Auricularia auricula-judae Attenuates the Progression of Metabolic Syndrome in High-Fat Diet-Induced Obese Rats: Enzymatic Pre-Digestion Technology Is Superior to Superfine Grinding Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of Basic Components
2.3. Animal Grouping and Treatments
2.4. Collection of Blood and Tissue Samples
2.5. Biochemical Assays
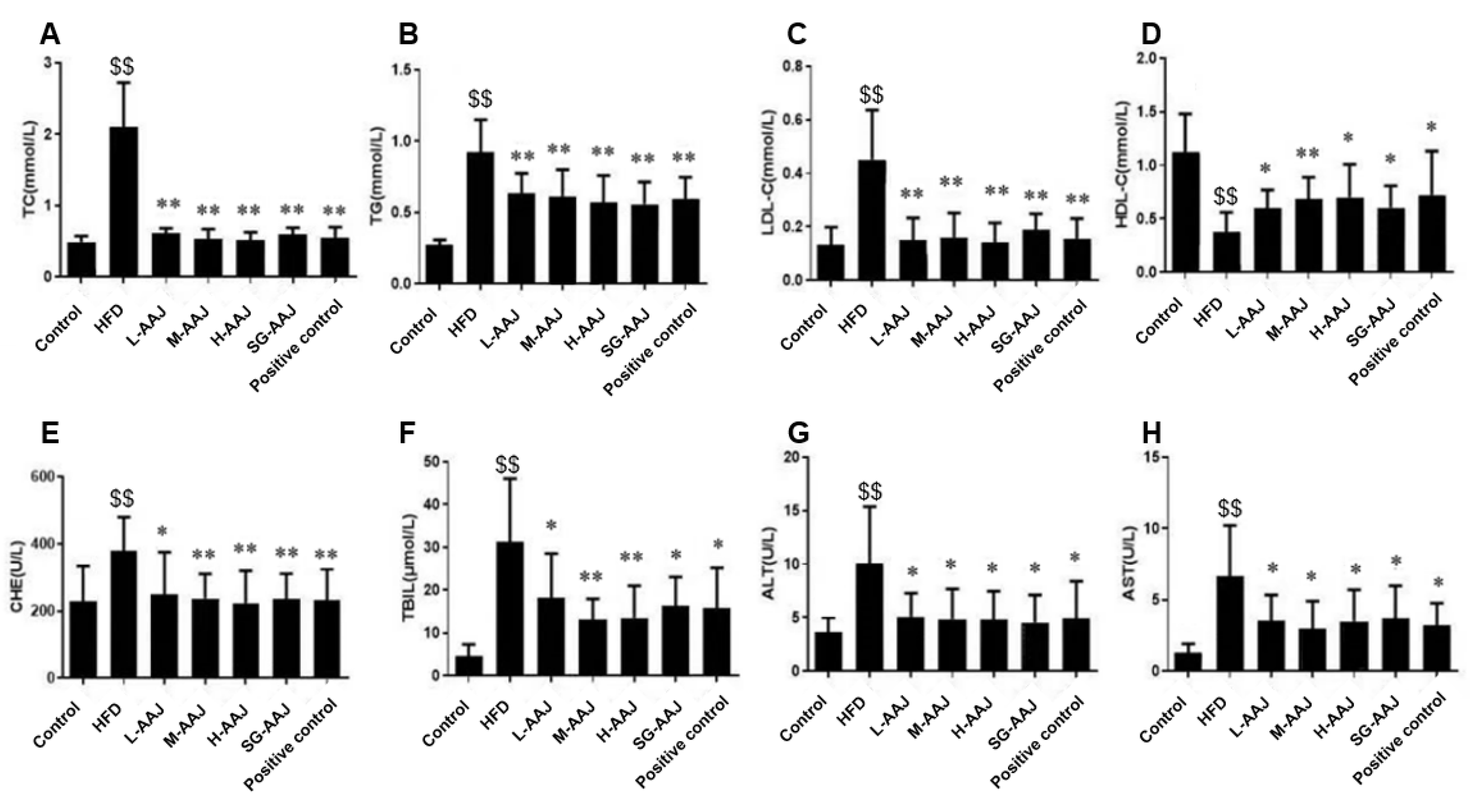
2.5.1. Serum Biochemistry
2.5.2. Liver Biochemistry
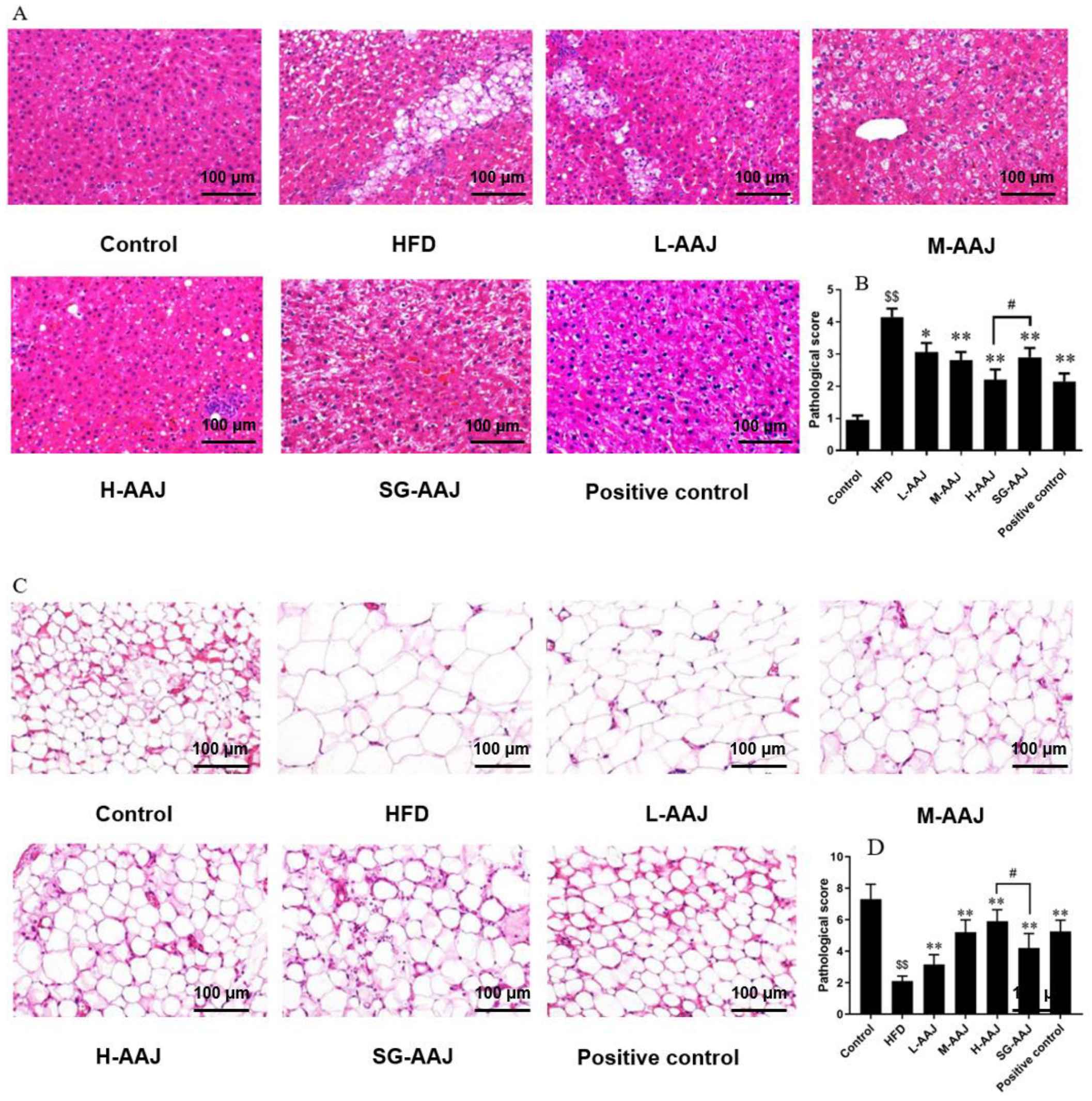
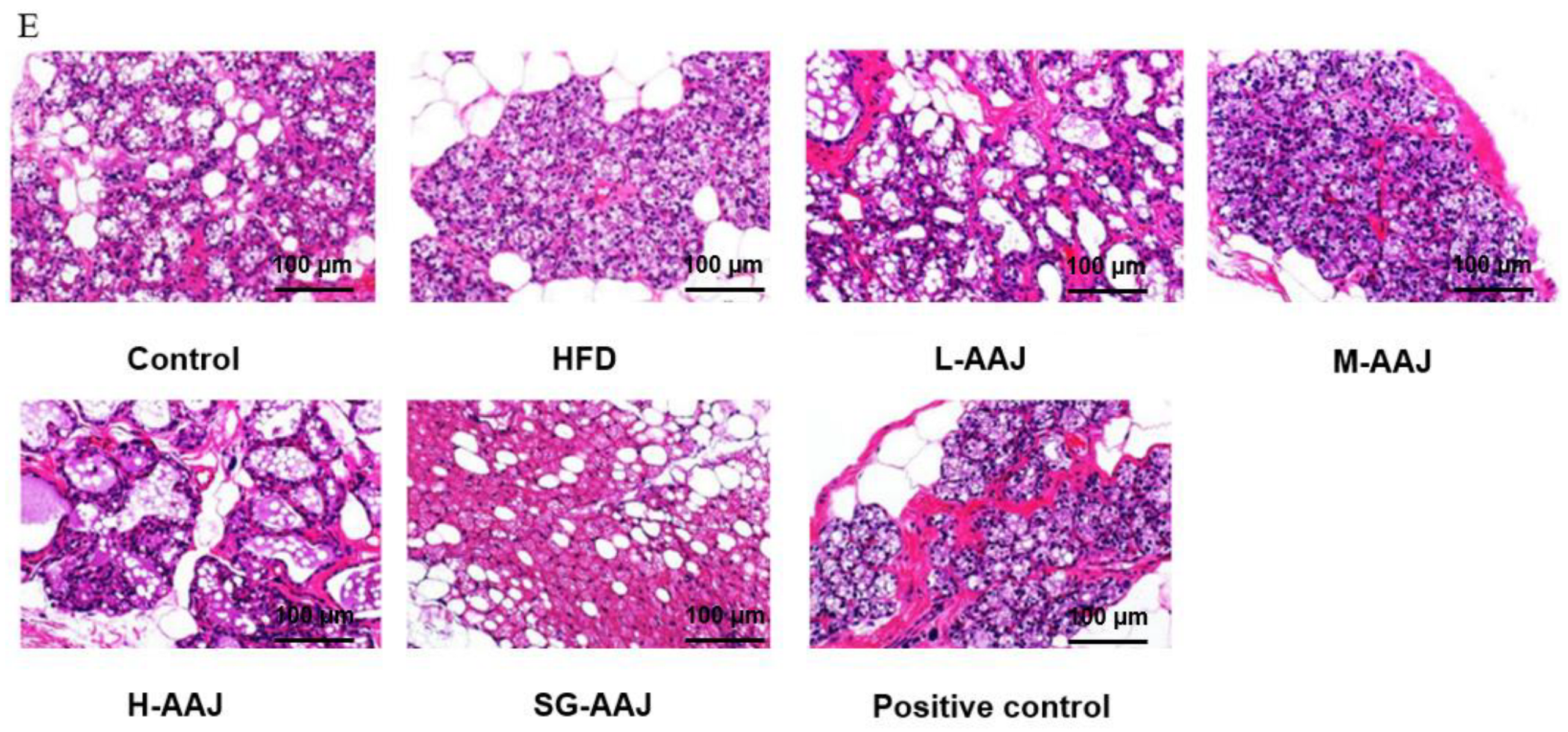
2.6. Histological Analyses of Liver and Fat Tissues
2.7. Transcriptome Sequencing
2.8. Real-Time Reverse Transcription PCR Analysis
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Analysis of the Basic Components
3.2. Body Weight and Food/Water Intakes
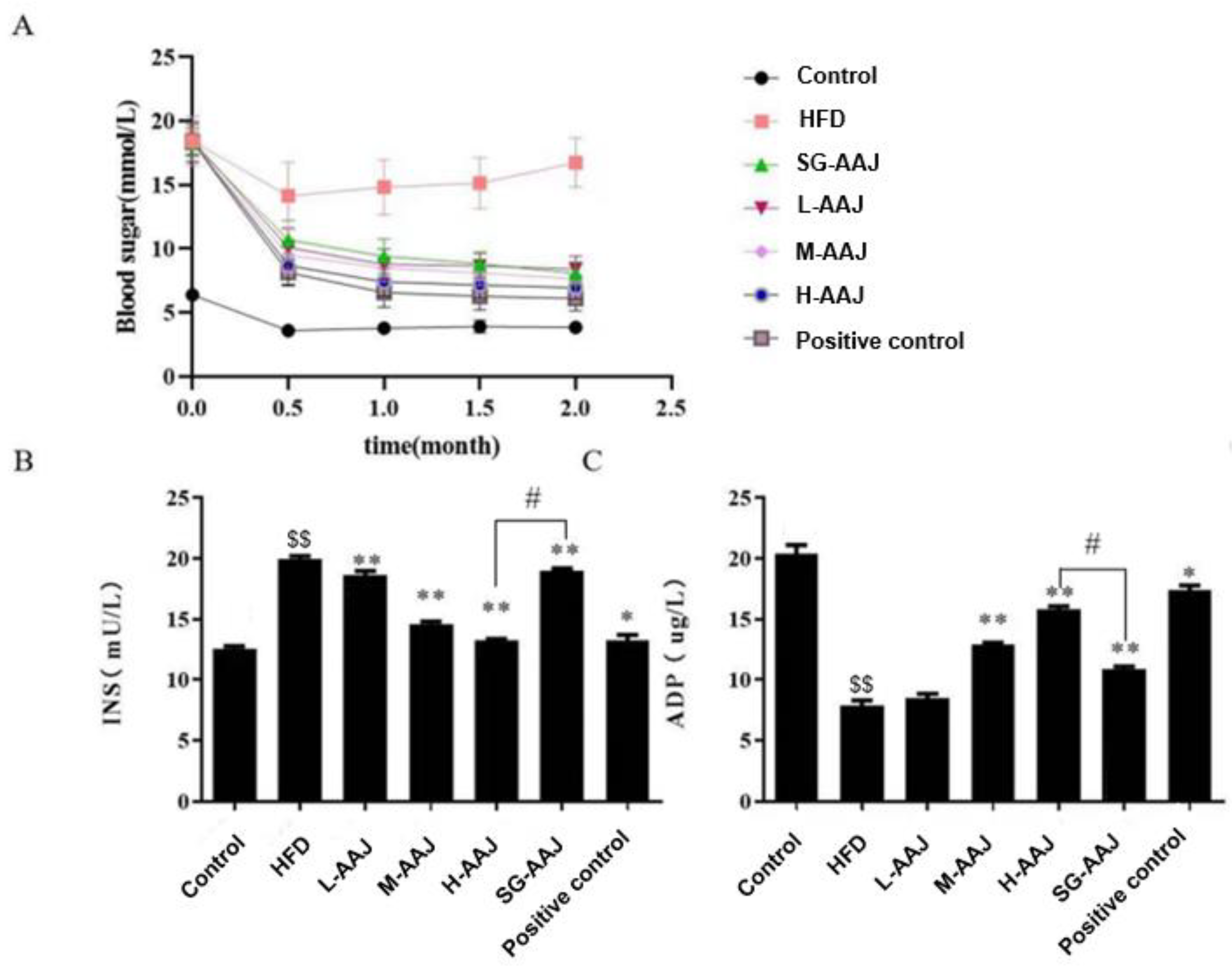
3.3. Serum Biochemistry
3.4. Indexes of the Liver, White Fat, and Brown Fat
3.5. Pathological Analysis of Liver and Fat
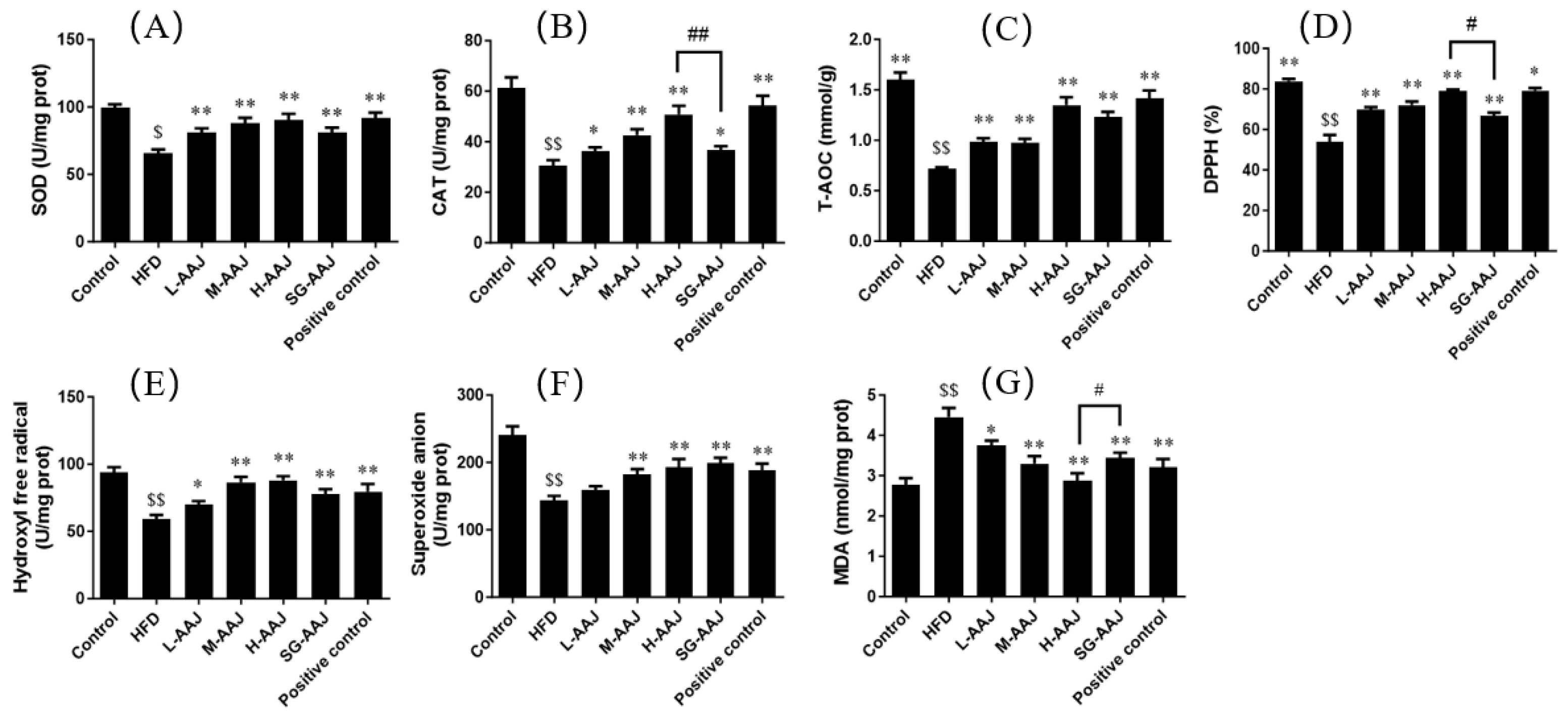
3.6. Antioxidative Indicators in Liver
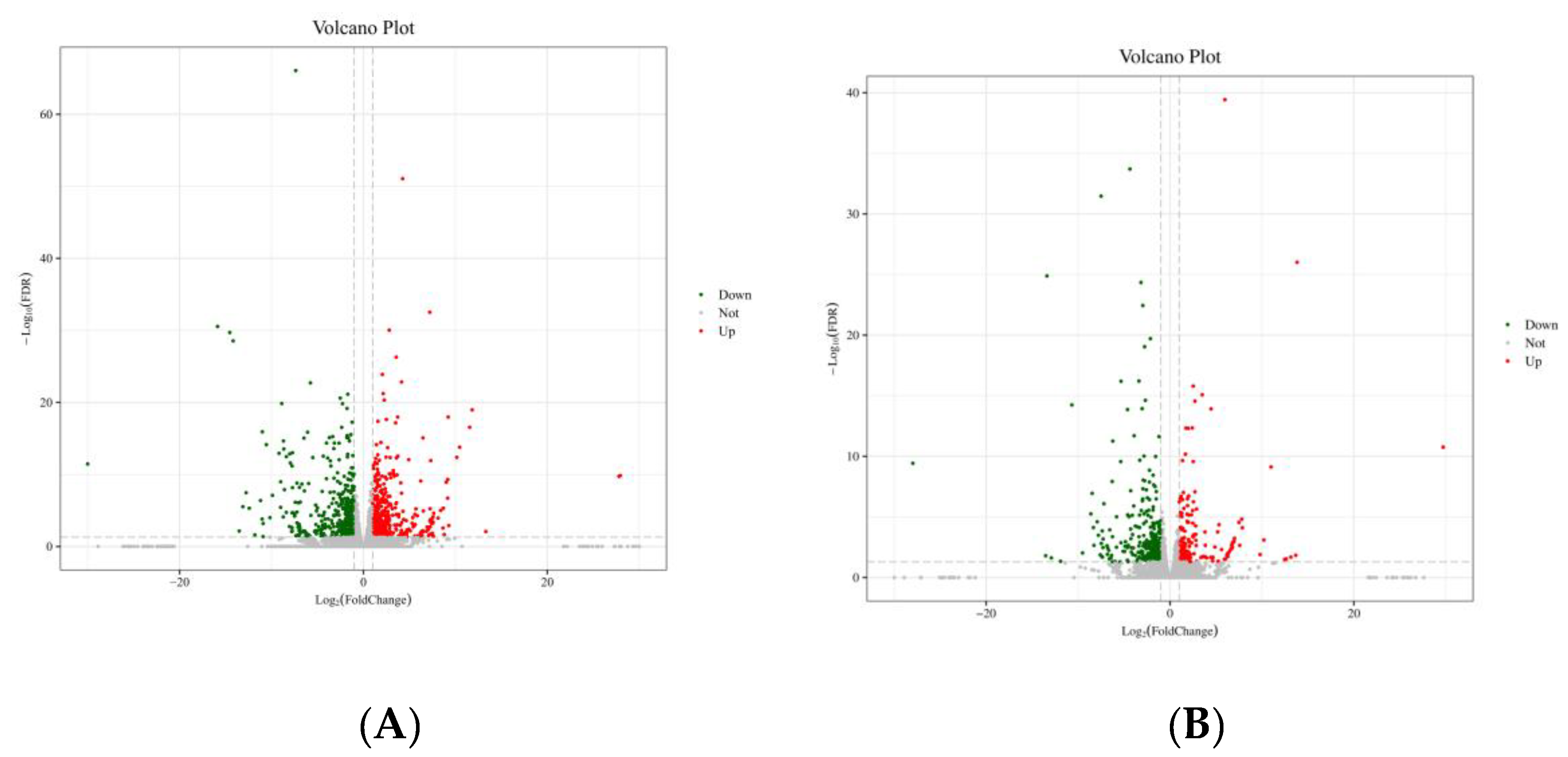
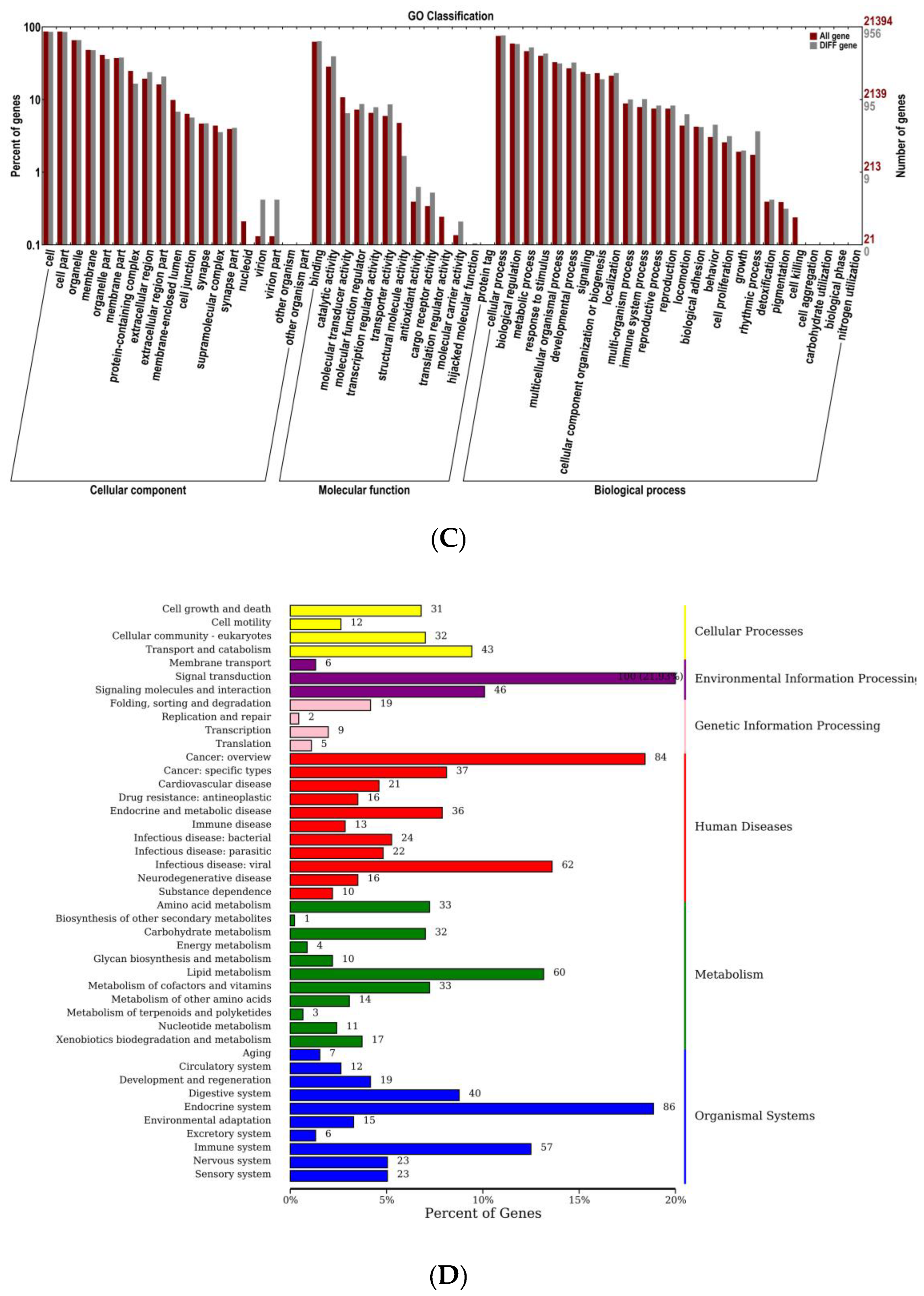
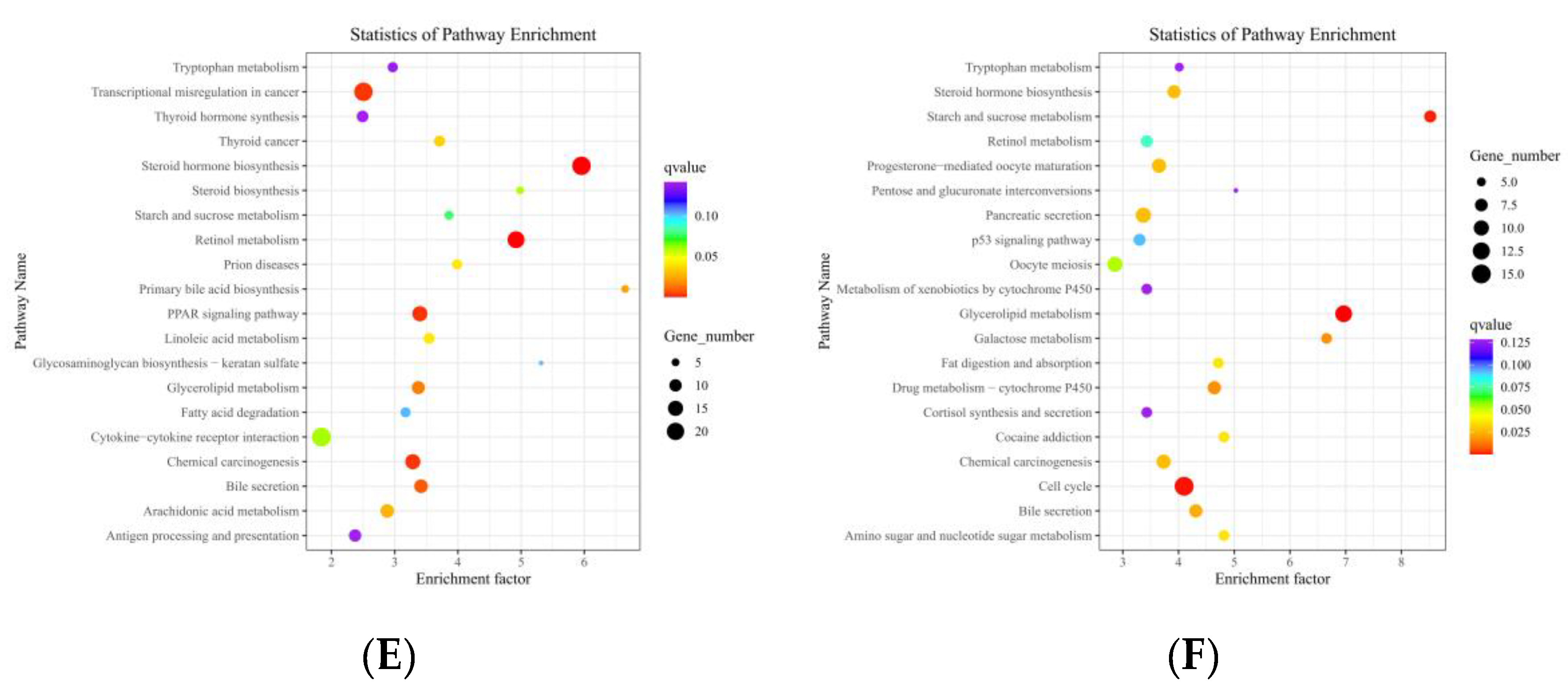
3.7. Transcriptome Sequencing
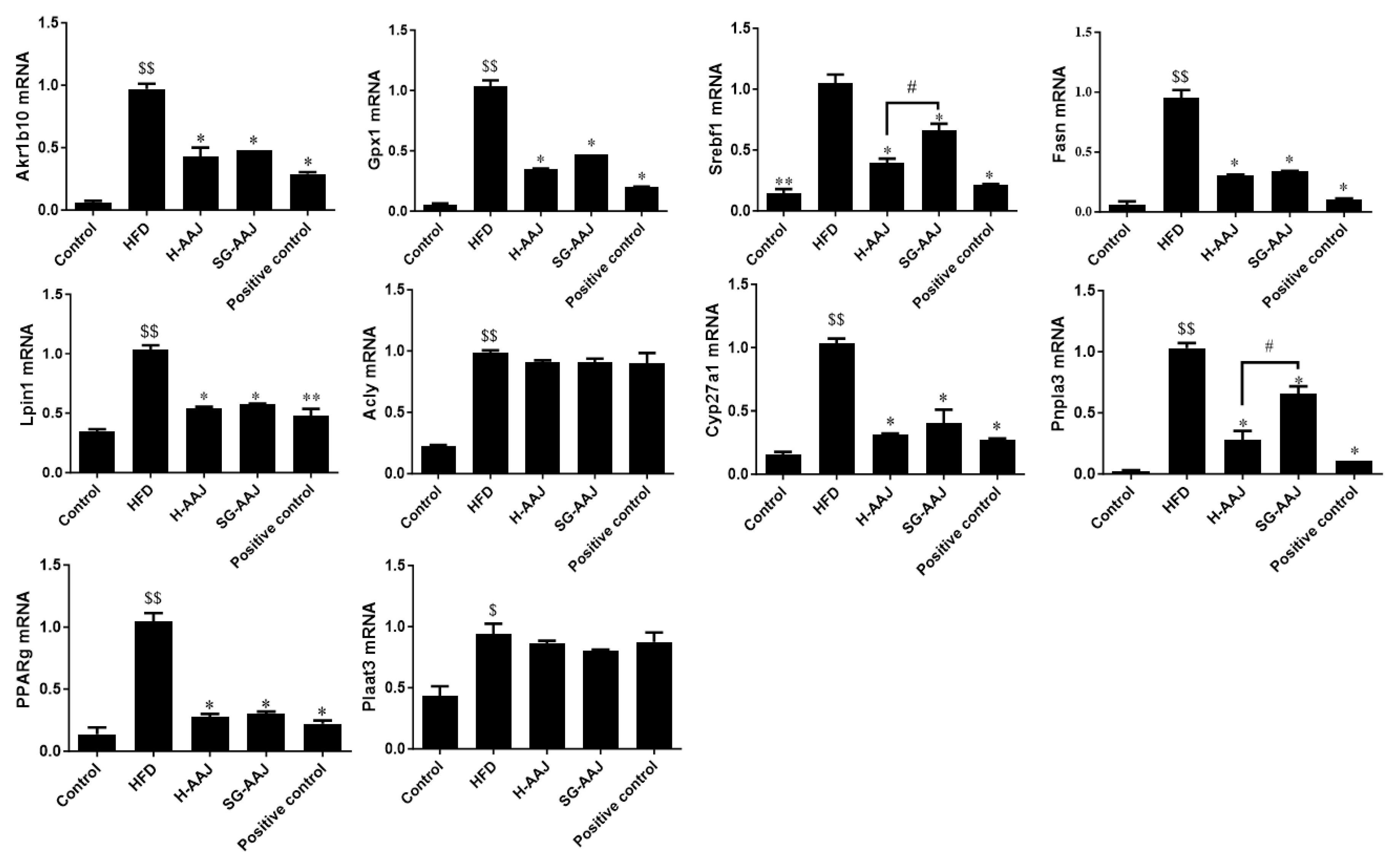
3.8. Effects of AAJ on Lipid Metabolism-Related Gene Transcriptions and Protein Expressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, T.; Zhao, W.; Xie, B.; Liu, H. Effects of Auricularia auricula and its polysaccharide on diet-induced hyperlipidemia rats by modulating gut microbiota. Foods Funct. 2020, 72, 104038. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Data: Raised Cholesterol. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3236 (accessed on 10 July 2023).
