Applications of Near-Infrared Spectroscopy for Nondestructive Quality Analysis of Fish and Fishery Products
Abstract
1. Introduction
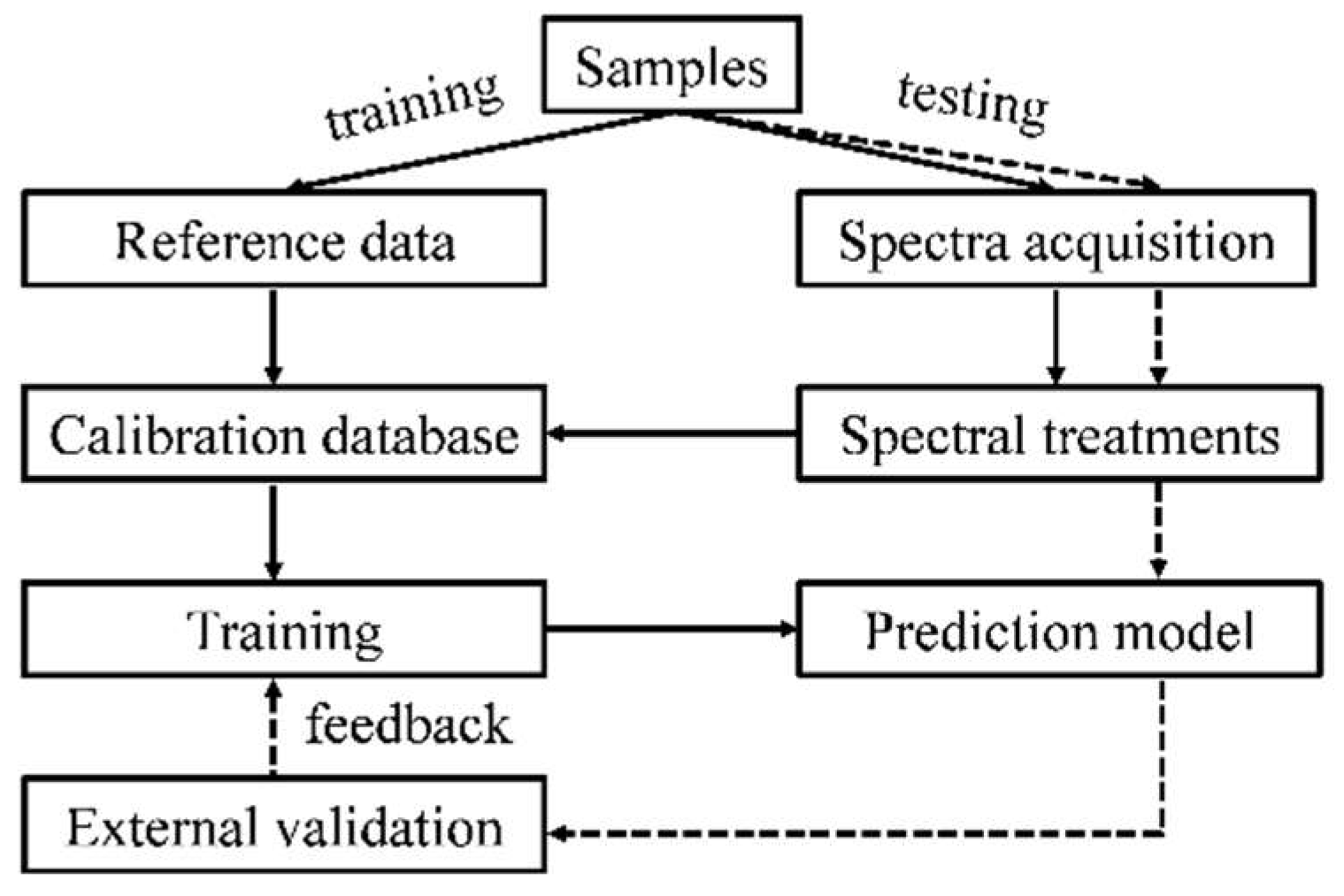
2. Fundamentals of NIRS
3. Applications in Fish and Fish Products Quality and Safety Analysis
3.1. Qualitative Analysis
| Samples | Applications | Spectral Range (nm) | Models | Accuracies | References |
|---|---|---|---|---|---|
| West African Goatfish | Classification | 300~1100 | PLS | 100% | [24] |
| Fresh and frozen/thawed fish | Classification | 1100~2500 | PLS | 80~91% | [25] |
| Fresh and frozen/thawed tuna fillets | Classification | 350~2500 | PLS | >82% | [26] |
| European sea bass | Classification | 1100~2500 | PCA + SIMCA | 90% | [28] |
| European seabass | Classification | 1100~2500 | PLS | [29] | |
| Atlantic cod | Classification | 950~1650 | LDA + SIMCA | 100% | [31] |
| Seven freshwater fish species | Classification | 1000~1799 | PCA + LDA | 100% | [37] |
| Fish meal | Classification | 1000–2500 | PLS-DA + CARS | 94.87% | [32] |
| European sea bass | Geographical origins | 1100–2500 | PCA + OPLS-DA | 85–100% | [34] |
| Tilapia fillets | Geographical origins | 1000–2500 | SIMCA | 75% | [36] |
| Salted ripened anchovies | Geographical origins | 1000–2400 | OPLS-DA | 99% | [9] |
3.2. Quantitative Quality Analysis of Fish and Fishery Products
| Samples | Parameters | Spectral Range (nm) | Model | Performance | Ref. |
|---|---|---|---|---|---|
| Silver carp | trimethylamine | 1000~2500 | PLS | R2P: 0.977, RMSEP: 5.10 | [43] |
| Parabramis pekinensis | TVB-N | 1000~2500 | ANN | Accuracy: 0.9333 | [44] |
| Atlantic salmon | microbial numbers | 800~2500 | PLS | R2P: 0.95, RMSEP: 0.12 | [45] |
| Flounder fillets | total bacteria | 600~1100 | ANN | R2P: 0.966, RMSEP: 0.083 | [46] |
| Tuna fish | histamine | 400~2500 | PLS | R2P: 0.74, R2cv: 0.88 | [47] |
| Mackerel | histamine | 400~2498 | WNN | R2P: 0.79, RMSEP: 70 | [48] |
| Silver chub | K value | 1000~2500 | SVM | R2P: 0.9374, RMSEP: 0.036525 | [50] |
| Silver carp | K value | 1000~2500 | PLS | R2P: 0.9786, RMSEP: 3.98 | [51] |
| Catfish fillets | TVB-N, thiobarbituric acid (TBA), drip loss, L*, and whiteness | 1000~2500 | PLSR | Accuracy: 0.86–0.90, residual predictive deviation (RPD): 2.66–3.19 | [52] |
| Bighead carp | pH, TVB-N, TBA, and K value | 1000~1799 | PLSR | R2P: 0.807–0.954, RMSEP: 0.081–6.509 | [53] |
| Silver carp flesh | textural properties | 1000~1799 | PLSR | R2P: 0.83–0.95, RMSEP: 0.08–2.63 | [54] |
4. Near-Infrared Hyperspectral Imaging Technology in Fish and Fishery Products
| Samples | Parameters | Spectral Range (nm) | Model | Performance | Ref. |
|---|---|---|---|---|---|
| Fish fillets | inspect substitution and mislabeling | 400~1000 and 900~1700 | SVM; SVM | Accuracy: 95.0% and 95.5% | [23] |
| Halibut fillets | fresh and frozen/thawed samples | 380~1030 | SVM | Accuracy: 97.22% | [57] |
| Grass carp fillets | fresh and frozen/thawed samples | 400~1000 | SVM | Accuracy: 94.29% | [58] |
| Crucian carp | fresh and froze/thawed samples | 400~1000 | PLS | Accuracy: over 92.5% | [59] |
| Atlantic cod | roe, milt, and liver | 400~1000 | Spectral Angle Mapper (SAM) | Sensitivity: 96%; specificity: 98% | [60] |
| Salmon fillets | organic and conventional salmon fillets | 400~1000 | SVM | Accuracy: 98.2% | [61] |
