and Genetic Variants for Estrogen Receptor-Positive Breast Cancer Risk. Eur. J. Nutr. 2019, 58, 1033–1045. [Google Scholar] [CrossRef]
- Kotemori, A.; Ishihara, J.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. Dietary Acrylamide Intake and the Risk of Endometrial or Ovarian Cancers in Japanese Women. Cancer Sci. 2018, 109, 3316–3325. [Google Scholar] [CrossRef]
- Acaroz, U.; Ince, S.; Arslan-Acaroz, D.; Gurler, Z.; Kucukkurt, I.; Demirel, H.H.; Arslan, H.O.; Varol, N.; Zhu, K. The Ameliorative Effects of Boron against Acrylamide-Induced Oxidative Stress, Inflammatory Response, and Metabolic Changes in Rats. Food Chem. Toxicol. 2018, 118, 745–752. [Google Scholar] [CrossRef]
- Ersoy, A.; Yasar, H.; Tanoglu, C.; Yazici, G.N.; Coban, T.A.; Arslan, Y.K.; Suleyman, H. The Effect of Taxifolin on Acrylamide-Induced Oxidative and Proinflammatory Brain Injury in Rats: A Biochemical and Histopathological Study. Indian J. Pharm. Educ. Res. 2021, 55, S765–S773. [Google Scholar] [CrossRef]
- Harahap, Y.; Elysia, C.; Starlin, Z.; Jayusman, A.M. Analysis of Acrylamide in Dried Blood Spots of Lung Cancer Patients by Ultrahigh-Performance Liquid Chromatography Tandem Mass Spectrometry. Int. J. Anal. Chem. 2020, 2020, 2015264. [Google Scholar] [CrossRef]
- de Conti, A.; Tryndyak, V.; VonTungeln, L.S.; Churchwell, M.I.; Beland, F.A.; Antunes, A.M.M.; Pogribny, I.P. Genotoxic and Epigenotoxic Alterations in the Lung and Liver of Mice Induced by Acrylamide: A 28 Day Drinking Water Study. Chem. Res. Toxicol. 2019, 32, 869–877. [Google Scholar] [CrossRef]
- Kumar, J.; Das, S.; Teoh, S.L. Dietary Acrylamide and the Risks of Developing Cancer: Facts to Ponder. Front. Nutr. 2018, 5, 323710. [Google Scholar] [CrossRef]
- Perloy, A.; Schouten, L.J.; van den Brandt, P.A.; Godschalk, R.; van Schooten, F.-J.; Hogervorst, J.G.F. The Role of Genetic Variants in the Association between Dietary Acrylamide and Advanced Prostate Cancer in the Netherlands Cohort Study on Diet and Cancer. Nutr. Cancer 2018, 70, 620–631. [Google Scholar] [CrossRef]
- Farag, O.M.; Abd-Elsalam, R.M.; Ogaly, H.A.; Ali, S.E.; El Badawy, S.A.; Alsherbiny, M.A.; Li, C.G.; Ahmed, K.A. Metabolomic Profiling and Neuroprotective Effects of Purslane Seeds Extract Against Acrylamide Toxicity in Rat’s Brain. Neurochem. Res. 2021, 46, 819–842. [Google Scholar] [CrossRef]
- Elblehi, S.S.; El Euony, O.I.; El-Sayed, Y.S. Apoptosis and Astrogliosis Perturbations and Expression of Regulatory Inflammatory Factors and Neurotransmitters in Acrylamide-Induced Neurotoxicity under Ω3 Fatty Acids Protection in Rats. Neurotoxicology 2020, 76, 44–57. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, L.; Lu, X.; Fan, L.; Zhu, Y.; Zhao, L. The Involvement of Oxidative Stress, Neuronal Lesions, Neurotransmission Impairment, and Neuroinflammation in Acrylamide-Induced Neurotoxicity in C57/BL6 Mice. Environ. Sci. Pollut. Res. 2022, 29, 41151–41167. [Google Scholar] [CrossRef]
- Elsawy, H.; Alzahrani, A.M.; Alfwuaires, M.; Sedky, A.; El-Trass, E.E.; Mahmoud, O.; Abdel-Moneim, A.M.; Khalil, M. Analysis of Silymarin-Modulating Effects against Acrylamide-Induced Cerebellar Damage in Male Rats: Biochemical and Pathological Markers. J. Chem. Neuroanat. 2021, 115, 101964. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Abdel-Fattah, A.-F.M.; Saied, E.M.; El-Seedi, H.R.; Abdel-Daim, M.M. Acrylamide-Induced Peripheral Neuropathy: Manifestations, Mechanisms, and Potential Treatment Modalities. Environ. Sci. Pollut. Res. 2021, 28, 13031–13046. [Google Scholar] [CrossRef]
