Milk Whey Protein Fibrils—Effect of Stirring and Heating Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.2. Process for mWPI Fibril Formation
2.3. Confirmation of the Presence of mWPI Fibrils
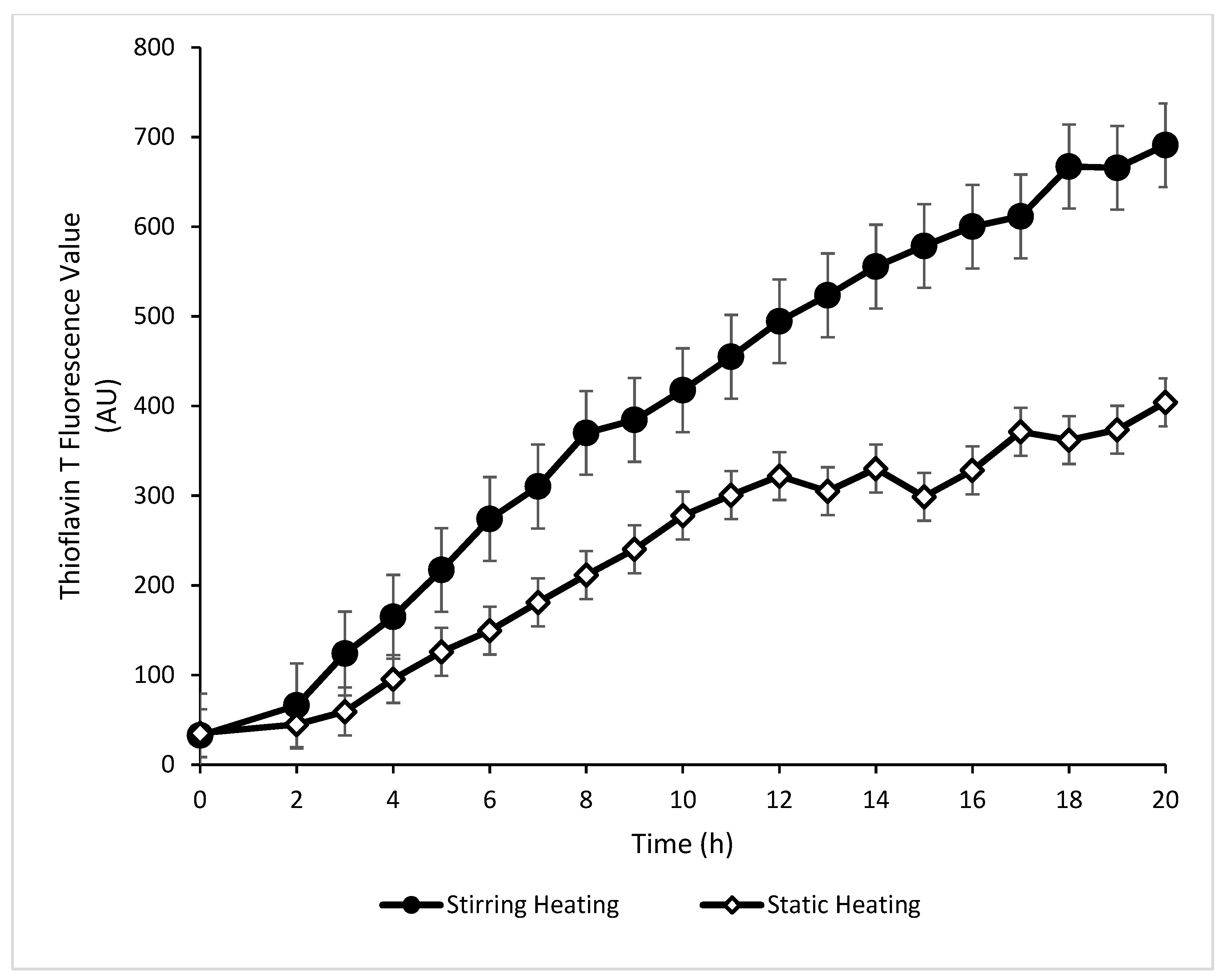
2.3.1. Thioflavin T (Th T) Fluorescence Value
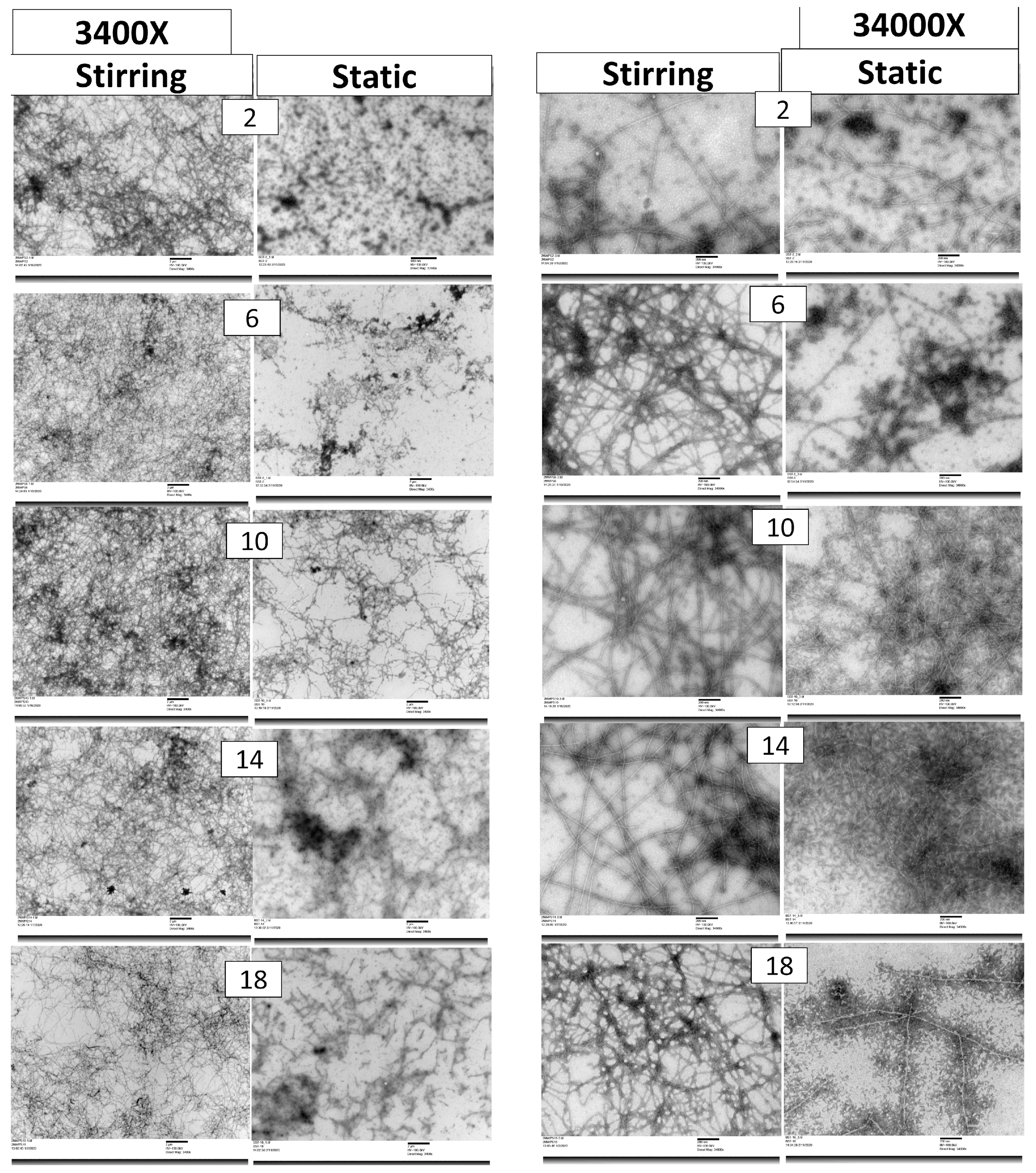
2.3.2. Transmission Electron Microscopy (TEM)
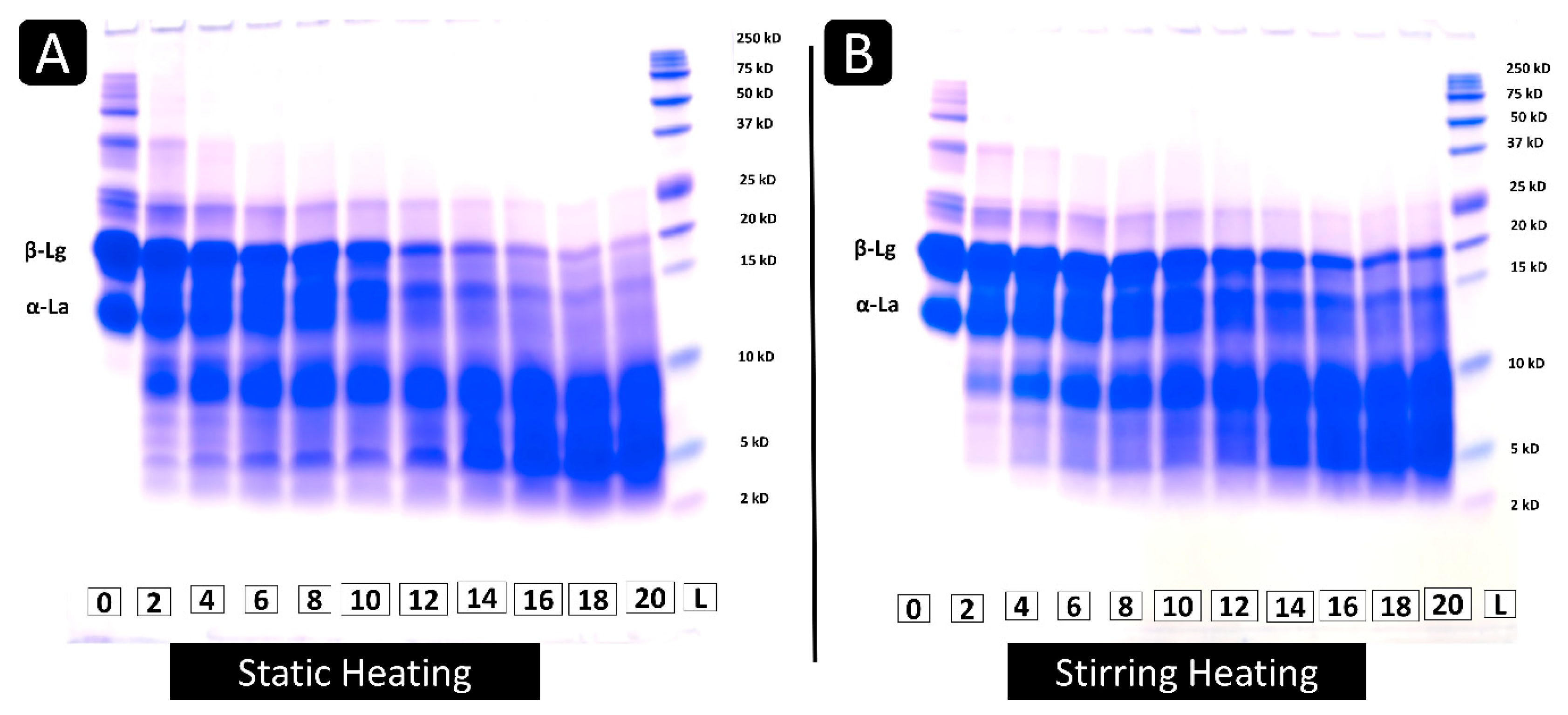
2.3.3. Tricine Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Rheology
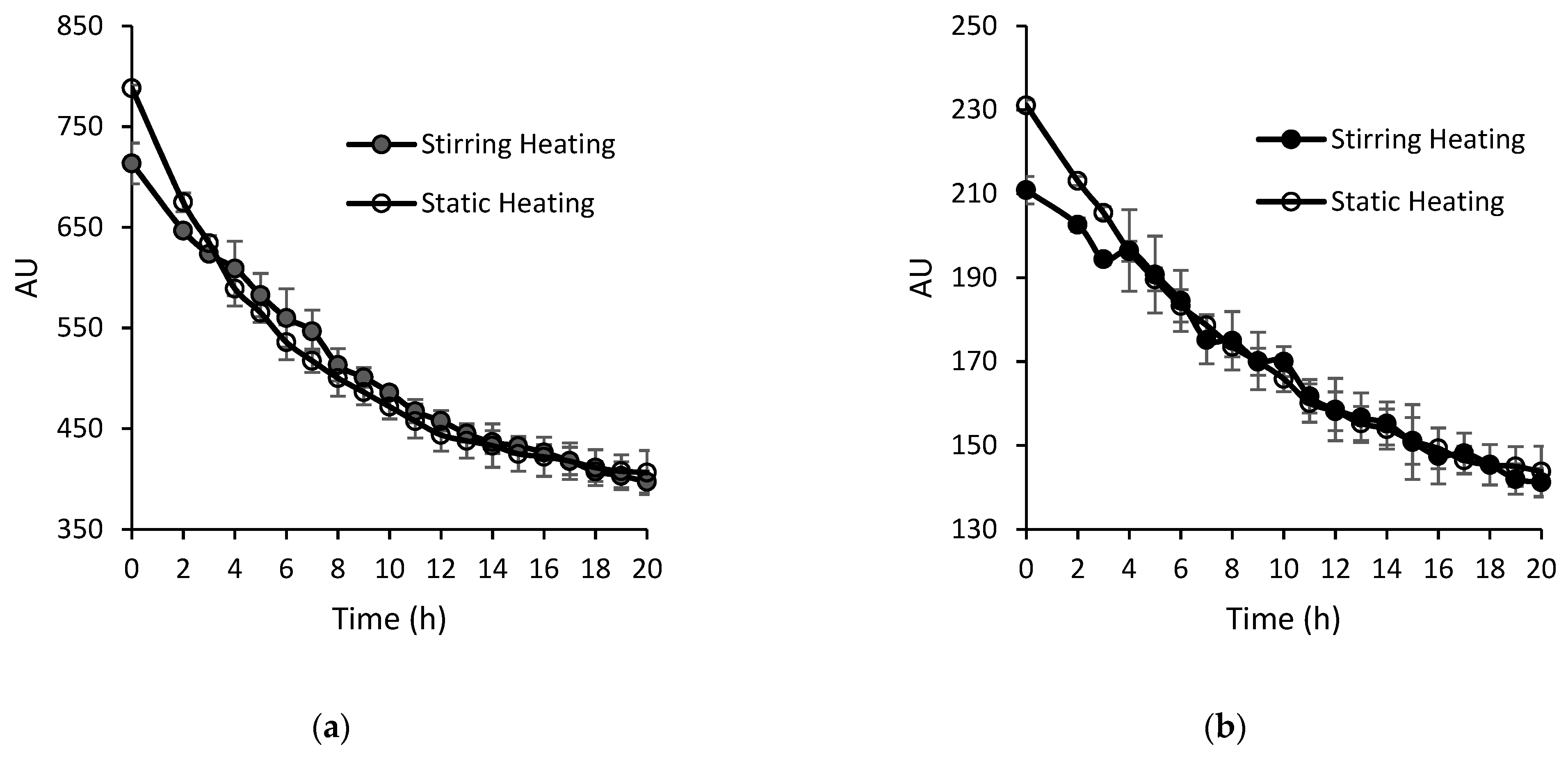
2.5. Protein Oxidation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Confirmation of the Presence of mWPI Fibrils
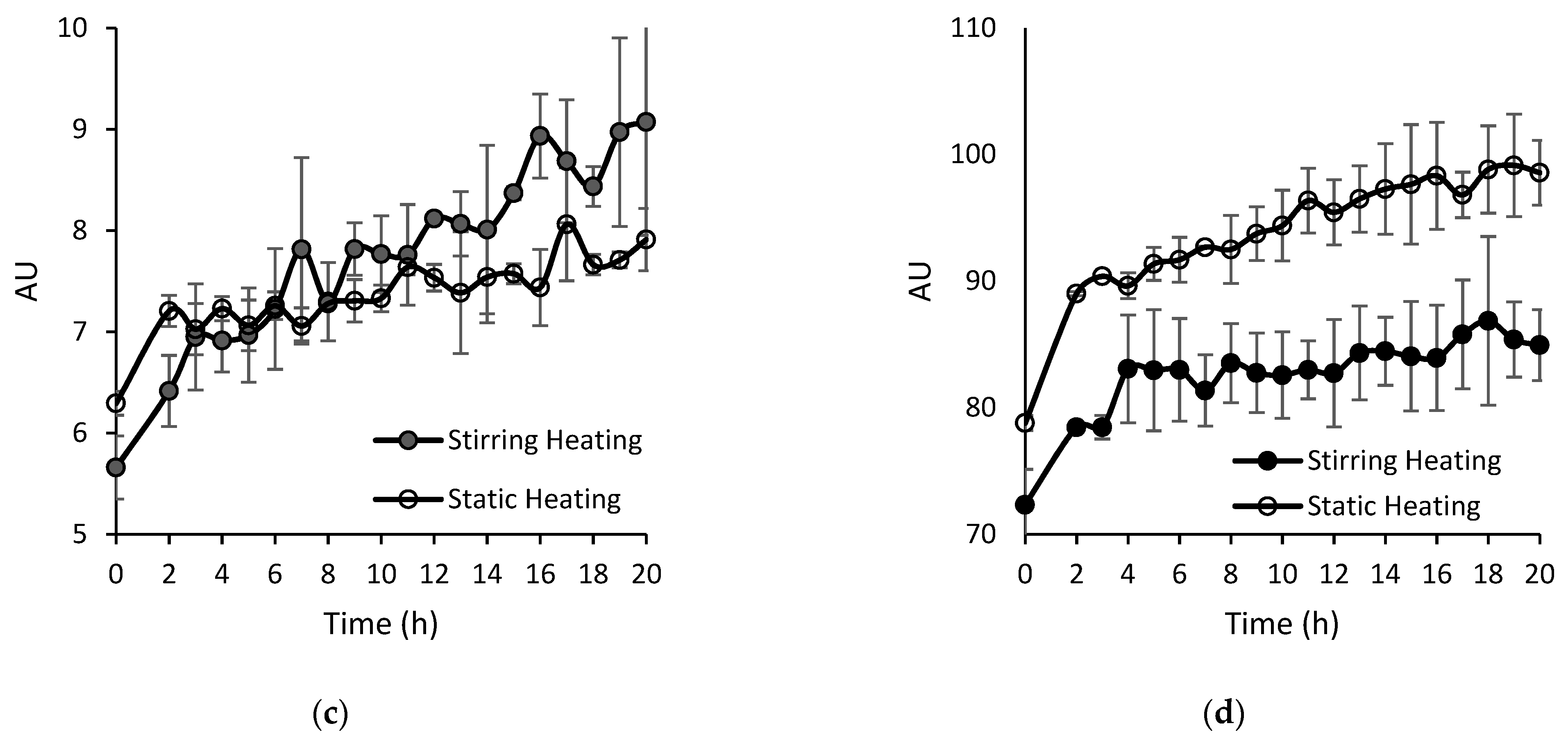
3.1.1. Th T Value
3.1.2. TEM
3.1.3. Tricine SDS-PAGE
3.2. Rheology
3.3. Protein Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damodaran, S. Food Proteins: An Overview. In Food Proteins and Their Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–24. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. The Functional Modification of Legume Proteins by Ultrasonication: A Review. Trends Food Sci. Technol. 2020, 98, 107–116. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Qi, G.; Li, Y.; Sun, X.S.; Qiu, D.; Li, Y. Formation and Physicochemical Properties of Amyloid Fibrils from Soy Protein. Int. J. Biol. Macromol. 2020, 149, 609–616. [Google Scholar] [CrossRef]