| Dried grass carp fillets | dehydrating and rehydrating mass changes | 400~1000 | PLSR | R2P: 0.8278, RMSEP: 9.79% | [62] |
| Salmon fillets | moisture | 400~1000; 900~1700; 400~1700 | PLSR | R2P: 0.893, RMSEP: 1.513%; R2P: 0.902, RMSEP: 1.450%; R2P: 0.849, RMSEP: 1.800%; | [63] |
| Salmon fillets | drip loss and pH distribution | 400~1700 | PLSR | R2P: 0.834, RMSEP: 0.067 (drip loss); R2P: 0.877, RMSEP: 0.046 (pH) | [64] |
| Salmon fillets | gross energy density | 900~1700 | PLSR | R2P: 0.908, RMSEP: 6.871% | [65] |
| Grass carp slices | moisture | 400~1000 | PLSR | R2P: 0.9021, RMSEP: 0.0561 | [66] |
| Frozen and thawed cod | liquid loss | 430~1000 | PLS | R2P: 0.88, RMSEP: 0.0062 | [67] |
| Salmon fillets | fat and moisture | 900~1700 | LS-SVM | R2P: 0.9685, RMSEP: 1.1750 (fat); R2P: 0.9688, RMSEP: 0.8021 (moisture) | [68] |
| Salmon fillets | fatty acids | 930~2500 | PLSR | R2P: 0.9, RMSEP: 0.86 | [69] |
| Tilapia fillet | TVB-N | 900~1700 | PLSR | R2P: 0.8524, RMSEP: 2.4487 | [70] |
| Rainbow trout fillets | TVB-N | 430~1010 | Linear Deep Neural Network (LDNN) | R2P: 0.853, RMSEP: 3.159 | [71] |
| Vacuum-packed smoked salmon | shelf life | 400~1000 | PLS | R2P: 0.97, RMSEP: 0.08 | [72] |
| Vacuum-packed chilled smoked salmon | shelf life | 400~1000 | support vector machine (SVM) | R2P: 0.89, RMSECV: 7.7 | [73] |
| Grass carp and silver carp fillets | K value | 400~1000 | SVM | R2P: 0.936, RMSEP: 5.21% | [74] |
| Salmon flesh | total viable counts | 400~1700 | PLSR | R2P: 0.985, residual predictive deviation (RPD): 5.127 | [75] |
| Salmon flesh | lactic acid bacteria | 900~1700 | SVM | R2P: 0.925, RMSEP: 0.531 | [76] |
| Salmon flesh | total counts of Enterobacteriaceae and Pseudomonas spp. (EPC) | 900~1700 | PLS | R2P: 0.964, RMSEP: 0.429 | [77] |
| Grass carp fillets | total viable counts | 400~1000 | PLSR | R2P: 0.90, RMSEP: 0.57 | [78] |
| Salmon fillets | tenderness | 400~1720 | SVM | R2P: 0.905, RMSEP: 1.089 | [80] |
| Grass carp fillets | firmness | 400~1000 | SVM | R2P: 0.941, RMSEP: 1.229 | [81] |
| Salmon fillets | color | 964~1631 | MLR | R2P: 0.876, 0.744, and 0.803 for L*, a*, and b*, respectively. | [82] |
| Large yellow croaker fillets | color | 400~1000 | PLSR | R2P: 0.908, 0.915, and 0.977 for L*, a*, and b*, respectively. | [83] |
5. Conclusions and Future Trends
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, P.; Zhu, K.; Cao, J.; Lin, X.; Shen, X.; Duan, Z.; Li, C. Relationship between Micromolecules and Quality Changes of Tilapia Fillets After Partial Freezing Treatment with Polyphenols. J. Agric. Food Chem. 2021, 69, 8213–8226. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Xu, Y.; Xia, W.; Jiang, Q. The impact of desmin on texture and water-holding capacity of ice-stored grass carp (Ctenopharyngodon idella) fillet. Int. J. Food Sci. Technol. 2016, 52, 464–471. [Google Scholar] [CrossRef]
- Sinthupachee, A.; Matan, N.; Matan, N. Development of smoke flavour-antimicrobial packaging from coconut fibre using Litsea cubeba essential oil and wood smoke for dried fish preservation and reduction of PAH. Food Control 2023, 148, 109629. [Google Scholar] [CrossRef]
- Yan, D.; Xu, W.; Yu, Q.; You, J.; Gao, R.; Bao, Y. Pre-rigor salting improves gel strength and water-holding of surimi gel made from snakehead fish (Channa argus): The role of protein oxidation. Food Chem. 2024, 450, 139269. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, L.; Zi, Y.; Chen, J.; Xu, J.; Shi, W.; Wang, X.; Zhong, J. Development of tilapia muscle-based Shanghai smoked fish and the effect of salt amounts in the preparation process. LWT 2023, 186, 115240. [Google Scholar] [CrossRef]