- Palus, K.; Całka, J. Influence of Acrylamide Administration on the Neurochemical Characteristics of Enteric Nervous System (ENS) Neurons in the Porcine Duodenum. Int. J. Mol. Sci. 2019, 21, 15. [Google Scholar] [CrossRef]
- Yan, D.; Yao, J.; Liu, Y.; Zhang, X.; Wang, Y.; Chen, X.; Liu, L.; Shi, N.; Yan, H. Tau Hyperphosphorylation and P-CREB Reduction Are Involved in Acrylamide-Induced Spatial Memory Impairment: Suppression by Curcumin. Brain Behav. Immun. 2018, 71, 66–80. [Google Scholar] [CrossRef]
- Karimi, M.Y.; Fatemi, I.; Kalantari, H.; Mombeini, M.A.; Mehrzadi, S.; Goudarzi, M. Ellagic Acid Prevents Oxidative Stress, Inflammation, and Histopathological Alterations in Acrylamide-Induced Hepatotoxicity in Wistar Rats. J. Diet. Suppl. 2020, 17, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, F.A.; Arafah, M.; Sharma, B.; Siddiqi, N.J. Effects of Alpha Lipoic Acid on Acrylamide-Induced Hepatotoxicity in Rats. Cell. Mol. Biol. 2017, 63, 1–6. [Google Scholar] [CrossRef]
- Erdemli, M.; Sahin, N.; Turkoz, Y.; Yilmaz, I.; Cinar, K.; Akgoz, M.; Cigremis, Y. The Possible Hepatoprotective Effect of Apricot against Acrylamide Induced Hepatotoxicity in Rats. J. Turgut Ozal Med. Cent. 2017, 24, 1. [Google Scholar] [CrossRef]
- Salman, A.; El-Ghazouly, D.E.-S.; El-Beltagy, M. Role of Ascorbic Acid Versus Silymarin in Amelioration of Hepatotoxicity Induced by Acrylamide in Adult Male Albino Rats: Histological and Immunohistochemical Study. Int. J. Morphol. 2020, 38, 1767–1778. [Google Scholar] [CrossRef]
- Altinoz, E.; Turkoz, Y.; Vardi, N. The Protective Effect of N-Acetylcysteine against Acrylamide Toxicity in Liver and Small and Large Intestine Tissues. Bratisl. Med. J. 2015, 116, 252–258. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Zheng, K.; Xin, Y.; Jia, S.; Zhao, X. Metabonomics Analysis of Liver in Rats Administered with Chronic Low-Dose Acrylamide. Xenobiotica 2020, 50, 894–905. [Google Scholar] [CrossRef]
- Huang, M.; Jiao, J.; Wang, J.; Xia, Z.; Zhang, Y. Characterization of Acrylamide-Induced Oxidative Stress and Cardiovascular Toxicity in Zebrafish Embryos. J. Hazard. Mater. 2018, 347, 451–460. [Google Scholar] [CrossRef]
- Huang, M.; Jiao, J.; Wang, J.; Xia, Z.; Zhang, Y. Exposure to Acrylamide Induces Cardiac Developmental Toxicity in Zebrafish during Cardiogenesis. Environ. Pollut. 2018, 234, 656–666. [Google Scholar] [CrossRef]
- Abd Al Haleem, E.N.; Hasan, W.Y.S.; Arafa, H.M.M. Therapeutic Effects of Thymoquinone or Capsaicin on Acrylamide-Induced Reproductive Toxicity in Rats Mediated by Their Effect on Oxidative Stress, Inflammation, and Tight Junction Integrity. Drug Chem. Toxicol. 2022, 45, 2328–2340. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Zou, F.; Bai, S.; Jiang, X.; Jiao, R.; Ou, S.; Zhang, H.; Su, Z.; Huang, Y.; et al. Glycidamide Inhibits Progesterone Production through Reactive Oxygen Species-Induced Apoptosis in R2C Rat Leydig Cells. Food Chem. Toxicol. 2017, 108, 563–570. [Google Scholar] [CrossRef]
- Lima, T.R.R.; Souza, N.P.; Cardoso, A.P.F.; Gomide, L.M.M.; Nascimento e Pontes, M.G.; Miot, H.A.; Arnold, L.L.; Cohen, S.M.; de Camargo, J.L.V. Testicular Alterations in Cryptorchid/Orchiopexic Rats Chronically Exposed to Acrylamide or Di-Butyl-Phthalate. J. Toxicol. Pathol. 2022, 35, 159–170. [Google Scholar] [CrossRef]