- Mantovani, R.A.; Fattori, J.; Michelon, M.; Cunha, R.L. Formation and PH-Stability of Whey Protein Fibrils in the Presence of Lecithin. Food Hydrocoll. 2016, 60, 288–298. [Google Scholar] [CrossRef]
- Munialo, C.D.; Martin, A.H.; Van Der Linden, E.; De Jongh, H.H.J. Fibril Formation from Pea Protein and Subsequent Gel Formation. J. Agric. Food Chem. 2014, 62, 2418–2427. [Google Scholar] [CrossRef]
- An, B.; Wu, X.; Li, M.; Chen, Y.; Li, F.; Yan, X.; Wang, J.; Li, C.; Brennan, C. Hydrophobicity-Modulating Self-Assembled Morphologies of α-Zein in Aqueous Ethanol. Int. J. Food Sci. Technol. 2016, 51, 2621–2629. [Google Scholar] [CrossRef]
- Ridgley, D.M.; Barone, J.R. Evolution of the Amyloid Fiber over Multiple Length Scales. ACS Nano 2013, 7, 1006–1015. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Huang, L.H. Effect of Heat-Induced Formation of Rice Bran Protein Fibrils on Morphological Structure and Physicochemical Properties in Solutions and Gels. Food Sci. Biotechnol. 2014, 23, 1417–1423. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Zhang, H.; Yang, H.Y.; Wang, L.; Qian, H.F. Formation of Heat-Induced Cottonseed Congossypin(7S) Fibrils at PH 2.0. J. Sci. Food Agric. 