- Chen, J.-N.; Zhang, Y.-Y.; Huang, X.; Wang, H.-P.; Dong, X.; Zhu, B.; Qin, L. Analysis of Lipid Molecule Profiling and Conversion Pathway in Mandarin Fish (Siniperca chuatsi) during Fermentation via Untargeted Lipidomics. J. Agric. Food Chem. 2023, 71, 8673–8684. [Google Scholar] [CrossRef]
- López-García, M.M.; Ramil-Novo, L.A.; Vázquez-Odériz, M.L.; Romero-Rodríguez, M.Á. A QIM-Based Evaluation of Sensory Quality of Frozen–Thawed Roundnose Grenadier (Coryphaenoides rupestris) and Its Applications. ACS Food Sci. Technol. 2021, 1, 392–398. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Smoking of Fish and Seafood: History, Methods and Effects on Physical, Nutritional and Microbiological Properties. Food Bioprocess Technol. 2011, 5, 831–853. [Google Scholar] [CrossRef]
- Varrà, M.O.; Ghidini, S.; Ianieri, A.; Zanardi, E. Near infrared spectral fingerprinting: A tool against origin-related fraud in the sector of processed anchovies. Food Control 2020, 123, 107778. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Sun, D.-W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Hassoun, A.; Sahar, A.; Lakhal, L.; Aït-Kaddour, A. Fluorescence spectroscopy as a rapid and non-destructive method for monitoring quality and authenticity of fish and meat products: Impact of different preservation conditions. LWT 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Chapman, J.; Elbourne, A.; Truong, V.K.; Newman, L.; Gangadoo, S.; Rajapaksha Pathirannahalage, P.; Cheeseman, S.; Cozzolino, D. Sensomics—From conventional to functional NIR spectroscopy—Shining light over the aroma and taste of foods. Trends Food Sci. Technol. 2019, 91, 274–281. [Google Scholar] [CrossRef]
- Teye, E.; Anyidoho, E.; Agbemafle, R.; Sam-Amoah, L.K.; Elliott, C. Cocoa bean and cocoa bean products quality evaluation by NIR spectroscopy and chemometrics: A review. Infrared Phys. Technol. 2020, 104, 103127. [Google Scholar] [CrossRef]
- Johnson, J.B. An overview of near-infrared spectroscopy (NIRS) for the detection of insect pests in stored grains. J. Stored Prod. Res. 2020, 86, 101558. [Google Scholar] [CrossRef]
- Yakubu, H.G.; Kovacs, Z.; Toth, T.; Bazar, G. The recent advances of near-infrared spectroscopy in dairy production—A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 810–831. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhang, Y.; Wang, D.; Wang, X.; Yu, L.; Zhang, W.; Li, P. Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils. Trends Food Sci. Technol. 2020, 101, 172–181. [Google Scholar] [CrossRef]
- Lin, X.; Sun, D.-W. Recent developments in vibrational spectroscopic techniques for tea quality and safety analyses. Trends Food Sci. Technol. 2020, 104, 163–176. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W. Partial Least Squares Regression (PLSR) Applied to NIR and HSI Spectral Data Modeling to Predict Chemical Properties of Fish Muscle. Food Eng. Rev. 2016, 9, 36–49. [Google Scholar] [CrossRef]
- Reis, M.M.; Van Beers, R.; Al-Sarayreh, M.; Shorten, P.; Yan, W.Q.; Saeys, W.; Klette, R.; Craigie, C. Chemometrics and hyperspectral imaging applied to assessment of chemical, textural and structural characteristics of meat. Meat Sci. 2018, 144, 100–109. [Google Scholar] [CrossRef]
