White Tea Aqueous Extract: A Potential Anti-Aging Agent Against High-Fat Diet-Induced Senescence in Drosophila melanogaster
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the WTAE
2.3. Chemical Analysis of the WTAE
2.4. Culture of Drosophila melanogaster
2.5. Lifespan Assay
2.6. Feeding Assay
2.7. Fly Weight Measurement
2.8. Climbing Ability Assay
2.9. In Vivo Antioxidant Activity
2.10. RNA-Seq
2.11. Analysis of Differential Genes and Functional Enrichment
2.12. Quantitative Real-Time PCR
2.13. Statistical Analysis
3. Results and Discussion
3.1. Chemical Compositions of the WTAE
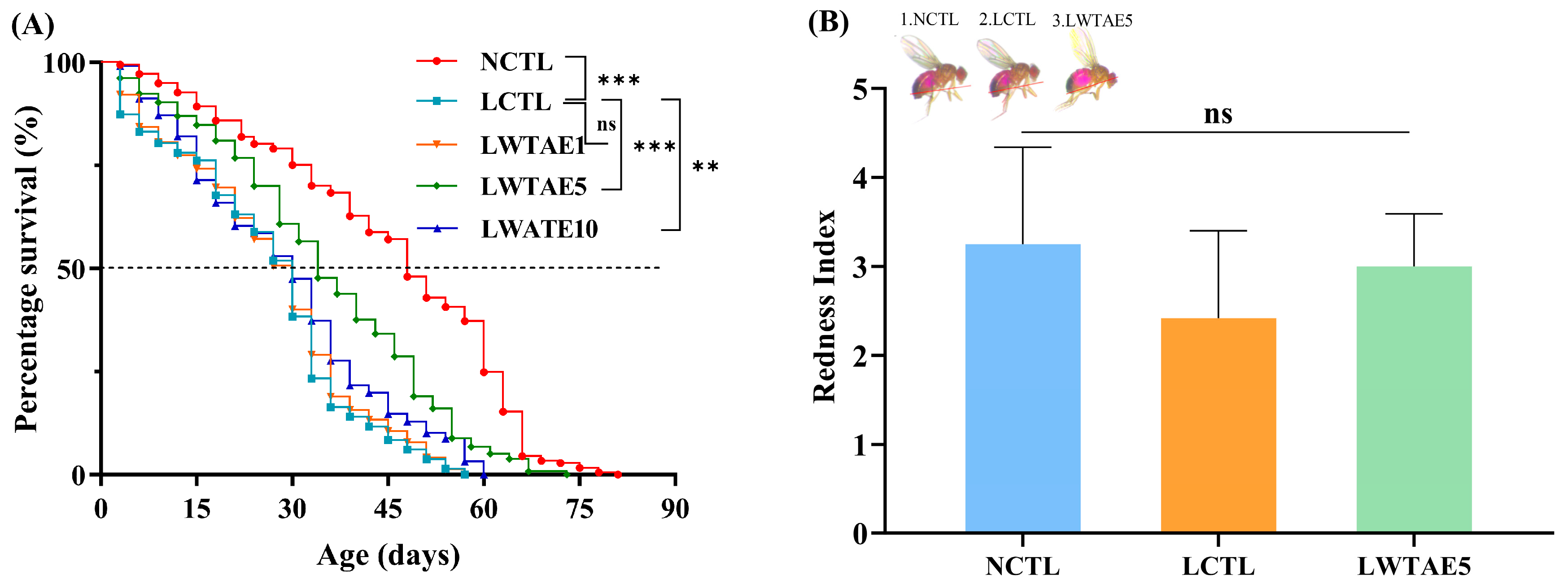
3.2. Effects of the WTAE on the Lifespan of Flies Fed a High-Fat Diet
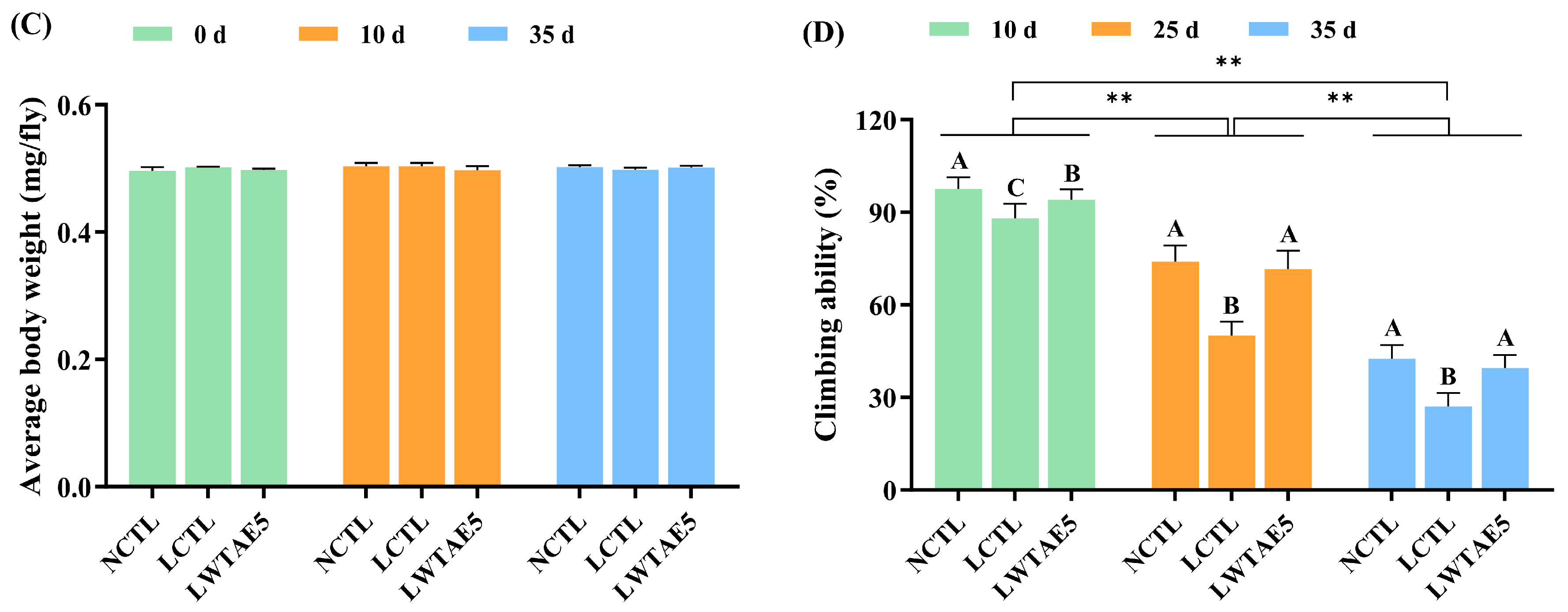
3.3. Effects of the WTAE on Feeding and the Average Body Weight of Flies
3.4. Effects of the WTAE on the Climbing Ability of Flies
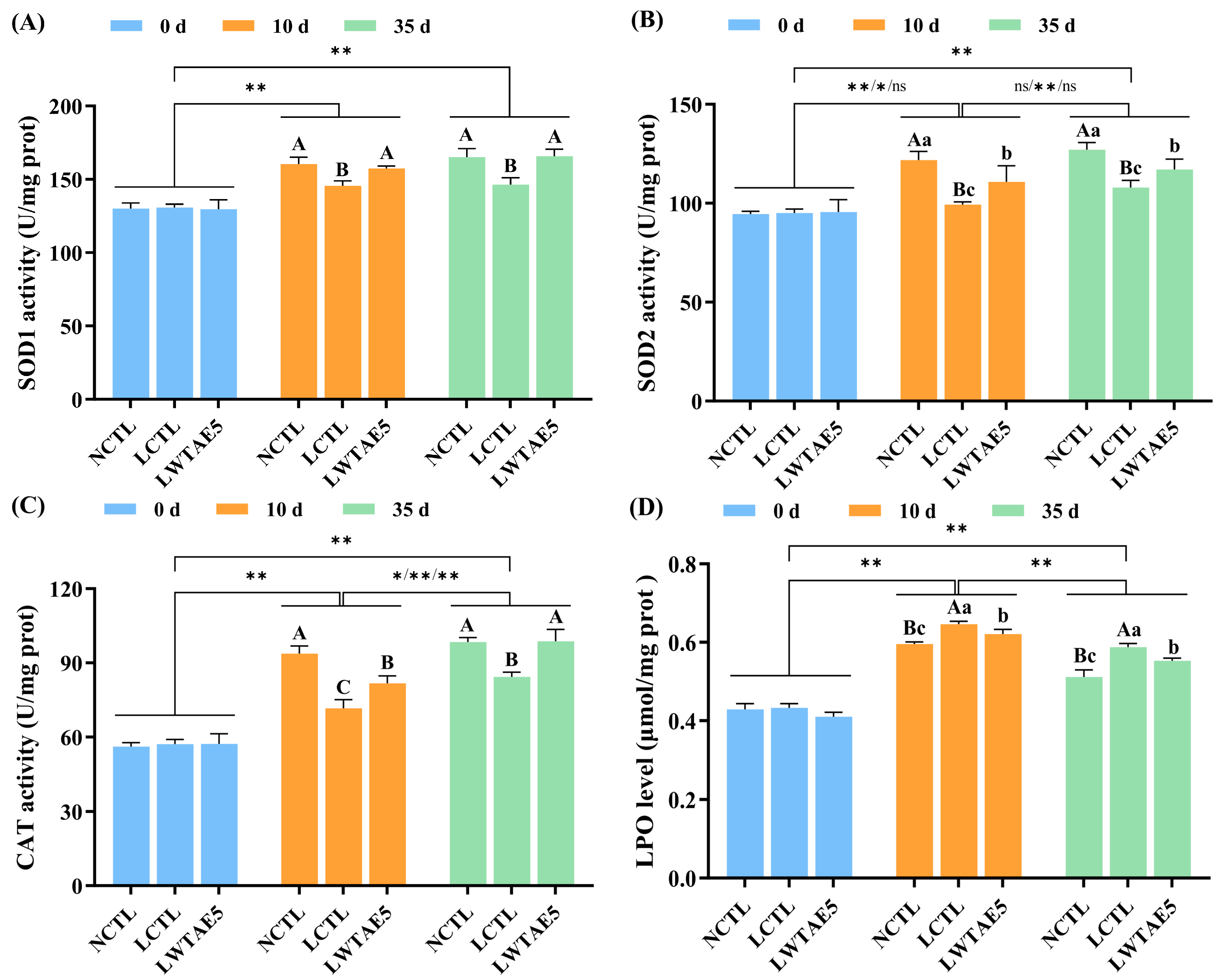
3.5. Effects of the WTAE on Antioxidant Enzyme Activity in Flies
3.6. Effects of the WTAE on LPO Levels in Flies
3.7. Transcriptome Profiles of Fruit Flies on Different Diets
3.8. Differentially Expressed Genes in Response to Different Diets
3.8.1. Attenuated Expression of Genes Related to Fat Accumulation in Flies
3.8.2. Suppressed Expression of Stress Response Genes in Flies
3.8.3. Regulation of Circadian Genes in Flies
3.8.4. Enhanced Expression of Energy Metabolism Genes in Flies
3.8.5. Enhanced Expression of Neuroregulation Genes and Other Genes
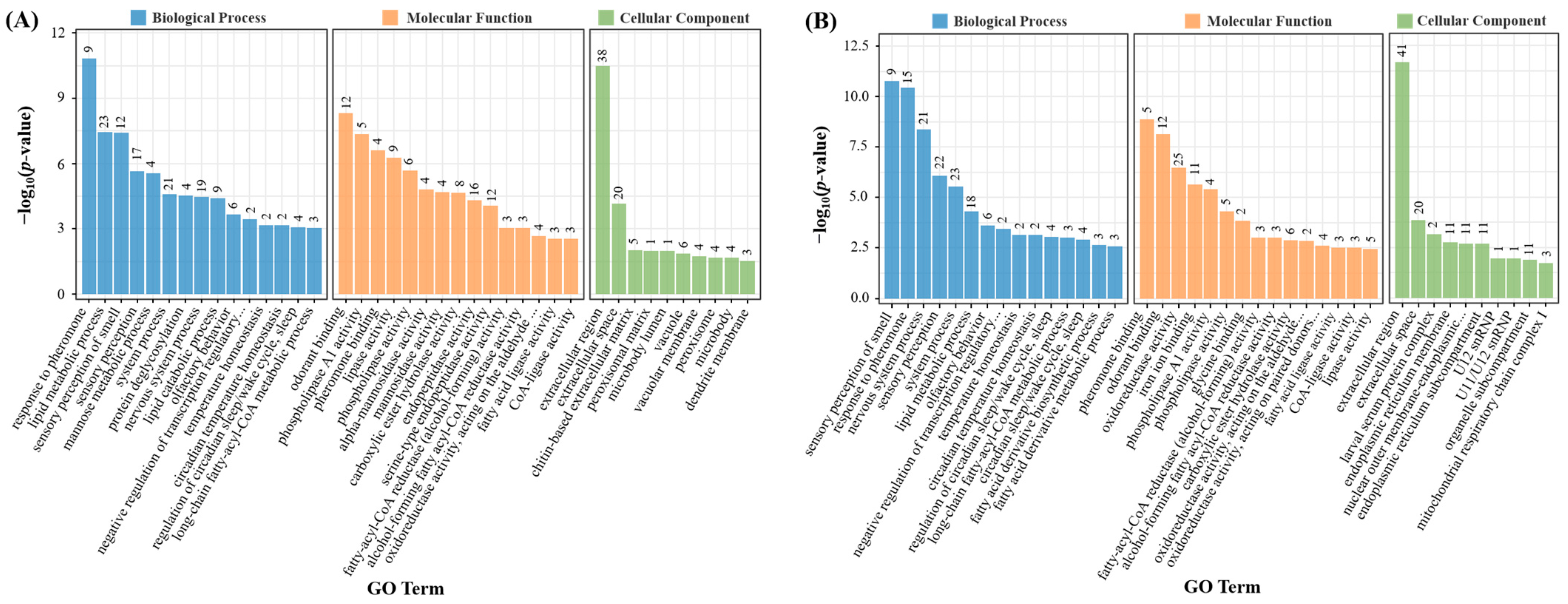
3.9. Enrichment Analysis for Differentially Expressed Genes
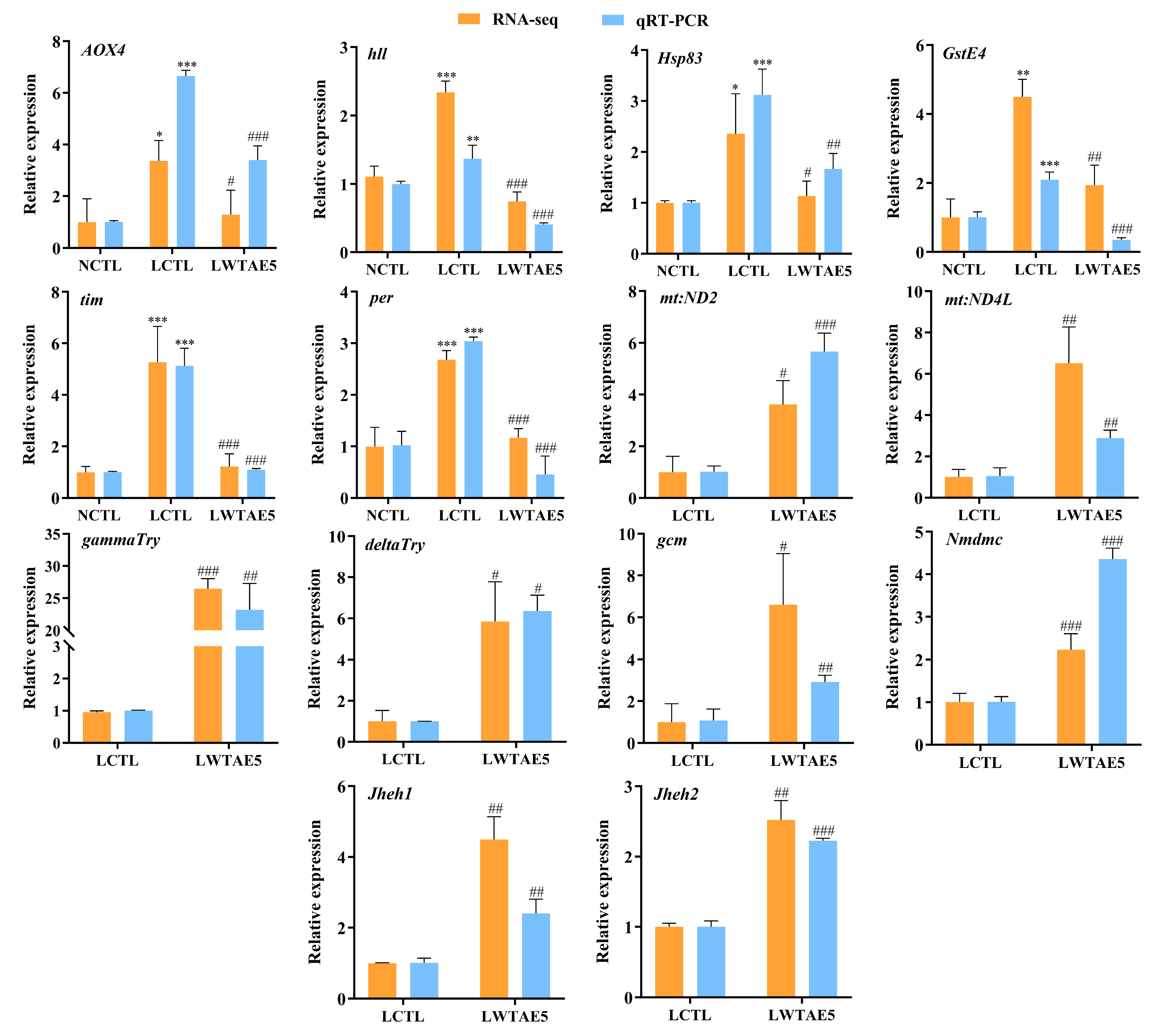
3.10. Validation of DEGs by qRT-PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular senescence: Aging, cancer, and injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Dan, A.; Chen, Y.; Tian, Y.; Wang, S. In vivo anti-aging properties on fat diet-induced high fat Drosophila melanogaster of n-butanol extract from Paecilomyces hepiali. Food Sci. Hum. Wellness 2023, 12, 1204–1211. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y. In vitro and in vivo antioxidant activities of polyphenol extracted from black garlic. Food Sci. Technol. 2017, 37, 681–685. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Zhang, Z.; Farre, J.C.; Li, X.; Wang, R.; Xia, Z.; Subramani, S.; Ma, C. The autophagic degradation of cytosolic pools of peroxisomal proteins by a new selective pathway. Autophagy 2020, 16, 154–166. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Thirumurugan, K. Longevity-promoting efficacies of rutin in high fat diet fed Drosophila melanogaster. Biogerontology 2020, 21, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.A.; Ross, C.N.; O’Brien, M.J. Obesity, metabolism, and aging: A multiscalar approach. Prog. Mol. Biol. Transl. Sci. 2018, 155, 25–42. [Google Scholar] [CrossRef]
- Guo, P.; Wang, P.; Liu, L.; Wang, P.; Lin, G.; Qu, Z.; Yu, Z.; Liu, N. Naringin alleviates glucose-induced aging by reducing fat accumulation and promoting autophagy in Caenorhabditis elegans. Nutrients 2023, 15, 907. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, Y.; Sun, L.; Lai, X.; Li, Q.; Zhang, W.; Xiang, L.; Sun, S.; Cao, F. Six types of tea reduce high-fat-diet-induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct. 2019, 10, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Nogueira, D.; Perez-Burillo, S.; Pastoriza De La Cueva, S.; Rufian-Henares, J.A. Green and white teas as health-promoting foods. Food Funct. 2021, 12, 3799–3819. [Google Scholar] [CrossRef]
- Sohle, J.; Knott, A.; Holtzmann, U.; Siegner, R.; Gronniger, E.; Schepky, A.; Gallinat, S.; Wenck, H.; Stab, F.; Winnefeld, M. White Tea extract induces lipolytic activity and inhibits adipogenesis in human subcutaneous (pre)-adipocytes. Nutr. Metab. 2009, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Staats, S.; Luersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a versatile model organism in food and nutrition research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, Q.; Chen, X.; Tian, Y.; Liu, Z.; Wang, S. Bioactive peptides derived from crimson snapper and in vivo anti-aging effects on fat diet-induced high fat Drosophila melanogaster. Food Funct. 