2014, 94, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M.; Anema, S.G.; Singh, H. β-Lactoglobulin Nanofibrils: The Long and the Short of It. Int. Dairy J. 2017, 67, 35–45. [Google Scholar] [CrossRef]
- Loveday, S.M.; Wang, X.L.; Rao, M.A.; Anema, S.G.; Singh, H. β-Lactoglobulin Nanofibrils: Effect of Temperature on Fibril Formation Kinetics, Fibril Morphology and the Rheological Properties of Fibril Dispersions. Food Hydrocoll. 2012, 27, 242–249. [Google Scholar] [CrossRef]
- Šarić, A.; Michaels, T.C.T.; Zaccone, A.; Knowles, T.P.J.; Frenkel, D. Kinetics of Spontaneous Filament Nucleation via Oligomers: Insights from Theory and Simulation. J. Chem. Phys. 2016, 145, 211926. [Google Scholar] [CrossRef] [PubMed]
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides Are Building Blocks of Heat-Induced Fibrillar Protein Aggregates of β-Lactoglobulin Formed at PH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef]
- Meng, Y.; Wei, Z.; Xue, C. Protein Fibrils from Different Food Sources: A Review of Fibrillation Conditions, Properties, Applications and Research Trends. Trends Food Sci. Technol. 2022, 121, 59–75. [Google Scholar] [CrossRef]