- Jerome Workman, J. A Brief review of near infrared in Petroleum product analysis. J. Near Infrared Spectrosc. 1996, 4, 69–74. [Google Scholar] [CrossRef]
- Louw, E.D.; Theron, K.I. Robust prediction models for quality parameters in Japanese plums (Prunus salicina L.) using NIR spectroscopy. Postharvest Biol. Technol. 2010, 58, 176–184. [Google Scholar] [CrossRef]
- Bogomolov, A.; Melenteva, A.; Dahm, D.J. Fat Globule Size Effect on Visible and Shortwave near Infrared Spectra of Milk. J. Near Infrared Spectrosc. 2013, 21, 435–440. [Google Scholar] [CrossRef]
- Qin, J.; Vasefi, F.; Hellberg, R.S.; Akhbardeh, A.; Isaacs, R.B.; Yilmaz, A.G.; Hwang, C.; Baek, I.; Schmidt, W.F.; Kim, M.S. Detection of fish fillet substitution and mislabeling using multimode hyperspectral imaging techniques. Food Control 2020, 114, 107234. [Google Scholar] [CrossRef]
- Ottavian, M.; Fasolato, L.; Serva, L.; Facco, P.; Barolo, M. Data Fusion for Food Authentication: Fresh/Frozen–Thawed Discrimination in West African Goatfish (Pseudupeneus prayensis) Fillets. Food Bioprocess Technol. 2013, 7, 1025–1036. [Google Scholar] [CrossRef]
- Ottavian, M.; Fasolato, L.; Facco, P.; Barolo, M. Foodstuff authentication from spectral data: Toward a species-independent discrimination between fresh and frozen–thawed fish samples. J. Food Eng. 2013, 119, 765–775. [Google Scholar] [CrossRef]
- Reis, M.M.; Martínez, E.; Saitua, E.; Rodríguez, R.; Pérez, I.; Olabarrieta, I. Non-invasive differentiation between fresh and frozen/thawed tuna fillets using near infrared spectroscopy (Vis-NIRS). LWT 2017, 78, 129–137. [Google Scholar] [CrossRef]
- Alamprese, C.; Casiraghi, E. Application of FT-NIR and FT-IR spectroscopy to fish fillet authentication. LWT Food Sci. Technol. 2015, 63, 720–725. [Google Scholar] [CrossRef]
- Trocino, A.; Xiccato, G.; Majolini, D.; Tazzoli, M.; Bertotto, D.; Pascoli, F.; Palazzi, R. Assessing the quality of organic and conventionally-farmed European sea bass (Dicentrarchus labrax). Food Chem. 2012, 131, 427–433. [Google Scholar] [CrossRef]
- Ottavian, M.; Facco, P.; Fasolato, L.; Novelli, E.; Mirisola, M.; Perini, M.; Barolo, M. Use of near-infrared spectroscopy for fast fraud detection in seafood: Application to the authentication of wild European sea bass (Dicentrarchus labrax). J. Agric. Food Chem. 2012, 60, 639–648. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Ottavian, M.; Concollato, A.; Serva, L.; Martelli, R.; Parisi, G. Authentication of raw and cooked freeze-dried rainbow trout (Oncorhynchus mykiss) by means of near infrared spectroscopy and data fusion. Food Res. Int. 2014, 60, 180–188. [Google Scholar] [CrossRef]
- Grassi, S.; Casiraghi, E.; Alamprese, C. Handheld NIR device: A non-targeted approach to assess authenticity of fish fillets and patties. Food Chem. 2018, 243, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Han, L.; Fan, X.; Liu, X.; Cao, Y.; Yang, Z. Classification of fish meal produced in China and Peru by online near infrared spectroscopy with characteristic wavelength variables. J. Near Infrared Spectrosc. 2017, 25, 63–71. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, H.; Yin, Y.; Yuan, Y.; Liang, H.; Li, F.; Li, Z. Determination of the Acid and Peroxide Values of Vegetable Oils by Raman Spectroscopy with Competitive Adaptive Reweighted Sampling (CARS) and Back Propagation Neural Network (BPNN). Anal. Lett. 2023, 57, 2289–2306. [Google Scholar] [CrossRef]
