1. Introduction
Figs (
Ficus carica L.) belong to the Moraceae family and are flavorful infructescences that develop on fig trees [
1]. Fig fruits are consumed worldwide, but more than 70% of global production is concentrated in Mediterranean countries [
2]. Italy is one of the major fig-producing countries, with production primarily concentrated in the southern regions, such as Calabria, where large cultivation areas exist and there is a very rich and diversified autochthonous fig germplasm in terms of genetic aspects [
3], tree morphological and functional characteristics [
4], phenological traits [
5], and fruit pomological and qualitative characteristics [
6].
Based on their bloom and maturity stage, Ficus fruits are classified as brebas and/or figs. Brebas are dormant figs that do not begin their development until the following spring. They are larger and juicier and are normally eaten fresh, while figs appear from the bloom of the year. These are smaller and can be eaten both fresh and dried [
7] or used to make jam [
8]. Ripe edible figs have a thick skin and a sweet flesh containing small seeds, which are usually unnoticeable but may provide a slight crunch when chewed [
9]. A fresh fig can be eaten either with or without its skin [
10]. The skin color of fig varieties can vary from green to black-violet, depending on the specific pigment compounds present [
11]. Figs are rich in essential nutrients, including dietary fibers, amino acids, vitamins, minerals, sugars, organic acids, carotenoids, and antioxidant polyphenols (primarily flavonoids and phenolic acids). Due to these beneficial properties, figs have been used in traditional medicine for centuries, particularly for their health-promoting effects in the treatment of gastrointestinal, respiratory, inflammatory, metabolic, and cardiovascular disorders; for this reason, figs are considered a good source of bioactive compounds [
12,
13,
14]. Furthermore, figs’ nutritional importance extends beyond basic nutrients.
Scientific research has proven that a diet based on fruit and vegetables has beneficial effects on human health. In particular, the intake of vegetable foods with bright colors is very important for the prevention of several diseases, for example, atherosclerosis, cardiac disorders, and cancer, because they are rich in bioactive compounds such as polyphenols and carotenoids [
15]. Fig fruits have a high antioxidant capacity and provide many benefits for human health, such as anti-inflammatory and antidiabetic properties and antibacterial activities [
16,
17]. The bioactive profile of fig fruits depends on many factors and is characterized by a wide range of phenolics that vary depending on cultivar and varietal type and on other factors such as soil and fruit maturity [
10,
18].
Figs contain two primary types of phytochemicals: polyphenols and carotenoids [
12]. Among phenolic compounds, phenolic acids and flavonoids (flavanols, flavonols, and anthocyanidins) have been identified, and, in addition, different agronomic parameters, such as fruit variety and harvest season, can influence the antioxidant profiles of the fruits [
17]. Quercetin rutinoside is one of the major individual phenolics [
12,
18].
An additional differentiation for fig trees is the partition into “biferous” trees, which produce two crops of figs per year—the first crop during the spring season (late May and the end of June) and the second crop in the summer season (mid-July and early September) [
19]—and “uniferous” trees, which produce a single crop of figs [
7].
The idea for this work stems from a previous study conducted by Mafrica et al. [
6], where forty fig accessions were analyzed for qualitative parameters. Of these forty, twenty-nine accessions were selected for the evaluation of antioxidant parameters. In particular, twenty-nine biferous and uniferous accessions of fig fruits (light- to dark-skinned) were studied for the evaluation of total polyphenols, total flavonoids, DPPH, ABTS, and individual phenolic compounds. To achieve these research objectives, the following methodology was employed.