2020, 11, 524–533. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, P.; Su, X.; Zheng, M.; Chi, W.; Lin, S.; Huang, Z.; Qin, S.; Zeng, S. Hsian-tsao (Mesona chinensis Benth.) extract improves the thermal tolerance of Drosophila melanogaster. Front. Nutr. 2022, 9, 819319. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Bai, H.; Dolezal, A.G.; Amdam, G.; Tatar, M. Juvenile hormone regulation of Drosophila aging. BMC Biol. 2013, 11, 85. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.M.; Lei, L.; Liu, Y.; Wang, X.; Ma, K.Y.; Zhang, C.; Zhu, H.; Zhao, A.; Chen, Z.Y. Purple sweet potato anthocyanin attenuates fat-induced mortality in Drosophila melanogaster. Exp. Gerontol. 2016, 82, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Z.; Zhang, Y.; Liu, E.; Han, S.; Gong, Z.; Xiao, W. Epigallocatechin-3-gallate + L-theanine/β-cyclodextrin inclusion complexes enhance epigallocatechin-3-gallate bioavailability and its lipid-lowering and weight loss effects. J. Funct. Foods 2022, 90, 104998. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Coso, J.D.; Espada, M.C.; Hernández-Beltrán, V.; Ferreira, C.C.; Varillas-Delgado, D.; Laiz, N.M.; Roberts, J.D.; Gamonales, J.M. Research Trends in the Effect of Caffeine Intake on Fat Oxidation: A Bibliometric and Visual Analysis. Nutrients 2023, 15, 4320. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.B.; Kim, J.H.; Young, L.; Clark, J.M.; Park, Y. Epigallocatechin gallate (EGCG) alters body fat and lean mass through sex-dependent metabolic mechanisms in Drosophila melanogaster. Int. J. Food Sci. Nutr. 2019, 70, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q.; Liu, J.; Wu, Y.; Niu, Y. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, A.; Yamazaki, T.; Ryan, C.; Ito, K.; Sato, S.; Tamura, K.; Sekiguchi, M.; Cao, S.; Shibata, S. Caffeine suppresses high-fat diet-induced body weight gain in mice depending on feeding timing. J. Funct. Foods 2022, 99, 105307. [Google Scholar] [CrossRef]