- Ghidini, S.; Varra, M.O.; Dall’Asta, C.; Badiani, A.; Ianieri, A.; Zanardi, E. Rapid authentication of European sea bass (Dicentrarchus labrax L.) according to production method, farming system, and geographical origin by near infrared spectroscopy coupled with chemometrics. Food Chem. 2019, 280, 321–327. [Google Scholar] [CrossRef]
- Wold, S.; SjÖStrÖM, M. SIMCA: A Method for Analyzing Chemical Data in Terms of Similarity and Analogy. In Chemometrics: Theory and Application; American Chemical Society: Washington, DC, USA, 1977; pp. 243–282. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, D.-h.; Wang, X.-c.; Liu, L.-p.; Fan, Y.-x.; Cao, J.-x. Prediction of chemical composition and geographical origin traceability of Chinese export tilapia fillets products by near infrared reflectance spectroscopy. LWT Food Sci. Technol. 2015, 60, 1214–1218. [Google Scholar] [CrossRef]
- Lv, H.; Xu, W.; You, J.; Xiong, S. Classification of freshwater fish species by linear discriminant analysis based on near infrared reflectance spectroscopy. J. Near Infrared Spectrosc. 2017, 25, 54–62. [Google Scholar] [CrossRef]
- Cozzolino, D.; Murray, I.; Chree, A.; Scaife, J.R. Multivariate determination of free fatty acids and moisture in fish oils by partial least-squares regression and near-infrared spectroscopy. LWT Food Sci. Technol. 2005, 38, 821–828. [Google Scholar] [CrossRef]
- Cascant, M.M.; Breil, C.; Fabiano-Tixier, A.S.; Chemat, F.; Garrigues, S.; de la Guardia, M. Determination of fatty acids and lipid classes in salmon oil by near infrared spectroscopy. Food Chem. 2018, 239, 865–871. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Tóth, I.V.; Portela, A.; Delgadillo, I.; Rangel, A.O.S.S. Monitoring sodium chloride during cod fish desalting process by flow injection spectrometry and infrared spectroscopy. Food Control 2011, 22, 277–282. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Fatahi, S.; Banan, A.; Makino, Y. Real-time nondestructive monitoring of Common Carp Fish freshness using robust vision-based intelligent modeling approaches. Comput. Electron. Agric. 2019, 159, 16–27. [Google Scholar] [CrossRef]
- Xiao-wei, H.; Zhi-hua, L.; Xiao-bo, Z.; Ji-yong, S.; Han-ping, M.; Jie-wen, Z.; Li-min, H.; Holmes, M. Detection of meat-borne trimethylamine based on nanoporous colorimetric sensor arrays. Food Chem. 2016, 197, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Agyekum, A.A.; Kutsanedzie, F.Y.H.; Mintah, B.K.; Annavaram, V.; Zareef, M.; Hassan, M.M.; Arslan, M.; Chen, Q. Rapid and Nondestructive Quantification of Trimethylamine by FT-NIR Coupled with Chemometric Techniques. Food Anal. Methods 2019, 12, 2035–2044. [Google Scholar] [CrossRef]
- Huang, X.; Xu, H.; Wu, L.; Dai, H.; Yao, L.; Han, F. A data fusion detection method for fish freshness based on computer vision and near-infrared spectroscopy. Anal. Methods 2016, 8, 2929–2935. [Google Scholar] [CrossRef]
- Tito, N.B.; Rodemann, T.; Powell, S.M. Use of near infrared spectroscopy to predict microbial numbers on Atlantic salmon. Food Microbiol. 2012, 32, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Chen, C.; Khan, M.N.; Liu, Y.; Zhang, R.; Lin, H.; Cao, L. Non-destructive determination of the total bacteria in flounder fillet by portable near infrared spectrometer. Food Control 2014, 42, 18–22. [Google Scholar] [CrossRef]