3. Results and Discussion
Based on the determination of the color parameters (L*, a*, b*, and chroma) of the figs’ skins, the twenty-nine fig accessions were divided into two main groups (
Table 3): light-skinned (LS) and dark-skinned (DS) figs. The skin color of brebas is one of the most important factors that influences consumer acceptability, as it is useful for assessing the status of ripening in fruits [
24,
25]. The obtained results revealed in this work showed great statistical variability (
p < 0.01) in the color of breba fruits, from light green to dark purple. Considering the high variability of the samples, we subdivided the samples into subgroups: two for LS, namely, yellow-green-skinned (YS) and light-green-skinned (SLG); and two for DS, namely, purple-skinned (PS) and dark-purple-skinned (DPS). For LS, the chromatic parameters ranged between L* 35.93 and 62.62, a* −3.85 and 12.05, b* 20.40 and 47.74, and C* 22.24 and 47.92, while for DS, they ranged between L* 21.48 and 37.48, a* 2.09 and 16.00, b* −0.36 and 9.66, and C* 2.31 and 19.12. The results obtained in this work fall within the ranges reported by other authors, such as Ercisli et al. [
20]. Chroma is one of the most important parameters used to describe the quality of food and has a considerable influence on acceptance by consumers [
26].
The chemical and morphological characteristics of breba fruits were reported in another of our papers [
5,
6].
The phytochemical composition of fig fruits is often affected by the cultivar, but also by other factors, such as the color, the part of the fruit, and the level of maturity [
27]. The antioxidant compounds and the phenolic profiles in particular that characterize the Breba accessions are very important for determining the quality of the final products in order to promote their consumption as fresh products, in addition to being useful for nutritional and health purposes. The phenolic profile mainly affects parameters such as the flavor and odor of the fruit [
