Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
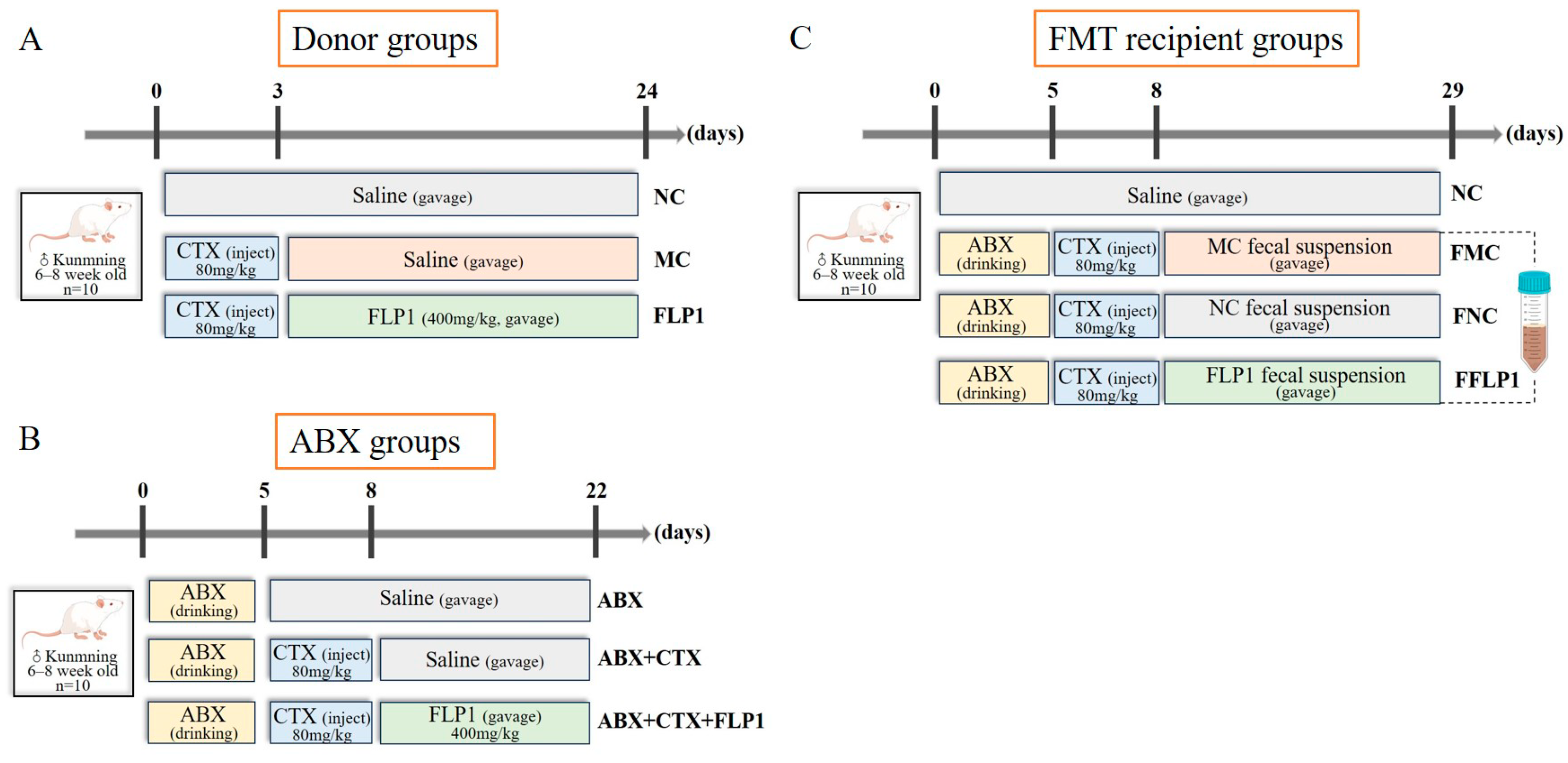
2.2. Animal Experiment Design
2.3. Histological Examination
2.4. Biochemical Analysis
2.5. Western Blot Assay
2.6. Gut Microbiota Analysis
2.7. Fecal Metabolomics Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of FLP1 on CTX-Induced Immunosuppression and Intestinal Injury in Mice
3.2. Effects of FLP1 on CTX-Induced Oxidative Stress and Intestine Immune Dysfunction
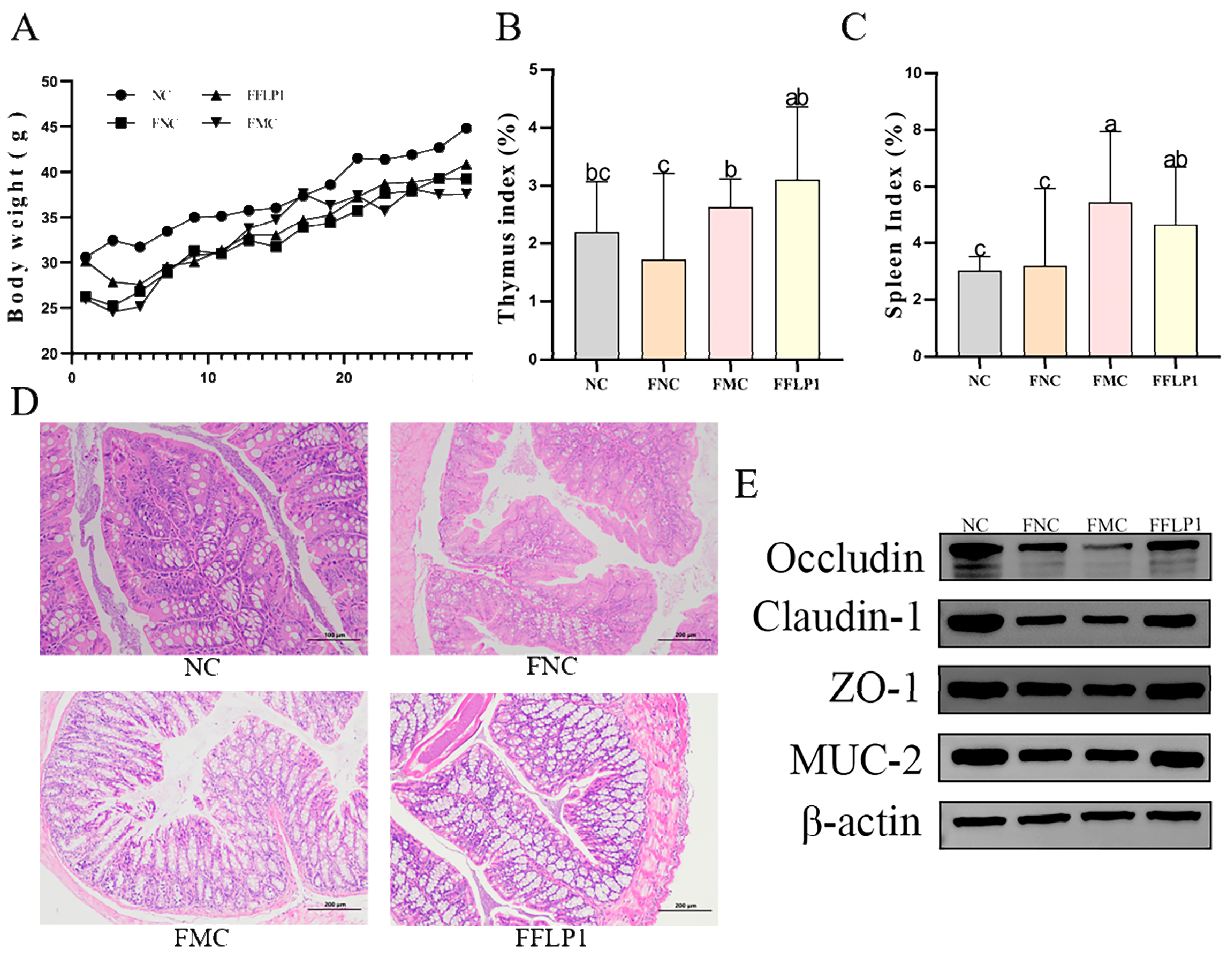
3.3. Effects of FFLP1 on CTX-Induced Immunosuppression and Intestinal Injury
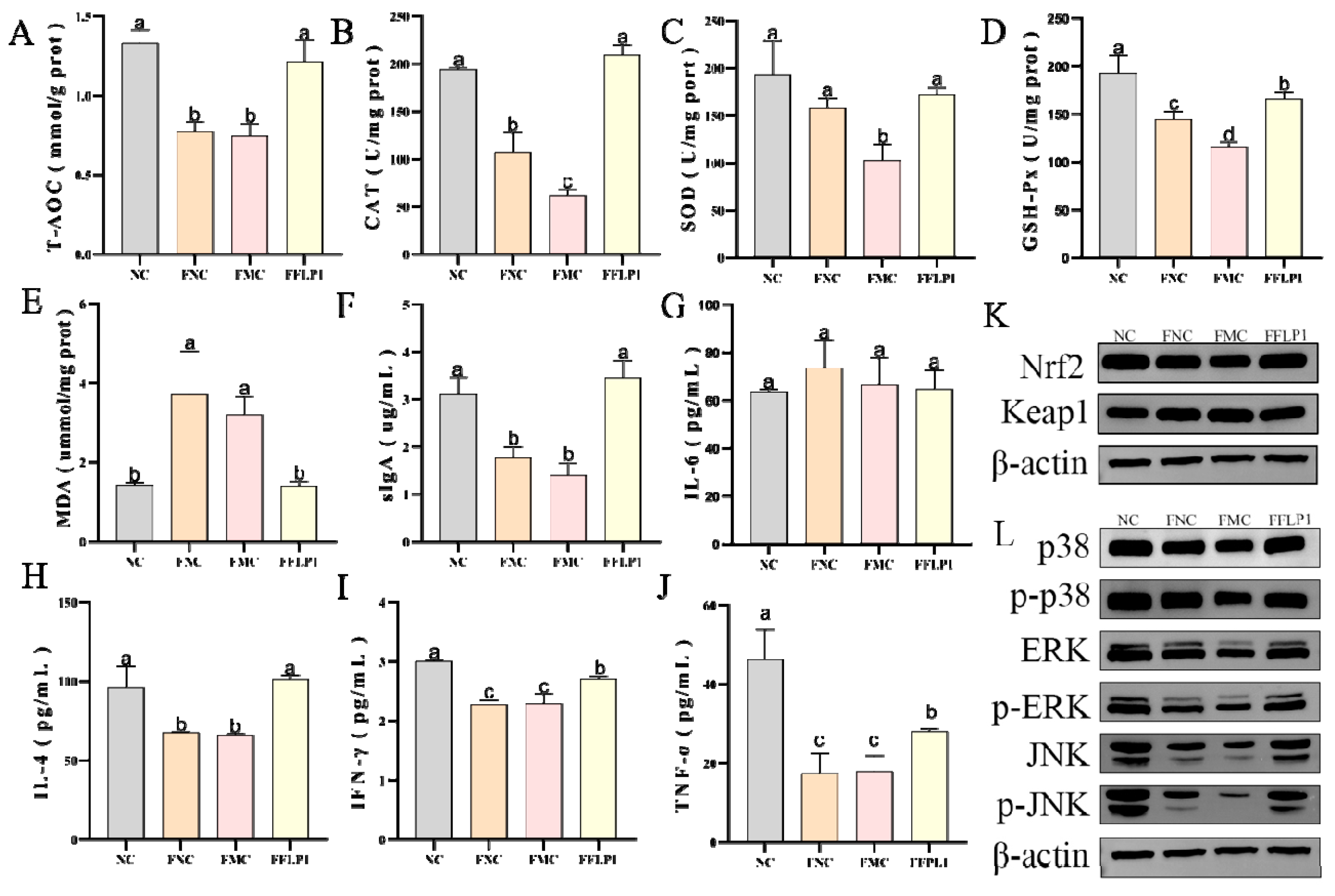
3.4. Effects of FMT on Oxidative Enzyme Secretion in CTX-Treated Groups
3.5. Effects of FMT on Intestine Immune Dysfunction Induced by CTX
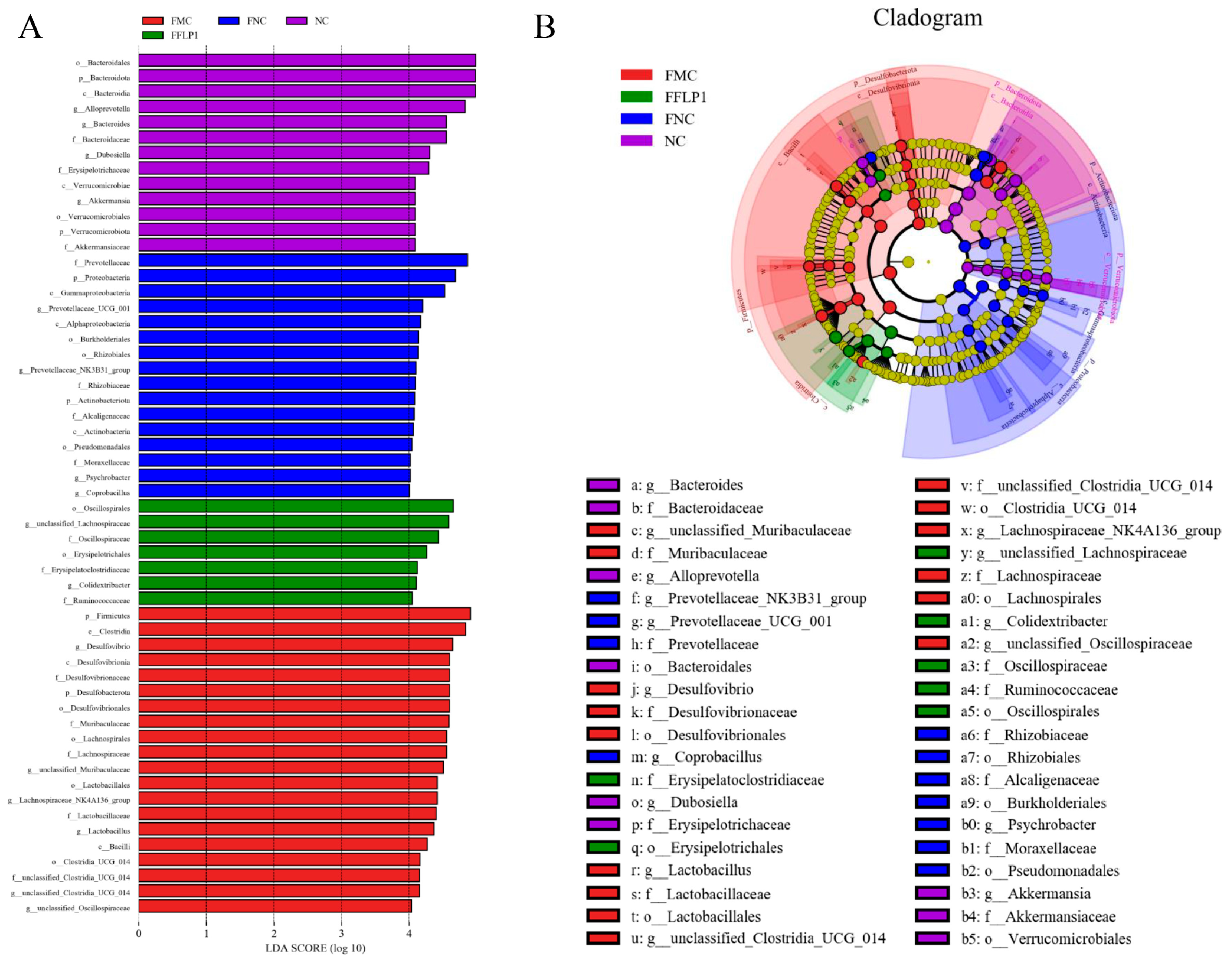
3.6. FMT Regulation of Intestinal Microbiota
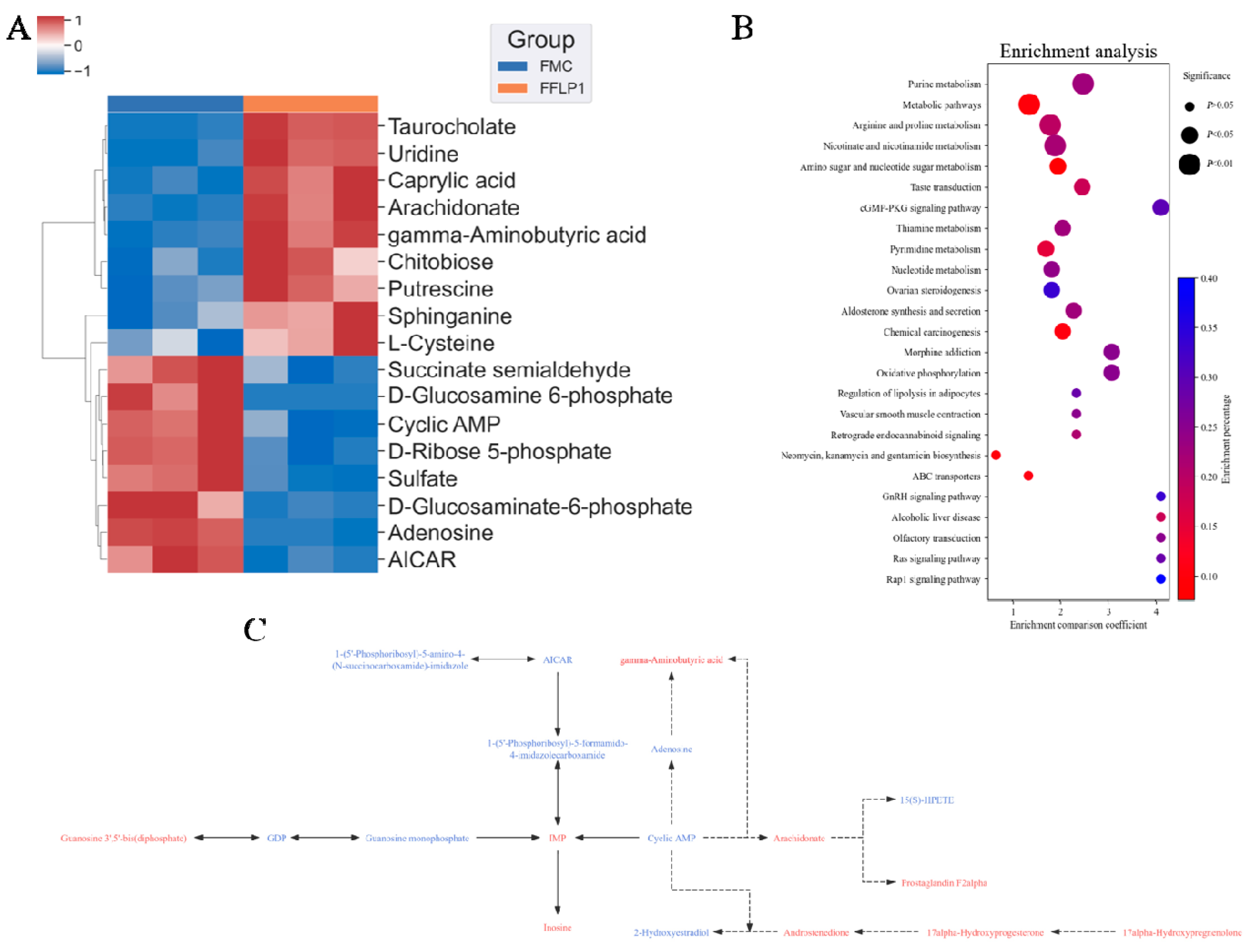
3.7. FMT Regulation of Metabolites in Fecal Sample