- Currò, S.; Savini, F.; Fasolato, L.; Indio, V.; Tomasello, F.; Rampazzo, G.; Zironi, E.; Pagliuca, G.; Gazzotti, T.; Prandini, L.; et al. Application of near-infrared spectroscopy as at line method for the evaluation of histamine in tuna fish (Thunnus albacares). Food Control 2025, 167, 110778. [Google Scholar] [CrossRef]
- Pauline, O.; Chang, H.-T.; Tsai, I.L.; Lin, C.-H.; Chen, S.; Chuang, Y.-K. Intelligent assessment of the histamine level in mackerel (Scomber australasicus) using near-infrared spectroscopy coupled with a hybrid variable selection strategy. LWT 2021, 145, 111524. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Ding, R.; Huang, X.; Han, F.; Dai, H.; Teye, E.; Xu, F. Rapid and nondestructive evaluation of fish freshness by near infrared reflectance spectroscopy combined with chemometrics analysis. Anal. Methods 2014, 6, 9675–9683. [Google Scholar] [CrossRef]
- Agyekum, A.A.; Kutsanedzie, F.Y.H.; Annavaram, V.; Mintah, B.K.; Asare, E.K.; Wang, B. FT-NIR coupled chemometric methods rapid prediction of K-value in fish. Vib. Spectrosc. 2020, 108, 103044. [Google Scholar] [CrossRef]
- Cao, L.; Huang, Z.; Wu, D.; Ruan, R.; Liu, Y. Rapid and nondestructive determination of qualities in vacuum-packaged catfish (Clarias leather) fillets during slurry ice storage. J. Food Process. Preserv. 2021, 45, e15262. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; Chen, Z.; You, J.; Xiong, S. Evaluation of freshness in freshwater fish based on near infrared reflectance spectroscopy and chemometrics. LWT 2019, 106, 145–150. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; You, J.; Xiong, S. Rapid determination of the textural properties of silver carp (Hypophthalmichthys molitrix) using near-infrared reflectance spectroscopy and chemometrics. LWT 2020, 129, 109545. [Google Scholar] [CrossRef]
- Washburn, K.E.; Stormo, S.K.; Skjelvareid, M.H.; Heia, K. Non-invasive assessment of packaged cod freeze-thaw history by hyperspectral imaging. J. Food Eng. 2017, 205, 64–73. [Google Scholar] [CrossRef]
- Pu, H.; Lin, L.; Sun, D.W. Principles of Hyperspectral Microscope Imaging Techniques and Their Applications in Food Quality and Safety Detection: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 853–866. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, D.; He, Y.; Liu, F.; Sun, D.-W. Application of Visible and Near Infrared Hyperspectral Imaging to Differentiate Between Fresh and Frozen–Thawed Fish Fillets. Food Bioprocess Technol. 2012, 6, 2931–2937. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Pu, H.-B.; Chen, X.; Liu, Y.; Zhang, H.; Li, J.-L. Integration of classifiers analysis and hyperspectral imaging for rapid discrimination of fresh from cold-stored and frozen-thawed fish fillets. J. Food Eng. 2015, 161, 33–39. [Google Scholar] [CrossRef]
- Shan, J.; Wang, X.; Russel, M.; Zhao, J.; Zhang, Y. Comparisons of Fish Morphology for Fresh and Frozen-Thawed Crucian Carp Quality Assessment by Hyperspectral Imaging Technology. Food Anal. Methods 2018, 11, 1701–1710. [Google Scholar] [CrossRef]
- Paluchowski, L.A.; Misimi, E.; Grimsmo, L.; Randeberg, L.L. Towards automated sorting of Atlantic cod (Gadus morhua) roe, milt, and liver—Spectral characterization and classification using visible and near-infrared hyperspectral imaging. Food Control 2016, 62, 337–345. [Google Scholar] [CrossRef]