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Arnoldi, A. Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: Focus on dietary proteins. Atherosclerosis 2009, 203, 8–17. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Liu, S.; Zhang, B.; Hu, Y.; Ma, H.; Wang, S. Green tea leaf powder prevents dyslipidemia in high-fat diet-fed mice by modulating gut microbiota. Food Nutr. Res. 2020, 64, 3672. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee pollen: Current status and therapeutic potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Mensink, R.P.; Plat, J. Plant sterols and stanols in the treatment of dyslipidemia: New insights into targets and mechanisms related to cardiovascular risk. Curr. Pharm. Des. 2011, 17, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Javid, A.Z.; Mohammadi, F.; Shirbeigi, E. Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: A randomized controlled clinical trial. J. Res. Med. Sci. 2014, 19, 31–536. [Google Scholar]
- Diane, A.; Borthwick, F.; Wu, S.; Lee, J.; Brown, P.N.; Dickinson, T.A.; Croft, K.D.; Vine, D.F.; Proctor, S.D. Hypolipidemic and cardioprotective benefits of a novel fireberry hawthorn fruit extract in the JCR:LA-cp rodent model of dyslipidemia and cardiac dysfunction. Food Funct. 2016, 7, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Hosseinzadeh, H. A review on the effects of Allium sativum (Garlic) in metabolic syndrome. J. Endocrinol. Investig. 2015, 38, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ma, R.; Li, S.; Fei, Y.; Lei, J.; Li, R.; Pan, Y.; Liu, S.; Wang, L. Dietary supplementation of Auricularia auricula-judae polysaccharides alleviate nutritional obesity in mice via regulating inflammatory response and lipid metabolism. Foods 2022, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Ganesan, K.; Xu, B. Insights into health-promoting effects of Jew’s ear (Auricularia auricula-judae). Trends Food Sci. Technol. 2021, 114, 552–569. [Google Scholar] [CrossRef]