- Doctor Arastoye Marandi, M.; Yadegari, M.; Shahedi, A.; Pourentezari, M.; Anvari, M.; Shahrokhi Raeini, A.; Dortaj, H. Effects of Icariin on Histomorphometric Changes of Testis and Prostate Induced by Acrylamide in Mice. Int. J. Med. Lab. 2020, 7, 197–211. [Google Scholar] [CrossRef]
- Wei, Q.; Li, J.; Li, X.; Zhang, L.; Shi, F. Reproductive Toxicity in Acrylamide-Treated Female Mice. Reprod. Toxicol. 2014, 46, 121–128. [Google Scholar] [CrossRef]
- Duan, X.; Wang, Q.-C.; Chen, K.-L.; Zhu, C.-C.; Liu, J.; Sun, S.-C. Acrylamide Toxic Effects on Mouse Oocyte Quality and Fertility in Vivo. Sci. Rep. 2015, 5, 11562. [Google Scholar] [CrossRef]
- Nagata, C.; Konishi, K.; Wada, K.; Tamura, T.; Goto, Y.; Koda, S.; Mizuta, F.; Iwasa, S. Maternal Acrylamide Intake during Pregnancy and Sex Hormone Levels in Maternal and Umbilical Cord Blood and Birth Size of Offspring. Nutr. Cancer 2019, 71, 77–82. [Google Scholar] [CrossRef]
- Erdemli, M.E.; Aksungur, Z.; Gul, M.; Yigitcan, B.; Bag, H.G.; Altinoz, E.; Turkoz, Y. The Effects of Acrylamide and Vitamin E on Kidneys in Pregnancy: An Experimental Study. J. Matern.-Fetal Neonatal Med. 2019, 32, 3747–3756. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecińska, I.; Jasińska-Melon, E.; Winiarek, J.; Sawicki, W. Are AAMA and GAMA Levels in Urine after Childbirth a Suitable Marker to Assess Exposure to Acrylamide from Passive Smoking during Pregnancy?—A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7391. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Q.; Qiao, B.; Jiang, W.; Zhang, L.; Shen, X.; Xie, L.; Liu, H.; Zhang, D.; Yang, B.; et al. Exposure to Acrylamide Inhibits Uterine Decidualization via Suppression of Cyclin D3/P21 and Apoptosis in Mice. J. Hazard. Mater. 2020, 388, 121785. [Google Scholar] [CrossRef]
- Hogervorst, J.G.F.; Saenen, N.D.; Nawrot, T.S. Gestational Acrylamide Exposure and Biomarkers of Fetal Growth: Probing the Mechanism Underlying the Association between Acrylamide and Reduced Fetal Growth. Environ. Int. 2021, 155, 106668. [Google Scholar] [CrossRef]
- Erdemli, Z.; Erdemli, M. Vitamin E Plays a Protective Role While Acrylamide Administration Disrupted the Placenta Structure in Pregnancy: An Experimental Study. Ann. Med. Res. 2020, 27, 3217. [Google Scholar] [CrossRef]
- Kadawathagedara, M.; Botton, J.; de Lauzon-Guillain, B.; Meltzer, H.M.; Alexander, J.; Brantsaeter, A.L.; Haugen, M.; Papadopoulou, E. Dietary Acrylamide Intake during Pregnancy and Postnatal Growth and Obesity: Results from the Norwegian Mother and Child Cohort Study (MoBa). Environ. Int. 2018, 113, 325–334. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I.; Donaldson, J.; Muszyński, S. Acrylamide-Induced Prenatal Programming of Bone Structure in Mammal Model. Ann. Anim. Sci. 2020, 20, 1257–1287. [Google Scholar] [CrossRef]
- Das, J.; Mishra, H.N. Recent Advances in Sensors for Detecting Food Pathogens, Contaminants, and Toxins: A Review. Eur. Food Res. Technol. 2022, 248, 1125–1148. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Rodrigues, N.; Marx, Í.M.G.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Assessing Acrylamide Content in Sterilized Californian-Style Black Table Olives Using HPLC-MS-QQQ and a Potentiometric Electronic Tongue. LWT 2020, 129, 109605. [Google Scholar] [CrossRef]