- Xu, J.-L.; Riccioli, C.; Sun, D.-W. Comparison of hyperspectral imaging and computer vision for automatic differentiation of organically and conventionally farmed salmon. J. Food Eng. 2017, 196, 170–182. [Google Scholar] [CrossRef]
- Ma, J.; Qu, J.-H.; Sun, D.-W. Developing hyperspectral prediction model for investigating dehydrating and rehydrating mass changes of vacuum freeze dried grass carp fillets. Food Bioprod. Process. 2017, 104, 66–76. [Google Scholar] [CrossRef]
- He, H.-J.; Wu, D.; Sun, D.-W. Non-destructive and rapid analysis of moisture distribution in farmed Atlantic salmon (Salmo salar) fillets using visible and near-infrared hyperspectral imaging. Innov. Food Sci. Emerg. Technol. 2013, 18, 237–245. [Google Scholar] [CrossRef]
- He, H.-J.; Wu, D.; Sun, D.-W. Rapid and non-destructive determination of drip loss and pH distribution in farmed Atlantic salmon (Salmo salar) fillets using visible and near-infrared (Vis–NIR) hyperspectral imaging. Food Chem. 2014, 156, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-L.; Riccioli, C.; Sun, D.-W. Development of an alternative technique for rapid and accurate determination of fish caloric density based on hyperspectral imaging. J. Food Eng. 2016, 190, 185–194. [Google Scholar] [CrossRef]
- Qu, J.-H.; Sun, D.-W.; Cheng, J.-H.; Pu, H. Mapping moisture contents in grass carp (Ctenopharyngodon idella) slices under different freeze drying periods by Vis-NIR hyperspectral imaging. LWT 2017, 75, 529–536. [Google Scholar] [CrossRef]
- Anderssen, K.E.; Stormo, S.K.; Skåra, T.; Skjelvareid, M.H.; Heia, K. Predicting liquid loss of frozen and thawed cod from hyperspectral imaging. LWT 2020, 133, 110093. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Chen, Y.; Luo, W.; Huang, Y.; Tao, D.; Zhan, B.; Liu, X. Non-destructive determination of fat and moisture contents in Salmon (Salmo salar) fillets using near-infrared hyperspectral imaging coupled with spectral and textural features. J. Food Compos. Anal. 2020, 92, 103567. [Google Scholar] [CrossRef]
- Lintvedt, T.A.; Andersen, P.V.; Afseth, N.K.; Heia, K.; Lindberg, S.-K.; Wold, J.P. Raman spectroscopy and NIR hyperspectral imaging for in-line estimation of fatty acid features in salmon fillets. Talanta 2023, 254, 124113. [Google Scholar] [CrossRef]
- Yu, H.-D.; Qing, L.-W.; Yan, D.-T.; Xia, G.; Zhang, C.; Yun, Y.-H.; Zhang, W. Hyperspectral imaging in combination with data fusion for rapid evaluation of tilapia fillet freshness. Food Chem. 2021, 348, 129129. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Khoshnoudi-Nia, S.; Azimifar, Z.; Kamyab, S. Evaluation of the total volatile basic nitrogen (TVB-N) content in fish fillets using hyperspectral imaging coupled with deep learning neural network and meta-analysis. Sci. Rep. 2021, 11, 5094. [Google Scholar] [CrossRef]
- Ivorra, E.; Girón, J.; Sánchez, A.J.; Verdú, S.; Barat, J.M.; Grau, R. Detection of expired vacuum-packed smoked salmon based on PLS-DA method using hyperspectral images. J. Food Eng. 2013, 117, 342–349. [Google Scholar] [CrossRef]