- Bolder, S.G.; Sagis, L.M.C.; Venema, P.; Van Der Linden, E. Effect of Stirring and Seeding on Whey Protein Fibril Formation. J. Agric. Food Chem. 2007, 55, 5661–5669. [Google Scholar] [CrossRef]
- Rathod, G.; Amamcharla, J.K. Process Development for a Novel Milk Protein Concentrate with Whey Proteins as Fibrils. J. Dairy Sci. 2021, 104, 4094–4107. [Google Scholar] [CrossRef] [PubMed]
- Rathod, G.; Kapoor, R.; Meletharayil, G.H.; Amamcharla, J.K. Development of Spray Dried Functional Milk Protein Concentrate Containing Whey Proteins as Fibrils. Int. Dairy J. 2023, 145, 105719. [Google Scholar] [CrossRef]
- Scheidegger, D.; Pecora, R.P.; Radici, P.M.; Kivatinitz, S.C. Protein Oxidative Changes in Whole and Skim Milk after Ultraviolet or Fluorescent Light Exposure. J. Dairy Sci. 2010, 93, 5101–5109. [Google Scholar] [CrossRef] [PubMed]
- Keppler, J.K.; Heyn, T.R.; Meissner, P.M.; Schrader, K.; Schwarz, K. Protein Oxidation during Temperature-Induced Amyloid Aggregation of Beta-Lactoglobulin. Food Chem. 2019, 289, 223–231. [Google Scholar] [CrossRef]
- Loveday, S.M.; Wang, X.L.; Rao, M.A.; Anema, S.G.; Creamer, L.K.; Singh, H. Tuning the Properties of β-Lactoglobulin Nanofibrils with PH, NaCl and CaCl2. Int. Dairy J. 2010, 20, 571–579. [Google Scholar] [CrossRef]