- Ghalebi, M.; Hamidi, S.; Nemati, M. High-Performance Liquid Chromatography Determination of Acrylamide after Its Extraction from Potato Chips. Pharm. Sci. 2019, 25, 338–344. [Google Scholar] [CrossRef]
- Başaran, B.; Aydın, F.; Kaban, G. The Determination of Acrylamide Content in Brewed Coffee Samples Marketed in Turkey. Food Addit. Contam. Part A 2020, 37, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Sáez-Escudero, L.; Morales, F.J.; Delgado-Andrade, C. Reassessment of Acrylamide Content in Breakfast Cereals. Evolution of the Spanish Market from 2006 to 2018. Food Control 2019, 105, 94–101. [Google Scholar] [CrossRef]
- Hai, Y.D.; Tran-Lam, T.-T.; Nguyen, T.Q.; Vu, N.D.; Ma, K.H.; Le, G.T. Acrylamide in Daily Food in the Metropolitan Area of Hanoi, Vietnam. Food Addit. Contam. Part B 2019, 12, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xu, X.; Ye, X.; Zhou, F.; Qian, C.; Chen, J.; Zhang, T.; Ding, Z. Determination and Risk Assessment of Acrylamide in Thermally Processed Atractylodis Macrocephalae Rhizoma. Food Chem. 2021, 352, 129438. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Acrylamide Formation and Antioxidant Activity in Coffee during Roasting—A Systematic Study. Food Chem. 2021, 343, 128514. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A. Investigation and Determination of Acrylamide in the Main Group of Cereal Products Using Advanced Microextraction Method Coupled with Gas Chromatography-Mass Spectrometry. J. Cereal Sci. 2019, 87, 157–164. [Google Scholar] [CrossRef]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Hadian, Z.; Ghasemi, J.; Mohammadi, A. Investigation and Determination of Acrylamide in 24 Types of Roasted Nuts and Seeds Using Microextraction Method Coupled with Gas Chromatography–Mass Spectrometry: Central Composite Design. J. Food Meas. Charact. 2020, 14, 1249–1260. [Google Scholar] [CrossRef]
- Yadav, N.; Chhillar, A.K.; Pundir, C.S. Preparation, Characterization and Application of Haemoglobin Nanoparticles for Detection of Acrylamide in Processed Foods. Int. J. Biol. Macromol. 2018, 107, 1000–1013. [Google Scholar] [CrossRef]
- Navarro, K.M.; Silva, J.C.; Ossick, M.V.; Nogueira, A.B.; Etchegaray, A.; Mendes, R.K. Low-Cost Electrochemical Determination of Acrylamide in Processed Food Using a Hemoglobin-Iron Magnetic Nanoparticle-Chitosan Modified Carbon Paste Electrode. Anal. Lett. 2021, 54, 1180–1192. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; Zhu, J.; Zhou, B.; Jing, J.; Wang, A.; Xu, R.; Wen, Z.; Shi, X.; Guo, S. Simple and Sensitive Detection of Acrylamide Based on Hemoglobin Immobilization in Carbon Ionic Liquid Paste Electrode. Food Control 2020, 109, 106764. [Google Scholar] [CrossRef]
- Asnaashari, M.; Esmaeilzadeh Kenari, R.; Farahmandfar, R.; Taghdisi, S.M.; Abnous, K. Fluorescence Quenching Biosensor for Acrylamide Detection in Food Products Based on Double-Stranded DNA and Gold Nanoparticles. Sens. Actuators B Chem. 2018, 265, 339–345. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, P.; Liu, T.; Pu, H.; Sun, D.-W. A Fluorescence Biosensor Based on Single-Stranded DNA and Carbon Quantum Dots for Acrylamide Detection. Food Chem. 2021, 356, 129668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, S.; Wang, S.; Wang, P.; Su, X.