- Liu, E.; Ji, Y.; Zhang, F.; Liu, B.; Meng, X. Review on Auricularia auricula-judae as a functional food: Growth, chemical composition, and biological activities. J. Agric. Food Chem. 2021, 69, 1739–1750. [Google Scholar] [CrossRef]
- Li, G.; Guo, W.; Gao, X.; Wang, Y.; Sun, S. Effect of superfine grinding on physicochemical and antioxidant properties of soybean residue powder. Food Sci. Nutr. 2020, 8, 1208–1214. [Google Scholar] [CrossRef]
- Gong, P.; Huang, Z.; Guo, Y.; Wang, X.; Yue, S.; Yang, W.; Chen, F.; Chang, X.; Chen, L. The effect of superfine grinding on physicochemical properties of three kinds of mushroom powder. J. Food Sci. 2022, 87, 3528–3541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, F.; Liu, R.; Tang, X.; Zhang, Q.; Zhang, Z. Effects of superfine grinding on physicochemical and antioxidant properties of Lycium barbarum polysaccharides. LWT Food Sci. Technol. 2014, 58, 594–601. [Google Scholar] [CrossRef]
- Tang, C.H.; Wang, X.S.; Yang, X.Q. Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chem. 2009, 114, 1484–1490. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Aleem, S.; Khan, M.R.; Ashraf, S.; Busquets, R. Quantitative estimation of protein in sprouts of Vigna radiate (Mung Beans), Lens culinaris (Lentils), and Cicer arietinum (Chickpeas) by Kjeldahl and Lowry methods. Molecules. 2022, 27, 814. [Google Scholar] [CrossRef] [PubMed]
- Merten, D.; Erman, L.; Marabelli, G.P.; Leners, B.; Ney, Y.; Nasim, M.J.; Jacob, C.; Tchoumtchoua, J.; Cajot, S.; Bohn, T. Potential health effects of brewers’ spent grain as a functional food ingredient assessed by markers of oxidative stress and inflammation following gastro-intestinal digestion and in a cell model of the small intestine. Food Funct. 