- Akkermans, C.; Venema, P.; Rogers, S.S.; Van Der Goot, A.J.; Boom, R.M.; Van Der Linden, E. Shear Pulses Nucleate Fibril Aggregation. Food Biophys. 2006, 1, 144–150. [Google Scholar] [CrossRef]
- Loveday, S.M.; Su, J.; Rao, M.A.; Anema, S.G.; Singh, H. Whey Protein Nanofibrils: The Environment-Morphology-Functionality Relationship in Lyophilization, Rehydration, and Seeding. J. Agric. Food Chem. 2012, 60, 5229–5236. [Google Scholar] [CrossRef]
- Kroes-Nijboer, A.; Venema, P.; Linden, E. Van Der Fibrillar Structures in Food. Food Funct. 2012, 3, 221–227. [Google Scholar] [CrossRef]
- Arunkumar, A.; Etzel, M.R. Fractionation of α-Lactalbumin from β-Lactoglobulin Using Positively Charged Tangential Flow Ultrafiltration Membranes. Sep. Purif. Technol. 2013, 105, 121–128. [Google Scholar] [CrossRef]
- Bolder, S.G.; Hendrickx, H.; Sagis, L.M.C.; van der Linden, E. Ca2+-Induced Cold-Set Gelation of Whey Protein Isolate Fibrils. Appl. Rheol. 2006, 16, 258–264. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Technological Functionality and Biological Properties of Food Protein Nanofibrils Formed by Heating at Acidic Condition. Trends Food Sci. Technol. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Akkermans, C.; Van der Goot, A.J.; Venema, P.; Van der Linden, E.; Boom, R.M. Properties of Protein Fibrils in Whey Protein Isolate Solutions: Microstructure, Flow Behaviour and Gelation. Int. Dairy J. 2008, 18, 1034–1042. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of Whey Protein Isolate to Oxidation and Changes in Physicochemical, Structural, and Digestibility Characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef] [PubMed]
- Headlam, H.A.; Davies, M.J. Markers of Protein Oxidation: Different Oxidants Give Rise to Variable Yields of Bound and Released Carbonyl Products. Free Radic. Biol. Med. 2004, 36, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Baum, F.; Vollmer, G.; Pischetsrieder, M. Distribution of Protein Oxidation Products in the Proteome of Thermally Processed Milk. J. Agric. Food Chem. 2012, 60, 7306–7311. [Google Scholar] [CrossRef]

| Time (h) | Apparent Viscosity (m.Pa.s) at 100 s−1 | Consistency Coefficient (mPa.sn) | Flow Behavior Index | |||
|---|---|---|---|---|---|---|
| Stirring Heating | Static Heating | Stirring Heating | Static Heating | Stirring Heating | Static Heating | |
| 0 | 1.08 ± 0.03 A | 1.09 ± 0.03 A | 1.1 ± 0.2 A | 1.2 ± 0.1 A | 0.99 ± 0.03 A | 0.99 ± 0.01 A |
| 2 | 1.37 ± 0.19 A | 1.16 ± 0.03 B | 2.2 ± 0.7 A | 1.2 ± 0.1 B | 0.91 ± 0.04 B | 0.99 ± 0.01 A |