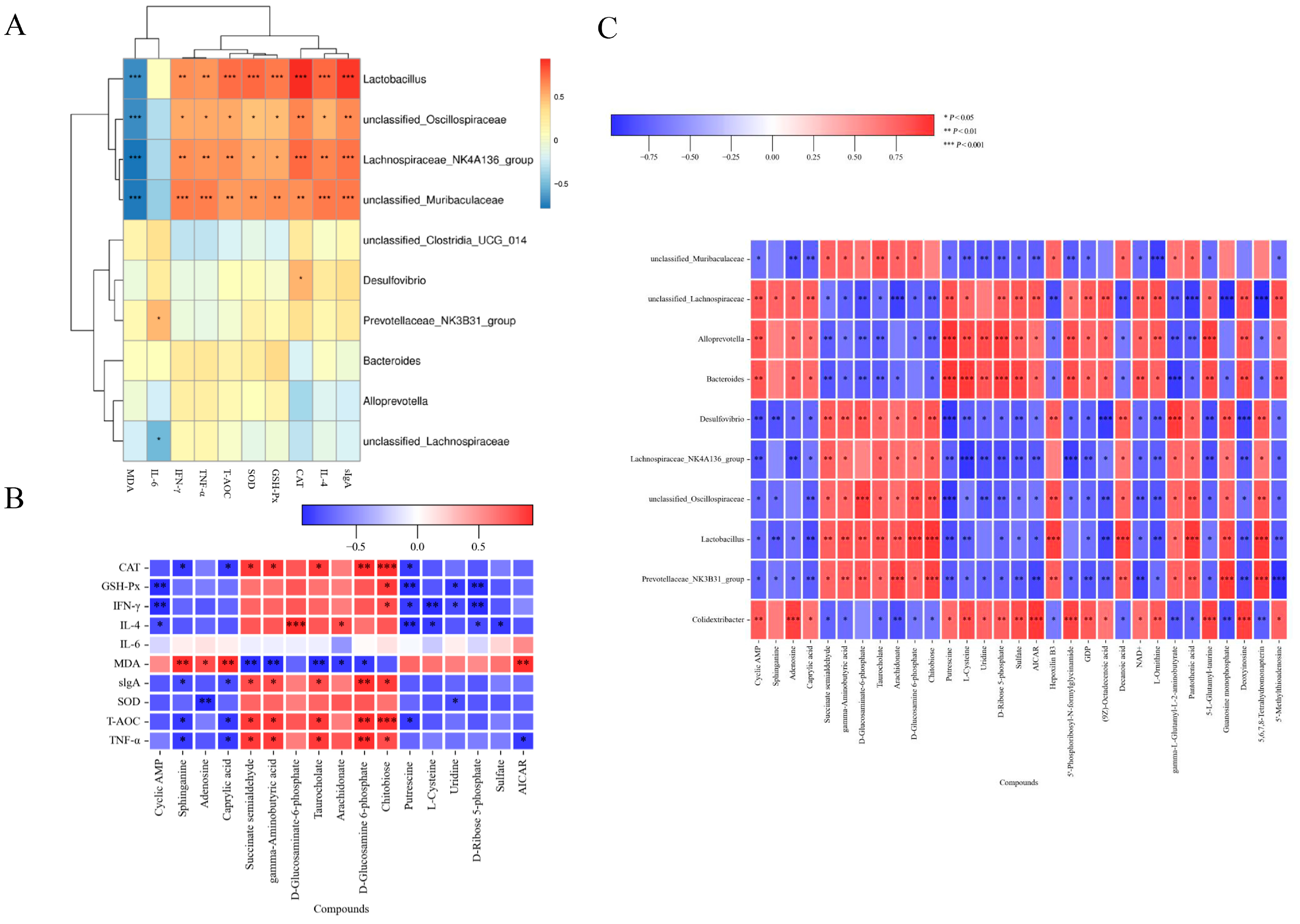
3.8. Correlation Between Gut Microbiota, Metabolites and Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinda, D.; Takada, T.; Mikami, T.; Shimizu, K.; Oana, K.; Arai, T.; Akitaya, K.; Sakuraba, H.; Katto, M.; Nagara, Y.; et al. Spatial distribution of live gut microbiota and bile acid metabolism in various parts of human large intestine. Sci. Rep. 2022, 12, 3593. [Google Scholar] [CrossRef] [PubMed]
- Serek, P.; Oleksy-Wawrzyniak, M. The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity. Int. J. Mol. Sci. 2021, 22, 11359. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Forsythe, S.J.; El-Nezami, H. Probiotics interaction with foodborne pathogens: A potential alternative to antibiotics and future challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 3320–3333. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions Between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, T.; Liu, C.; Shan, X.; Xu, Z.; Hong, H.; Lin, H.; Chen, J.; Sun, H. Cyclophosphamide induced physiological and biochemical changes in mice with an emphasis on sensitivity analysis. Ecotoxicol. Environ. Saf. 2021, 211, 111889. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides From American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, H.; Zhang, X.; Chen, Q. The Genomic and Transcriptomic Analyses of Floccularia luteovirens, a Rare Edible Fungus in the Qinghai–Tibet Plateau, Provide Insights into the Taxonomy Placement and Fruiting Body Formation. J. Fungi 2021, 7, 887. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Cao, L.; Li, W.; Zhang, Q.; Feng, R.; Zhao, Z.; Zhao, X. The Research Status and Prospects of Floccularia luteovirens: A Mycorrhizal Fungus with Edible Fruiting Bodies. J. Fungi 2023, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Liu, Q.H.; Ng, T.B.; Liu, H.Z.; Li, J.Q.; Chen, G.; Sheng, H.Y.; Xie, Z.L.; Wang, H.X. Isolation and characterization of a novel lectin from the mushroom Armillaria luteo-virens. Biochem. Biophys. Res. Commun. 2006, 345, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, J.; Guo, X.; Li, S.; Wang, F.; Wang, M. Armillaria luteo-virens Sacc Ameliorates Dextran Sulfate Sodium Induced Colitis through Modulation of Gut Microbiota and Microbiota-Related Bile Acids. Nutrients 2021, 13, 3926. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Wang, S.; Li, C.; Chen, C.; Wan, X.; Li, D.; Li, Y. Polysaccharides of Floccularia luteovirens Alleviate Oxidative Damage and Inflammatory Parameters of Diabetic Nephropathy in db/db Mice. FBL 2023, 28, 82. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Mueed, A.; Liu, D.; Ali, A.; Wang, T.; Ibrahim, M.; Su, L.; Wang, Q. Polysaccharides of Floccularia luteovirens regulate intestinal immune response, and oxidative stress activity through MAPK/Nrf2/Keap1 signaling pathway in immunosuppressive mice. Int. J. Biol. Macromol. 2024, 277, 134140. [Google Scholar] [CrossRef]