2022, 13, 5327–5342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Niu, L.L.; Yang, X.H.; Li, L. Analysis of water-soluble polysaccharides in an edible medicinal plant Epimedium: Method development, validation, and application. J. AOAC Int. 2014, 97, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.M.; Xiang, X.S.; Li, Y.; Zhao, J.P.; Huang, Y.; Di, S.S.; Zhuo, Q.; Nie, H.G. Analysis of metabolite distribution in rat liver of high-fat model by mass spectrometry imaging. Metabolites 2023, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. 2006, 9, 281–291. [Google Scholar]
- Ren, Q.; Liu, X.Q.; Zhou, X.W.; Zhou, X.; Fang, G.; Wang, B.; Wang, Y.P.; Peng, D.H.; Li, X.T. Effects of Huatan Jiangzhuo decoction on diet-induced hyperlipidemia and gene expressions in rats. Chin. J. Nat. Med. 2021, 19, 100–111. [Google Scholar] [CrossRef]
- Khan, T.J.; Kuerban, A.; Razvi, S.S.; Mehanna, M.G.; Khan, K.A.; Almulaiky, Y.Q.; Faidallah, H.M. In vivo evaluation of hypolipidemic and antioxidative effect of ‘Ajwa’ (Phoenix dactylifera L.) date seed-extract in high-fat diet-induced hyperlipidemic rat model. Biomed. Pharmacother. 2018, 107, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, F.; Wang, Y.; Ma, X.; Zhao, M.; Zhao, C. Metabonomics study of the therapeutic mechanism of Gynostemma pentaphyllum and atorvastatin for hyperlipidemia in rats. PLoS ONE 2013, 8, e78731. [Google Scholar] [CrossRef]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.P.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar] [PubMed]
- Steinberg, H.O.; Bayazeed, B.; Hook, G.; Johnson, A.; Cronin, J.; Baron, A.D. Endothelial dysfunction is associated with cholesterol levels in the high normal range in humans. Circulation 1997, 96, 3287–3293. [Google Scholar] [CrossRef]
- Lv, X.C.; Guo, W.L.; Lia, L.; Yu, X.D.; Liu, B. Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats. J. Funct. Foods. 2019, 57, 48–58. [Google Scholar] [CrossRef]