- Fortunato, I.M.; Pereira, Q.C.; Oliveira, F.S.; Alvarez, M.C.; Santos, T.W.D.; Ribeiro, M.L. Metabolic Insights into Caffeine’s Anti-Adipogenic Effects: An Exploration through Intestinal Microbiota Modulation in Obesity. Int. J. Mol. Sci. 2024, 25, 1803. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, Y.F.; Liu, A.L. Comparative and combined effects of epigallocatechin-3-gallate and caffeine in reducing lipid accumulation in Caenorhabditis elegans. Plant Foods Hum. Nutr. 2022, 77, 279–285. [Google Scholar] [CrossRef]
- Tian, J.; Geiss, C.; Zarse, K.; Madreiter-Sokolowski, C.T.; Ristow, M. Green tea catechins EGCG and ECG enhance the fitness and lifespan of Caenorhabditis elegans by complex I inhibition. Aging 2021, 13, 22629–22648. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jasper, H. Studying aging in Drosophila. Methods 2014, 68, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Amcoff, M.; Nassel, D.R. Impact of high-fat diet on lifespan, metabolism, fecundity and behavioral senescence in Drosophila. Insect Biochem. Mol. Biol. 2021, 133, 103495. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Feng, H.; Yu, Y.; Sun, M.; Liu, Y.; Li, T.; Sun, X.; Liu, S.; Sun, M. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohydr. Polym. 2017, 157, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Huang, Q.; Wang, S. Isolation of a novel lutein-protein complex from Chlorella vulgaris and its functional properties. Food Funct. 2015, 6, 1893–1899. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, M.; Feng, Z.; Zhu, Y.; Chen, J. Antiaging effects of rice protein hydrolysates on Drosophila melanogaster. J. Food Biochem. 2021, 45, e13602. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, J.; Zhou, B.; Wang, Z.; Huang, Y.; Ma, C.; Li, X. Impact of harvest season on bioactive compounds, amino acids and in vitro antioxidant capacity of white tea through multivariate statistical analysis. LWT-Food Sci. Technol. 2022, 164, 113655. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Pan, J.; Zhao, Q.; Li, Y.; Gao, W.; Zhang, Z. Anti-aging effect of brown black wolfberry on Drosophila melanogaster and D-galactose-induced aging mice. J. Funct. Foods 2020, 65, 103724. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: Oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Wu, L.; Tong, A.; Zhao, L.; Liu, B.; Zhao, C. Physicochemical characterization of polysaccharides from Chlorella pyrenoidosa and its anti-ageing effects in Drosophila melanogaster. Carbohydr. Polym. 2018, 185, 120–126. [Google Scholar] [CrossRef]
- Fleming, J.E.; Reveillaud, I.; Niedzwiecki, A. Role of oxidative stress in Drosophila aging. Mutat. Res. 1992, 275, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Terao, M.; Barzago, M.M.; Kurosaki, M.; Fratelli, M.; Bolis, M.; Borsotti, A.; Bigini, P.; Micotti, E.; Carli, M.; Invernizzi, R.W. Mouse aldehyde-oxidase-4 controls diurnal rhythms, fat deposition and locomotor activity. Sci. Rep. 2016, 6, 30343. [Google Scholar] [CrossRef]
- Thimgan, M.S.; Seugnet, L.; Turk, J.; Shaw, P.J. Identification of genes associated with resilience/vulnerability to sleep deprivation and starvation in Drosophila. Sleep 2015, 38, 801–814. [Google Scholar] [CrossRef][Green Version]
- Bolhassani, A.; Agi, E. Heat shock proteins in infection. Clin. Chim. Acta 2019, 498, 90–100. [Google Scholar] [CrossRef]
- Aaron, C.; Tae, K.Y. Protein quality control by molecular chaperones in neurodegeneration. Front. Neurosci. 2017, 11, 185. [Google Scholar] [CrossRef]
- Paula, M.T.D.; Silva, M.R.P.; Araujo, S.M.; Bortolotto, V.C.; Meichtry, L.B.; Zemolin, A.P.; Wallau, G.L.; Jesse, C.R.; Franco, J.L.; Posser, T.; et al. High-fat diet induces oxidative stress and MPK2 and HSP83 gene expression in Drosophila melanogaster. Oxid. Med. Cell Longev. 2016, 2016, 4018157. [Google Scholar] [CrossRef]
- Yong, C.; Xin, L.; Jiang, C.; Liang, J.; Ordovas, J.M.; Lai, C.Q.; Shen, L. Curcumin supplementation increases survival and lifespan in Drosophila under heat stress conditions. Biofactors 2018, 44, 577–587. [Google Scholar] [CrossRef]