7]. Breba fruits contain high amounts of polyphenols and flavonoids and at the same time exhibit higher antioxidant activity. Moreover, as reported by Viuda-Martos et al. [
28], the total amount of phenolic compounds (phenolic acids, flavonoids, and anthocyanins) present in fig fruits could be considered an important parameter for revealing their antioxidant capacity with the aim of promoting the fruits as a natural source of antioxidants.
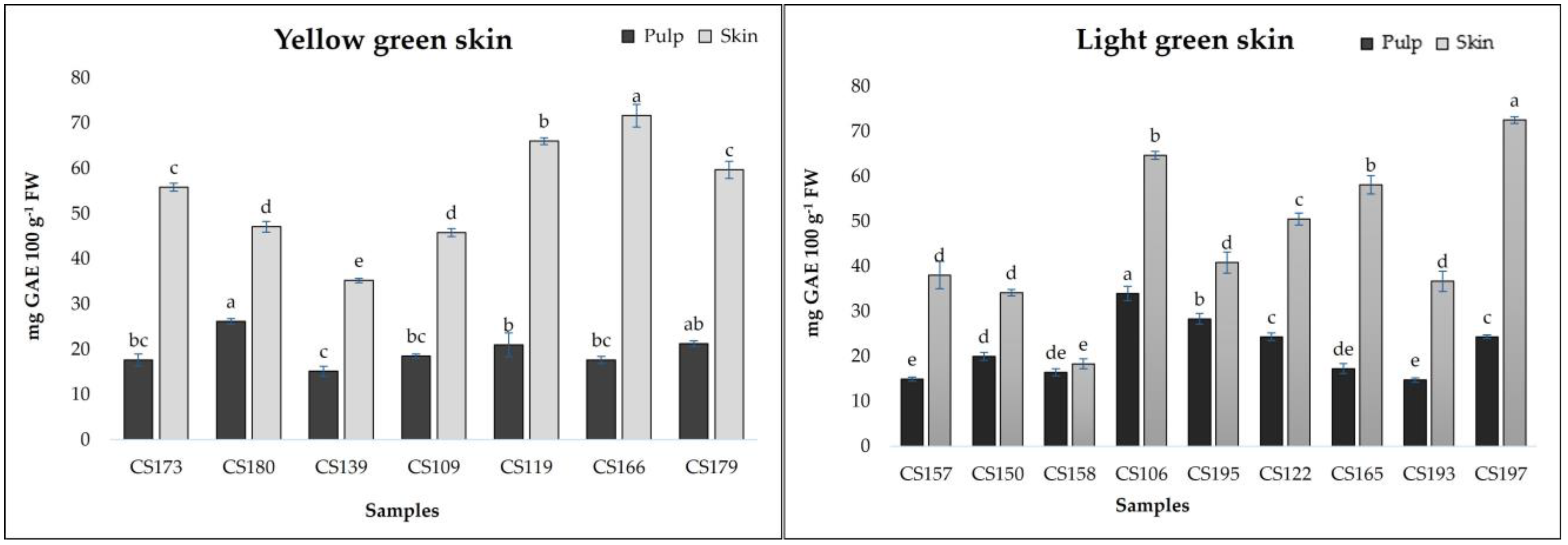
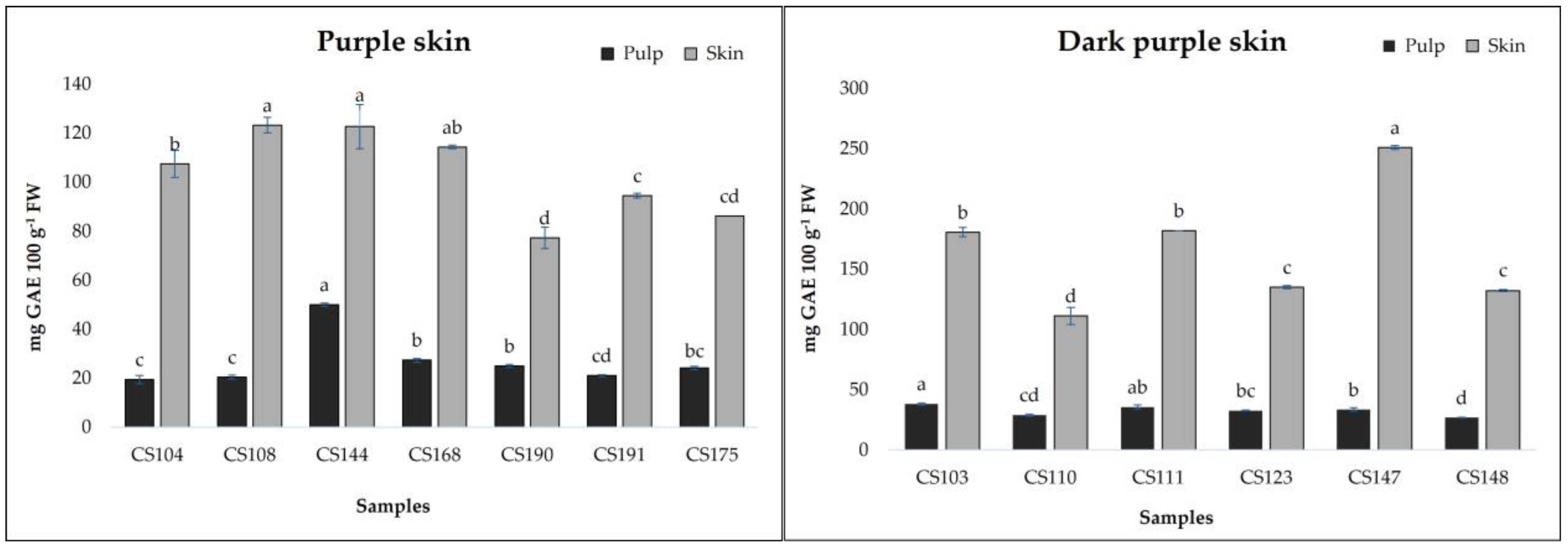
Figure 2 and
Figure 3 show the results related to total phenolic contents measured in breba fruits. TPCs ranged from 14.72 to 49.92 mg GAE 100 g
−1 FW (pulp) and from 18.30 to 251.81 mg GAE 100 g
−1 FW (skin). The obtained results are comparable to those obtained by Vallejo et al. [
14] for the skin of fresh figs (19.1 mg/100 g for to 140.2 mg/100 g FW) and by Ercisli et al. [
20] (237 mg/100 g GAE FW). The analyzed fig samples revealed statistical differences (
p < 0.01) not only among the different typologies of breba fruit (LS and DS), but also within the subgroups (YS, LGS, PS, and DPS). As can be seen in
Figure 2 and
Figure 3, it is clear that the skin showed the highest TPCs, with a major concentration in the sample
CS147 (250.81 mg GAE 100 g
−1 FW); however, almost all the “bifera nera” samples exhibited high TPCs in their skin (>100 mg GAE 100 g
−1 FW). On the other hand, even the pulp showed good TPC values, ranging from 14.72 (
CS193) to 49.92 in
CS144, with higher results for “bifera nera”. Also, other authors found that fig fruit with dark skin showed higher TPC values [
20,
29,
30]. Building upon these findings, we next examined the individual phenolic compounds.