| 3 | 1.89 ± 0.05 A | 1.34 ± 0.07 B | 4.6 ± 0.2 A | 1.9 ± 0.5 B | 0.81 ± 0.01 B | 0.94 ± 0.05 A |
| 4 | 2.23 ± 0.19 A | 1.75 ± 0.55 A | 6.8 ± 0.9 A | 6.3 ± 5.7 A | 0.76 ± 0.02 A | 0.79 ± 0.14 A |
| 5 | 2.77 ± 0.31 A | 3.58 ± 1.23 A | 9.8 ± 1.2 A | 38.6 ± 36.4 A | 0.72 ± 0.01 A | 0.55 ± 0.14 B |
| 6 | 3.29 ± 0.24 A | 4.01 ± 1.55 A | 13.3 ± 1.3 A | 63.1 ± 62.8 A | 0.7 ± 0.01 A | 0.49 ± 0.16 B |
| 7 | 3.68 ± 0.21 A | 4.79 ± 1.53 A | 16.5 ± 2.2 B | 92.2 ± 73.8 A | 0.68 ± 0.02 A | 0.42 ± 0.12 B |
| 8 | 4.19 ± 0.39 A | 4.21 ± 0.35 A | 18.0 ± 2.1 B | 58.0 ± 16.4 A | 0.68 ± 0.02 A | 0.44 ± 0.05 B |
| 9 | 4.44 ± 0.29 A | 4.20 ± 0.56 A | 21.9 ± 1.5 B | 61.9 ± 21.9 A | 0.66 ± 0.01 A | 0.43 ± 0.05 B |
| 10 | 4.72 ± 0.13 A | 2.96 ± 1.01 B | 22.7 ± 1.4 A | 32.8 ± 29.4 A | 0.66 ± 0.02 A | 0.54 ± 0.12 B |
| 11 | 4.92 ± 0.16 A | 5.22 ± 1.56 A | 24.6 ± 0.9 B | 101.1 ± 64.4 A | 0.65 ± 0.02 A | 0.4 ± 0.09 B |
| 12 | 5.20 ± 0.14 A | 3.69 ± 1.21 A | 27.9 ± 2.9 A | 42.8 ± 33.1 A | 0.64 ± 0.02 A | 0.51 ± 0.1 B |
| 13 | 5.27 ± 0.16 A | 5.63 ± 1.63 A | 27.2 ± 1.1 B | 133.1 ± 49.0 A | 0.65 ± 0.02 A | 0.33 ± 0.03 B |
| 14 | 5.31 ± 0.07 A | 5.01 ± 0.91 A | 27.7 ± 1.0 B | 108.9 ± 32.0 A | 0.64 ± 0.01 A | 0.35 ± 0.06 B |
| 15 | 5.49 ± 0.06 A | 5.64 ± 2.58 A | 29.4 ± 2.0 A | 1564.2 ± 2.9 A | 0.64 ± 0.02 A | 0.41 ± 0.17 A |