- Mashek, D.G. Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD. Mol. Metab. 2021, 50, 101115. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, K.L.; Ravussin, E. Brown adipose tissue: An update on recent findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.S.; Seol, D.J.; Cho, I.J.; Ku, S.K.; Choi, J.S.; Lee, H.J. Antiobesity and fatty liver-preventing activities of Lonicera caerulea in high-fat diet-fed mice. Int. J. Mol. Med. 2018, 42, 3047–3064. [Google Scholar]
- Fujita, H.; Fujishima, H.; Koshimura, J.; Hosoba, M.; Yoshioka, N.; Shimotomai, T.; Morii, T.; Narita, T.; Kakei, M.; Ito, S. Effects of antidiabetic treatment with metformin and insulin on serum and adipose tissue adiponectin levels in db/db mice. Endocr. J. 2005, 52, 427–433. [Google Scholar]
- Kang, S.J.; Lee, J.E.; Lee, E.K.; Jung, D.H.; Song, C.H.; Park, S.J.; Kang, S.J.; Lee, J.E.; Lee, E.K.; Jung, D.H.; et al. Fermentation with Aquilariae lignum enhances the anti-diabetic activity of green tea in type II diabetic db/db mouse. Nutrients 2014, 6, 3536–3571. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, Q.Z.; Wang, Y.R.; Fu, L.N.; Han, J.S.; Zhang, J.; Wang, B.M. Astragaloside IV improves high-fat diet-induced hepatic steatosis in nonalcoholic fatty liver disease rats by regulating inflammatory factors level via TLR4/NF-κB signaling pathway. Front. Pharmacol. 2021, 11, 605064. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yan, R.; Zu, X.; Cheng, J.M.; Rao, K.; Liao, D.F.; Cao, D. Aldo-keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl-CoA carboxylase-alpha in breast cancer cells. J. Biol. Chem. 2008, 283, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Grim, J.M.; Hyndman, K.A.; Kriska, T.; Girotti, A.W.; Crockett, E.L. Relationship between oxidizable fatty acid content and level of antioxidant glutathione peroxidases in marine fish. J. Exp. Biol. 2011, 214 Pt 22, 3751–3759. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea-Alonso, M.T.; Ericsson, J. SREBP in signal transduction: Cholesterol metabolism and beyond. Curr. Opin. Cell Biol. 2007, 19, 215–222. [Google Scholar] [CrossRef]