Cluster analysis highlighted the variation among the fig samples in terms of total phenolic contents. Indeed,
Figure 4 shows the obtained dendrogram, in which the fig fruits are divided into two groups with similar total phenolic contents.
The cluster analysis (
Figure 4) classified the fig accessions into two main groups (Group 1 and Group 2) based on total polyphenol content (TPC) and other distinguishing characteristics, such as skin color. Each group was further divided into subgroups, reflecting finer differences among the accessions.
Group 1 comprised a total of 19 accessions characterized by a lower TPC. This group predominantly included figs with lighter skin tones and featured both uniferous and biferous types. It was further divided into two subgroups.
Subgroup 1a included nine accessions with the lowest TPC values recorded in the entire dataset.
Subgroup 1b comprised 10 accessions with slightly higher TPC levels than those in Subgroup 1a but whose levels were still lower than those in Group 2. This subgroup exhibited greater diversity in skin color, ranging from light to intermediate tones, and included a mix of uniferous and biferous types.
Group 2 consisted of 10 accessions characterized by higher TPCs. This group was distinctly marked by a predominance of dark-skinned figs, a trait visually and biochemically associated with a higher polyphenol content. This group was also subdivided into two subgroups:
Subgroup 2a consisted of two specific accessions (Bifera Nera CS103 and CS111), which exhibited the highest TPC values among all the analyzed accessions.
Subgroup 2b included eight accessions, primarily of the Bifera Nera type, with a single exception (Unifera Nera CS168). These accessions exhibited high TPC levels but lower levels than those observed in Subgroup 2a. The limited variability within this subgroup reflects small differences in TPC content that may be attributed to genetic or environmental factors.
The cluster analysis results highlight a clear separation of fig accessions based on polyphenol content and morphological/visual traits, such as skin color and cropping type (uniferous vs. biferous).
Total flavonoid contents (TFCs) were measured spectrophotometrically, and the results are reported in
Table 4. Flavonoids represent a subgroup of phenolic compounds in breba fruit accessions, and their presence was confirmed with the TFC/TPC ratio values. In general, the highest TFC/TPC ratios were shown in the skin of the samples with values of about 0.17–0.39 in LS and 0.22–0.45 in DS. Significant statistical differences (
p < 0.01) were found among all samples, between LS and DS and between pulp and skin. Most flavonoid compounds are present in the fruit skin, the values for which ranged between 5.81 and 26.25 in LS and between 20.08 and 65.26 mg CE 100 g
−1 FW in DS accessions. Thus, the highest TFC results were shown in the subgroup of DPS (
CS147 sample). Instead, the highest TFC content in fig pulp was evidenced in the samples
CS144 (15.30 mg CE 100 g
−1 FW) and
CS179 (12.22 mg CE 100 g
−1 FW). The TFC data obtained in this study are similar and sometimes higher than the data reported by other authors [
30,
31,
32].
The antioxidant activity of different breba fruits (pulp and skin) was quantified by two methods: DPPH and ABTS assays (
Table 5 and
Table 6), and we found significant differences among the accessions (
p < 0.01).