- Wang, T.; Jia, Z.; An, C.; Wang, Z.; Mueed, A.; Liu, Y.; Ma, H.; Guan, L.; Li, Y.; Su, L. The protective effect of Auricularia auricula polysaccharides on cyclophosphamide-induced immunosuppression and intestinal injury: A fecal microbiota transplantation study. Food Biosci. 2024, 62, 105416. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Jarade, A.; Garcia, Z.; Marie, S.; Demera, A.; Prinz, I.; Bousso, P.; Di Santo, J.P.; Serafini, N. Inflammation triggers ILC3 patrolling of the intestinal barrier. Nat. Immunol. 2022, 23, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, Y.; Wang, Y.; Fan, R.; Hu, X.; Zhang, F.; Yang, J.; Chen, J. The Role of Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Front. Immunol. 2022, 13, 871713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Dong, S.; Zhang, A.; Lu, Y.; Li, Y.; Lv, S.; Zhang, J. Role of oxidative stress in cardiotoxicity of antineoplastic drugs. Life Sci. 2019, 232, 116526. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, J.; Hou, L.; Gao, Y.; Sun, J.; Zhang, N.; Fan, B.; Wang, F. The Small Molecule Fractions of Floccularia luteovirens Induce Apoptosis of NSCLC Cells through Activating Caspase-3 Activity. Int. J. Mol. Sci. 2021, 22, 10609. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, M.; Yang, Z. Structural characterization, anti-tumor and immunomodulatory activity of intracellular polysaccharide from Armillaria luteo-virens. Carbohydr. Res. 2023, 534, 108945. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.-H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e2. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, J.; Xu, J.; Huangfu, W.; Zhang, Y.; Ali, Q.; Liu, B.; Li, D.; Cui, Y.; Wang, Z.; et al. Fecal microbiota transplantation alleviates intestinal inflammatory diarrhea caused by oxidative stress and pyroptosis via reducing gut microbiota-derived lipopolysaccharides. Int. J. Biol. Macromol. 