- Flavin, R.; Peluso, S.; Nguyen, P.L.; Loda, M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010, 6, 551–562. [Google Scholar] [CrossRef]
- Jiang, L.; Bi, Z.; Zhou, J. The role of lipin-1 in the pathogenesis of alcoholic fatty liver. Alcohol Alcohol. 2015, 50, 146–151. [Google Scholar]
- Zaidi, N.; Royaux, I.; Swinnen, J.V.; Smans, K. ATP citrate lyase knockdown induces growth arrest and apoptosis through different cell- and environment-dependent mechanisms. Mol. Cancer Ther. 2012, 11, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Zurkinden, L.; Sviridov, D.; Vogt, B.; Escher, G. Downregulation of Cyp7a1 by cholic acid and chenodeoxycholic acid in Cyp27a1/ApoE double knockout mice: Differential cardiovascular outcome. Front. Endocrinol. 2020, 11, 586980. [Google Scholar] [CrossRef] [PubMed]
- Basu Ray, S. PNPLA3-I148M: A problem of plenty in non-alcoholic fatty liver disease. Adipocyte 2019, 8, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Tsuboi, K.; Ueda, N. An involvement of phospholipase A/acyltransferase family proteins in peroxisome regulation and plasmalogen metabolism. FEBS Lett. 2017, 591, 2745–2760. [Google Scholar] [CrossRef] [PubMed]


| Gene Symbol | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Length (bps) |
|---|---|---|---|
| GAPDH | 5′-CCTCTGACTTCAACAGCGACAC-3′ | 5′-CTGTTGCTGTAGCCAAATTCGT-3′ | 121 |
| Akr1b10 | 5′-CAAGTCTGTGACACCCTCCC-3′ | 5′-GCCCTCCAGTTCCTGTTGAA-3′ | 108 |
| Gpx1 | 5′-CCGGGACTACACCGAAATGA-3′ | 5′-TGCCATTCTCCTGATGTCCG-3′ | 104 |
| Srebf1 | 5′-CGTTTCTTCGTGGATGGGGA-3′ | 5′-ACAGTTCAATGCTCGCTCCA-3′ | 141 |