In agreement with the above data, total antioxidant activity revealed higher values in DS accessions than in LS accessions. Regarding the determinations carried out for the “light-skinned breba accessions”, the results are shown in
Table 5. The DPPH assay results ranged between 2.06% (
CS173) and 10.02% (
CS158) for pulp and from 6% (
CS157) to 17.99% (
CS166) for the skin of brebas. The results obtained for “dark-skinned breba accessions” (DS) are reported in
Table 5. The DPPH assay results ranged from 3.91% (
CS104) to 9.18% (
CS147) in the pulp and from 13.88% (
CS190) to 52.95% (
CS147) in the skin of brebas.
In general, accessions with dark skin revealed higher total antioxidant capacities than those with light skin, and the sample that showed the highest antioxidant capacity in the DPPH assay was
CS147 (Black Biferous), both in the flesh and the skin. The findings of this study support previous reports, confirming that figs with a dark skin exhibit higher antioxidant capacity compared to white figs [
33,
34].
For the ABTS assay, the samples that showed higher results were
CS166 for LS pulp and
CS173 for skin (
Table 5), while for DS,
CS144 showed the highest value for pulp and
CS147 showed the highest value for skin (
Table 6). The latter sample (
CS147) showed higher antioxidant activity in both assays.
Pearson correlation analysis revealed significant relationships between total phenolic content and antioxidant activity assays (DPPH and ABTS), with notable differences between the skin and the pulp of the fig samples.
For the skin, TPC showed a very high correlation with the DPPH assay, with coefficients ranging from r = 0.838 (LGS) to r = 0.947 (DPS). This highlights the skin’s substantial contribution to antioxidant capacity, driven by its phenolic content.
In the pulp, a stronger correlation was observed between TPC and the ABTS assay, with the PS sample showing the highest coefficient.
Correlations between total flavonoid content and antioxidant activity were weaker, with exceptions in DPS pulp (r = 0.760) and LGS skin (0.814), indicating a lesser but still relevant contribution of flavonoids.
The most representative individual phenolic compounds present in the studied breba fruits are shown in
Table 7. A total of five phenolic compounds were detected, which belong to different chemical classes, such as phenolic acids (chlorogenic acid), flavonoids (catechin and epicatechin), and flavonols (quercetin and rutin). These compounds, as reported by other authors, are among the most representative bioactive compounds present in the peel, flesh, and whole fruits of figs [
35]. The identification and quantification of these phenolic compounds through UHPLC analysis confirmed the results obtained from spectrophotometric determinations, providing robust evidence of their presence and distribution in the breba samples. Higher concentrations of individual phenolic compounds were found in the breba fruit samples, with statistical differences among the samples (
p < 0.01). The two most prevalent compounds determined were catechin and rutin, followed by the other compounds, epicatechin, chlorogenic acid, and quercetin. Regarding the LS samples, rutin was the most abundant compound present in the skin samples and ranged between 0.98 and 38.88 mg 100 g
−1 FW, while catechin contents were high both in flesh and in skin (ranging between 1.37 and 35.52 and between 2.60 and 30.77 mg 100 g
−1 FW, respectively). Meanwhile, the DS breba accessions showed the highest contents of catechin in the skin of the fruits, with values that ranged between 54.87 and 597.81 mg 100 g
−1 FW.
Concerning the LS fig fruits, a higher content of catechin was highlighted in the
CS195 sample, prevailing in the pulp (35.52 mg 100 g
−1 FW); moreover, among the light-skinned samples, this variety of fruit is the richest in individual phenolic compounds, while the
CS158 sample showed a lower amount of individual phenolic compounds, both in the pulp and in the skin. Rutin contents were higher in the LS samples compared with the DS samples, while catechin contents were higher in the dark figs, particularly in the skin, with values of 448.37 mg 100 g
−1 FW. The data obtained for the characterization of individual phenolic compounds confirmed the results reported by several other authors, namely, that the skin contains higher concentrations of phenolic compounds compared to the pulp [
17,
36,
37] and that fruit color also influences the concentration and composition of these antioxidant active compounds [
29,
38].