-O.; Xie, J. Rapid and Sensitive Detection of Acrylamide in Fried Food Using Dispersive Solid-Phase Extraction Combined with Surface-Enhanced Raman Spectroscopy. Food Chem. 2019, 276, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, W.; Liu, C.; Foda, M.F.; Zhu, Y. Strawberry-like SiO2/Ag Nanocomposites Immersed Filter Paper as SERS Substrate for Acrylamide Detection. Food Chem. 2020, 328, 127106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, M.; Adhikari, B. Recent Developments in Frying Technologies Applied to Fresh Foods. Trends Food Sci. Technol. 2020, 98, 68–81. [Google Scholar] [CrossRef]
- Belkova, B.; Hradecky, J.; Hurkova, K.; Forstova, V.; Vaclavik, L.; Hajslova, J. Impact of Vacuum Frying on Quality of Potato Crisps and Frying Oil. Food Chem. 2018, 241, 51–59. [Google Scholar] [CrossRef]
- Akkurt, K.; Mogol, B.A.; Gökmen, V. Mitigation of Acrylamide in Baked Potato Chips by Vacuum Baking and Combined Conventional and Vacuum Baking Processes. LWT 2021, 144, 111211. [Google Scholar] [CrossRef]
- Hu, H.; Liu, X.; Jiang, L.; Zhang, Q.; Zhang, H. The Relationship between Acrylamide and Various Components during Coffee Roasting and Effect of Amino Acids on Acrylamide Formation. J. Food Process. Preserv. 2021, 45, e15421. [Google Scholar] [CrossRef]
- Sharif, R.; Shahar, S.; Rajab, N.F.; Fenech, M. Dietary Pattern, Genomic Stability and Relative Cancer Risk in Asian Food Landscape. Nutr. Cancer 2022, 74, 1171–1187. [Google Scholar] [CrossRef]
- Jia, R.; Wan, X.; Geng, X.; Xue, D.; Xie, Z.; Chen, C. Microbial L-Asparaginase for Application in Acrylamide Mitigation from Food: Current Research Status and Future Perspectives. Microorganisms 2021, 9, 1659. [Google Scholar] [CrossRef]
- Liyanage, D.W.K.; Yevtushenko, D.P.; Konschuh, M.; Bizimungu, B.; Lu, Z.-X. Processing Strategies to Decrease Acrylamide Formation, Reducing Sugars and Free Asparagine Content in Potato Chips from Three Commercial Cultivars. Food Control 2021, 119, 107452. [Google Scholar] [CrossRef]
- Wang, X.; Xu, L. Influence Factors on the Formation of Acrylamide in the Amino Acid/Sugar Chemical Model System. J. Food Nutr. Res. 2014, 2, 344–348. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Hu, H.; McClements, D.J.; Nie, S.; Shen, M.; Li, C.; Huang, Y.; Zhong, Y.; Chen, J.; Zeng, M.; et al. PH and Lipid Unsaturation Impact the Formation of Acrylamide and 5-Hydroxymethylfurfural in Model System at Frying Temperature. Food Res. Int. 2019, 123, 403–413. [Google Scholar] [CrossRef]
- Albedwawi, A.; Al Sakkaf, R.; Yusuf, A.; Osaili, T.; Al-Nabulsi, A.; Liu, S.-Q.; Palmisano, G.; Ayyash, M. Acrylamide Elimination by Lactic Acid Bacteria: Screening, Optimization, In Vitro Digestion, and Mechanism. Microorganisms 2022, 10, 557. [Google Scholar] [CrossRef]
- Nachi, I.; Fhoula, I.; Smida, I.; Ben Taher, I.; Chouaibi, M.; Jaunbergs, J.; Bartkevics, V.; Hassouna, M. Assessment of Lactic Acid Bacteria Application for the Reduction of Acrylamide Formation in Bread. LWT 2018, 92, 435–441. [Google Scholar] [CrossRef]
- Akıllıoglu, H.G.; Gökmen, V. Mitigation of Acrylamide and Hydroxymethyl Furfural in Instant Coffee by Yeast Fermentation. Food Res. Int. 2014, 61, 252–256. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Q.; Fan, D.; Xiao, J.; Zhao, Y.; Cheng, K.-W.; Wang, M. Novel Roles of Hydrocolloids in Foods: Inhibition of Toxic Maillard Reaction Products Formation and Attenuation of Their Harmful Effects. Trends Food Sci. Technol. 2021, 111, 706–715. [Google Scholar] [CrossRef]