| Fasn | 5′-GCTTGGTGAACTGTCTCCGA-3′ | 5′-GTGAGATGTGCTGCTGAGGT-3′ | 83 |
| Acly | 5′-CAGTGGGAGCACATCGACAC-3′ | 5′-ACTCTTTCCTTGGGGCATGG-3′ | 115 |
| Cyp27a1 | 5′-GCTCCAGGCGCTGAACAAG-3′ | 5′-CACTGCTCCATGCTGTCTCT-3′ | 150 |
| Pnpla3 | 5′-GGGGCTACGCTATGTCTGAG-3′ | 5′-GATCGAGAGGGAGACTGCAC-3′ | 121 |
| Pparg | 5′-TCCCGTTCACAAGAGCTGAC-3′ | 5′-ATAATAAGGCGGGGACGCAG-3′ | 107 |
| Plaat3 | 5′-TGGCTCCCCCAAGTGAAATC-3′ | 5′-GGTACTTGTCCTTCCCAGCC-3′ | 117 |
| Lpin1 | 5′-AGCTCCTCTACTTCTGGCGA-3′ | 5′-TGTGGCGTTTCTTCCTCTCC-3′ | 133 |
| Total Protein (g/100 g) | Dietary Fiber (g/100 g) | Crude Polysaccharides (g/100 g) | Total Flavonoids (mg/100 g) | |
|---|---|---|---|---|
| PD product of AAJ | 0.53 | 1.78 | 0.28 | 410 |
| SG product of AAJ | 0.52 | 1.10 | 0.20 | 8.4 |
| Groups | Control | HFD | SG-AAJ | L-AAJ | M-AAJ | H-AAJ | Positive Control |
|---|---|---|---|---|---|---|---|
| 0 Week | 193.8 ± 11.4 | 192.7 ± 6.5 | 194.3 ± 8.4 | 191.5 ± 8.1 | 190.4 ± 10.1 | 191.9 ± 9.7 | 189.4 ± 6.0 |
| 1 Week | 204.8 ± 12.9 | 214.5 ± 6.7 | 210.7 ± 10.2 | 209.9 ± 9.8 | 206.3 ± 9.4 | 210.2 ± 9.8 | 209.4 ± 6.7 |
| 2 Week | 218.3 ± 12.6 | 233.5 ± 8.2 $ | 231.3 ± 10.2 | 230.0 ± 12.4 | 227.1 ± 12.1 | 231.8 ± 8.7 | 231.2 ± 7.6 |
| 3 Week | 229.6 ± 12.8 | 254.8 ± 9.6 $ | 249.3 ± 11.7 | 253.5 ± 13.4 | 248.2 ± 14.3 | 255.7 ± 10.4 | 250.2 ± 10.2 |
| 4 Week | 243.9 ± 14.0 | 281.0 ± 11.3 $$ | 266.6 ± 12.8 * | 273.5 ± 16.3 | 263.2 ± 15.8 * | 268.4 ± 11.0 * | 263.4 ± 10.1 ** |
| 5 Week | 254.7 ± 15.7 | 304.6 ± 11.9 $$ | 280.9 ± 14.1 ** | 292.1 ± 17.1 | 275.7 ± 14.9 ** | 281.3 ± 12.3 ** | 274.6 ± 10.8 ** |
| 6 Week | 267.6 ± 17.1 | 322.3 ± 13.8 $$ | 298.2 ± 15.6 ** | 307.7 ± 18.0 | 291.4 ± 15.3 ** | 294.7 ± 13.3 ** | 286.7 ± 13.1 ** |
| 7 Week | 277.5 ± 17.8 | 342.3 ± 15.2 $$ | 310.6 ± 16.5 ** | 325.2 ± 18.2 * | 306.3 ± 17.3 ** | 307.9 ± 13.8 ** | 300.8 ± 14.4 ** |