| 16 | 5.06 ± 0.90 B | 5.62 ± 1.10 A | 28.8 ± 6.8 B | 142.6 ± 0.0 A | 0.63 ± 0.02 A | 0.3 ± 0.07 B |
| 17 | 6.09 ± 0.14 A | 3.84 ± 0.83 B | 33.7 ± 3.8 A | 68.7 ± 0.1 A | 0.63 ± 0.02 A | 0.41 ± 0.11 B |
| 18 | 6.07 ± 0.11 A | 6.48 ± 1.23 A | 36.8 ± 0.6 B | 241.8 ± 0.1 A | 0.61 ± 0.01 A | 0.23 ± 0.08 B |
| 19 | 6.08 ± 0.09 A | 6.48 ± 1.10 A | 33.0 ± 2.3 B | 161.1 ± 0.0 A | 0.63 ± 0.02 A | 0.31 ± 0.04 B |
| 20 | 5.94 ± 0.13 A | 6.06 ± 1.01 A | 32.3 ± 4.0 B | 128.7 ± 0.0 A | 0.64 ± 0.02 A | 0.35 ± 0.06 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathod, G.; Amamcharla, J. Milk Whey Protein Fibrils—Effect of Stirring and Heating Time. Foods 2024, 13, 466. https://doi.org/10.3390/foods13030466
Rathod G, Amamcharla J. Milk Whey Protein Fibrils—Effect of Stirring and Heating Time. Foods. 2024; 13(3):466. https://doi.org/10.3390/foods13030466
Chicago/Turabian StyleRathod, Gunvantsinh, and Jayendra Amamcharla. 2024. "Milk Whey Protein Fibrils—Effect of Stirring and Heating Time" Foods 13, no. 3: 466. https://doi.org/10.3390/foods13030466
APA StyleRathod, G., & Amamcharla, J. (2024). Milk Whey Protein Fibrils—Effect of Stirring and Heating Time. Foods, 13(3), 466. https://doi.org/10.3390/foods13030466