- Zokaei, M.; Kamankesh, M.; Abedi, A.-S.; Moosavi, M.H.; Mohammadi, A.; Rezvani, M.; Shojaee-Aliabadi, S.; Khaneghah, A.M. Reduction in Acrylamide Formation in Potato Crisps: Application of Extract and Hydrocolloid-Based Coatings. J. Food Prot. 2020, 83, 754–761. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, R.; Jia, C.; Rong, J.; Hu, Y.; Liu, R. Hydrocolloid Effects on Nε-Carboxymethyllysine and Acrylamide of Deep-Fried Fish Nuggets. Food Biosci. 2021, 39, 100797. [Google Scholar] [CrossRef]
- Gülcan, Ü.; Candal Uslu, C.; Mutlu, C.; Arslan-Tontul, S.; Erbaş, M. Impact of Inert and Inhibitor Baking Atmosphere on HMF and Acrylamide Formation in Bread. Food Chem. 2020, 332, 127434. [Google Scholar] [CrossRef]



| Detection Method | Limit of Detection (LOD) | Range of Detection | Sample Model | References |
|---|---|---|---|---|
| E-tongue and nose | 2.5 × 10−3 μg kg−1 | 2.5 × 10−3–20×10−3 μg kg−1 | Olive and brine solution | [82] |
| Fluorescence biosensor | 0.5 × 10−6 nmol L−1 | 0.05 M–10−7 nmol L−1 | Potato fries | [94] |
| 2.41 × 10−2 nmol L−1 | 1 × 10−1–5 × 103 nmol L−1 | Bread crust | [95] | |
| HbNPs | 0.1 nmol L−1 | 0.05–100 nmol L−1 | Bread, nuts, potato crips, biscuits | [91] |
| 0.06 nmol L−1 | 10–171 nmol L−1 | French fries | [92] | |
| SERS | 2 µg kg−1 | 5–100 µg kg−1 | Fried food | [96] |
| 0.02 nmol L−1 | 0.1–5 × 104 nmol L−1 | Cookies, chips, and bread | [97] | |
| LC-MS/MS | 3 ng mL−1 | 16.8–72.8 ng mL−1 | Coffee | [84] |
| 6 µg kg−1 | 197–639 µg kg−1 | Breakfast cereals | [85] | |
| 2 μg kg−1 | 95.8–9826 μg kg−1 | Medicine homologous foods (Atractylodis Macrocephalae Rhizoma) | [87] | |
| GC-MS | 0.6 µg kg−1 | 1–500 µg kg−1 | Bread, biscuits, wafers, cakes, cookies, and crackers | [89] |
| 0.6 µg kg−1 | 33.36–250.90 µg kg−1 | Roasted nuts and seeds | [90] |
| Mitigation Strategies | Reduction Percentage (%) | Sample Model | Reference |
|---|---|---|---|
| Air- and vacuum-frying | 72–98% | Potato chips | [100] |
| Blanching | 65% and 96% | French fries and potato crisps | [27] |
| Additives | 30–60% | Amino acid/sugar chemical model | [105] |
| Fermentation | 70% | Roasted coffee | [109] |
| Hydrocolloid coating | 48% | French fries | [28] |
| Inhibitory and inert baking atmosphere | 50–99% | Bread | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindaraju, I.; Sana, M.; Chakraborty, I.; Rahman, M.H.; Biswas, R.; Mazumder, N. Dietary Acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts. Foods 2024, 13, 556. https://doi.org/10.3390/foods13040556
Govindaraju I, Sana M, Chakraborty I, Rahman MH, Biswas R, Mazumder N. Dietary Acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts. Foods. 2024; 13(4):556. https://doi.org/10.3390/foods13040556
Chicago/Turabian StyleGovindaraju, Indira, Maidin Sana, Ishita Chakraborty, Md. Hafizur Rahman, Rajib Biswas, and Nirmal Mazumder. 2024. "Dietary Acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts" Foods 13, no. 4: 556. https://doi.org/10.3390/foods13040556
APA StyleGovindaraju, I., Sana, M., Chakraborty, I., Rahman, M. H., Biswas, R., & Mazumder, N. (2024). Dietary Acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts. Foods, 13(4), 556. https://doi.org/10.3390/foods13040556