- Hemphill, W.; Rivera, O.; Talbert, M. RNA-sequencing of Drosophila melanogaster head tissue on high-sugar and high-fat diets. G3 2018, 8, 279–290. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Cheon, C. Functional characterization of Gomisin N in high-fat-induced Drosophila obesity models. Int. J. Mol. Sci. 2020, 21, 7209. [Google Scholar] [CrossRef]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2018, 88, 855S–858S. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Zhang, X.; Cheng, J.; Liu, D.; Yan, Y.; Wang, H. Transcriptomic analysis of the life-extending effect exerted by black rice anthocyanin extract in D. melanogaster through regulation of aging pathways. Exp. Gerontol. 2019, 119, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ulgherait, M.; Chen, A.; McAllister, S.F.; Kim, H.X.; Delventhal, R.; Wayne, C.R.; Garcia, C.J.; Recinos, Y.; Oliva, M.; Canman, J.C.; et al. Circadian regulation of mitochondrial uncoupling and lifespan. Nat. Commun. 2020, 11, 1927. [Google Scholar] [CrossRef]
- Nayak, N.; Mishra, M. High fat diet induced abnormalities in metabolism, growth, behavior, and circadian clock in Drosophila melanogaster. Life Sci. 2021, 281, 119758. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Oishi, K.; Atsumi, G.; Sugiyama, S.; Kodomari, I.; Kasamatsu, M.; Machida, K.; Ishida, N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in clock mutant mice. FEBS Lett. 2006, 580, 127–130. [Google Scholar] [CrossRef]
- Zickermann, V.; Kerscher, S.; Zwicker, K.; Tocilescu, M.A.; Radermacher, M.; Brandt, U. Architecture of complex I and its implications for electron transfer and proton pumping. Biochim. Biophys. Acta 2009, 1787, 574–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, J.; Vinothkumar, K.R.; Hirst, J. Architecture of mammalian respiratory complex I. Nature 2016, 515, 80–84. [Google Scholar] [CrossRef]