- Ivorra, E.; Sánchez, A.J.; Verdú, S.; Barat, J.M.; Grau, R. Shelf life prediction of expired vacuum-packed chilled smoked salmon based on a KNN tissue segmentation method using hyperspectral images. J. Food Eng. 2016, 178, 110–116. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W.; Pu, H.; Zhu, Z. Development of hyperspectral imaging coupled with chemometric analysis to monitor K value for evaluation of chemical spoilage in fish fillets. Food Chem. 2015, 185, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sun, D.-W. Potential of time series-hyperspectral imaging (TS-HSI) for non-invasive determination of microbial spoilage of salmon flesh. Talanta 2013, 111, 39–46. [Google Scholar] [CrossRef] [PubMed]
- He, H.-J.; Sun, D.-W.; Wu, D. Rapid and real-time prediction of lactic acid bacteria (LAB) in farmed salmon flesh using near-infrared (NIR) hyperspectral imaging combined with chemometric analysis. Food Res. Int. 2014, 62, 476–483. [Google Scholar] [CrossRef]
- He, H.-J.; Sun, D.-W. Inspection of harmful microbial contamination occurred in edible salmon flesh using imaging technology. J. Food Eng. 2015, 150, 82–89. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Sun, D.-W. Rapid and non-invasive detection of fish microbial spoilage by visible and near infrared hyperspectral imaging and multivariate analysis. LWT Food Sci. Technol. 2015, 62, 1060–1068. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W.; He, Y. Novel non-invasive distribution measurement of texture profile analysis (TPA) in salmon fillet by using visible and near infrared hyperspectral imaging. Food Chem. 2014, 145, 417–426. [Google Scholar] [CrossRef]
- He, H.-J.; Wu, D.; Sun, D.-W. Potential of hyperspectral imaging combined with chemometric analysis for assessing and visualising tenderness distribution in raw farmed salmon fillets. J. Food Eng. 2014, 126, 156–164. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Qu, J.-H.; Sun, D.-W.; Zeng, X.-A. Visible/near-infrared hyperspectral imaging prediction of textural firmness of grass carp (Ctenopharyngodon idella) as affected by frozen storage. Food Res. Int. 2014, 56, 190–198. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W.; He, Y. Application of long-wave near infrared hyperspectral imaging for measurement of color distribution in salmon fillet. Innov. Food Sci. Emerg. Technol. 2012, 16, 361–372. [Google Scholar] [CrossRef]
- Wang, S.; Das, A.K.; Pang, J.; Liang, P. Real-time monitoring the color changes of large yellow croaker (Larimichthys crocea) fillets based on hyperspectral imaging empowered with artificial intelligence. Food Chem. 2022, 382, 132343. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Liu, C.; Zhong, Y.; Luo, Z. Applications of Near-Infrared Spectroscopy for Nondestructive Quality Analysis of Fish and Fishery Products. Foods 2024, 13, 3992. https://doi.org/10.3390/foods13243992
Zhou J, Liu C, Zhong Y, Luo Z. Applications of Near-Infrared Spectroscopy for Nondestructive Quality Analysis of Fish and Fishery Products. Foods. 2024; 13(24):3992. https://doi.org/10.3390/foods13243992
Chicago/Turabian StyleZhou, Jiaojiao, Chen Liu, Yujun Zhong, and Zhihui Luo. 2024. "Applications of Near-Infrared Spectroscopy for Nondestructive Quality Analysis of Fish and Fishery Products" Foods 13, no. 24: 3992. https://doi.org/10.3390/foods13243992
APA StyleZhou, J., Liu, C., Zhong, Y., & Luo, Z. (2024). Applications of Near-Infrared Spectroscopy for Nondestructive Quality Analysis of Fish and Fishery Products. Foods, 13(24), 3992. https://doi.org/10.3390/foods13243992