| 8 Week | 286.3 ± 18.6 | 360.5 ± 16.4 $$ | 324.9 ± 18.1 ** | 339.8 ± 18.9 * | 317.6 ± 17.5 ** | 315.8 ± 16.1 ** | 310.1 ± 17.2 ** |
| 9 Week | 296.6 ± 20.2 | 381.9 ± 20.0 $$ | 338.7 ± 19.6 ** | 352.2 ± 23.1 ** | 331.6 ± 20.7 ** | 327.8 ± 17.6 ** | 320.6 ± 17.4 ** |
| 10 Week | 308.7 ± 21.2 | 400.2 ± 22.0 $$ | 351.9 ± 21.2 ** | 368.1 ± 22.5 ** | 344.1 ± 21.1 ** | 338.6 ± 19.4 ** | 328.7 ± 17.6 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Chen, K.; Du, M.; Zhao, W.; Chen, Y.; Cheng, J.; Zhao, L.; Liu, J.; Long, J. Auricularia auricula-judae Attenuates the Progression of Metabolic Syndrome in High-Fat Diet-Induced Obese Rats: Enzymatic Pre-Digestion Technology Is Superior to Superfine Grinding Method. Foods 2024, 13, 406. https://doi.org/10.3390/foods13030406
Jia Y, Chen K, Du M, Zhao W, Chen Y, Cheng J, Zhao L, Liu J, Long J. Auricularia auricula-judae Attenuates the Progression of Metabolic Syndrome in High-Fat Diet-Induced Obese Rats: Enzymatic Pre-Digestion Technology Is Superior to Superfine Grinding Method. Foods. 2024; 13(3):406. https://doi.org/10.3390/foods13030406
Chicago/Turabian StyleJia, Ying, Kun Chen, Menggang Du, Wanzhou Zhao, Yong Chen, Junhong Cheng, Lin Zhao, Jiankang Liu, and Jiangang Long. 2024. "Auricularia auricula-judae Attenuates the Progression of Metabolic Syndrome in High-Fat Diet-Induced Obese Rats: Enzymatic Pre-Digestion Technology Is Superior to Superfine Grinding Method" Foods 13, no. 3: 406. https://doi.org/10.3390/foods13030406
APA StyleJia, Y., Chen, K., Du, M., Zhao, W., Chen, Y., Cheng, J., Zhao, L., Liu, J., & Long, J. (2024). Auricularia auricula-judae Attenuates the Progression of Metabolic Syndrome in High-Fat Diet-Induced Obese Rats: Enzymatic Pre-Digestion Technology Is Superior to Superfine Grinding Method. Foods, 13(3), 406. https://doi.org/10.3390/foods13030406