- Azuma, M.; Le, T.D.; Yoshimoto, Y.; Hiraki, N.; Inoue, Y.H. RNA-seq analysis of diet-driven obesity and anti-obesity effects of quercetin glucoside or epigallocatechin gallate in Drosophila adults. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 857–876. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; An, T.; Zhai, M.; Huang, Y.; Wang, Q.; Wang, Y.; Zhang, R.; Wang, T.; Liu, J.; Zhang, Y.; et al. Mitochondrial miR-762 regulates apoptosis and myocardial infarction by impairing ND2. Cell Death Dis. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Lauss, M.; Kriegner, A.; Vierlinger, K.; Noehammer, C. Characterization of the drugged human genome. Pharmacogenomics 2007, 8, 1063–1073. [Google Scholar] [CrossRef]
- Liang, Y.; Zhan, X.; Wei, X.; Zhong, J.; Deng, J.; Chen, Y.; Pan, L.; Zhang, J.; Li, M.; Huang, R.; et al. Study on the material basis and mechanism of Hemerocallis citrina Baroni on sleep-improvement using Drosophila activity monitoring, metabolomic, targeted screening and transcriptomic. Food Res. Int. 2023, 172, 112562. [Google Scholar] [CrossRef]
- Yamazaki, M.; Tomita, J.; Takahama, K.; Ueno, T.; Mitsuyoshi, M.; Sakamoto, E.; Kume, S.; Kume, K. High calorie diet augments age-associats sleep impairment in Drosophila. Biochem. Biophys. Res. Commun. 2012, 417, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Altshuller, Y.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Frohman, M.A. Gcm1, a mammalian homolog of Drosophila glial cells missing. FEBS Lett. 1996, 393, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Wael, B.; Sara, M.; Claude, D.; Céline, R.; Angela, G.; Pierre, B.C. Gcm alleviates the inflammatory phenotype induced by toll activation in Drosophila hemocytes. bioRxiv 2023, 23, 558811. [Google Scholar] [CrossRef]
- McMullen, E.; Hertenstein, H.; Strassburger, K.; Deharde, L.; Brankatschk, M.; Schirmeier, S. Glycolytically impaired Drosophila glial cells fuel neural metabolism via β-oxidation. Nat. Commun. 2023, 14, 2996. [Google Scholar] [CrossRef]
- Yu, S.; Jang, Y. Nmdmc overexpression extends Drosophila lifespan and reduces levels of mitochondrial reactive oxygen species. Biochem. Biophys. Res. Commun. 2015, 465, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, Z.; Zheng, X.; Zheng, Z.; Yao, D.; Zhang, Y.; Aweya, J.J. The juvenile hormone epoxide hydrolase homolog in Penaeus vannamei plays immune-related functions. Dev. Comp. Immunol. 2022, 132, 104410. [Google Scholar] [CrossRef] [PubMed]



| Chemical Constituents | White Tea Aqueous Extract |
|---|---|
| Total polyphenols (%) | 22.92 ± 0.40 |
| Total flavonoids (%) | 2.24 ± 0.01 |
| Free amino acids (%) | 7.30 ± 0.02 |
| Soluble proteins (%) | 17.50 ± 0.23 |
| Soluble sugars (%) | 9.38 ± 0.43 |
| Group | 50% Survival (days) | Mean Lifespan (days) | Maximum Lifespan (days) |
|---|---|---|---|
| NCTL | 44.9 ± 5.9 | 45.4 ± 5.4 | 67.6 ± 4.6 |
| LCTL | 24.4 ± 0.9 ### | 25.9 ± 1.0 ### | 50.5 ± 2.9 ### |
| LWTAE1 | 25.0 ± 0.8 | 26.7 ± 0.8 | 51.8 ± 2.1 |
| LWTAE5 | 33.9 ± 4.5 *** | 35.2 ± 4.1 *** | 61.4 ± 6.8 *** |
| LWTAE10 | 27.6 ± 2.3 ** | 29.1 ± 2.2 ** | 55.8 ± 3.1 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; He, M.; Zhang, J.; Cheng, S.; Cheng, X.; Chen, H.; Wu, G.; Wang, F.; Zeng, S. White Tea Aqueous Extract: A Potential Anti-Aging Agent Against High-Fat Diet-Induced Senescence in Drosophila melanogaster. Foods 2024, 13, 4034. https://doi.org/10.3390/foods13244034
Huang Y, He M, Zhang J, Cheng S, Cheng X, Chen H, Wu G, Wang F, Zeng S. White Tea Aqueous Extract: A Potential Anti-Aging Agent Against High-Fat Diet-Induced Senescence in Drosophila melanogaster. Foods. 2024; 13(24):4034. https://doi.org/10.3390/foods13244034
Chicago/Turabian StyleHuang, Yan, Miaoyuan He, Jianming Zhang, Shilong Cheng, Xi Cheng, Haoran Chen, Guangheng Wu, Fang Wang, and Shaoxiao Zeng. 2024. "White Tea Aqueous Extract: A Potential Anti-Aging Agent Against High-Fat Diet-Induced Senescence in Drosophila melanogaster" Foods 13, no. 24: 4034. https://doi.org/10.3390/foods13244034
APA StyleHuang, Y., He, M., Zhang, J., Cheng, S., Cheng, X., Chen, H., Wu, G., Wang, F., & Zeng, S. (2024). White Tea Aqueous Extract: A Potential Anti-Aging Agent Against High-Fat Diet-Induced Senescence in Drosophila melanogaster. Foods, 13(24), 4034. https://doi.org/10.3390/foods13244034