2024, 261, 129696. [Google Scholar] [CrossRef]
- Chu Nathaniel, D.; Crothers Jessica, W.; Nguyen Le, T.T.; Kearney Sean, M.; Smith Mark, B.; Kassam, Z.; Collins, C.; Xavier, R.; Moses Peter, L.; Alm Eric, J. Dynamic Colonization of Microbes and Their Functions after Fecal Microbiota Transplantation for Inflammatory Bowel Disease. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Liu, D.; Mueed, A.; Ma, H.; Wang, T.; Su, L.; Wang, Q. Pleurocinus ostreatus Polysaccharide Alleviates Cyclophosphamide-Induced Immunosuppression through the Gut Microbiome, Metabolome, and JAK/STAT1 Signaling Pathway. Foods 2024, 13, 2679. [Google Scholar] [CrossRef]
- León, E.D.; Francino, M.P. Roles of Secretory Immunoglobulin A in Host-Microbiota Interactions in the Gut Ecosystem. Front. Microbiol. 2022, 13, 880484. [Google Scholar] [CrossRef]
- Li, C.; Duan, S.; Li, Y.; Pan, X.; Han, L. Polysaccharides in natural products that repair the damage to intestinal mucosa caused by cyclophosphamide and their mechanisms: A review. Carbohydr. Polym. 2021, 261, 117876. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Palm, N.W. Immunoglobulin A and the microbiome. Curr. Opin. Microbiol. 2020, 56, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qu, H.; Gao, Z.; Zeng, D.; Wang, J.; Baranenko, D.; Li, Y.; Lu, W. Protective effects of Ulva pertusa polysaccharide and polysaccharide-iron (III) complex on cyclophosphamide induced immunosuppression in mice. Int. J. Biol. Macromol. 2019, 133, 911–919. [Google Scholar] [CrossRef]
- Shen, M.; Chen, X.; Huang, L.; Yu, Q.; Chen, Y.; Xie, J. Sulfated Mesona chinensis Benth polysaccharide enhance the immunomodulatory activities of cyclophosphamide-treated mice. J. Funct. Foods 2021, 76, 104321. [Google Scholar] [CrossRef]
- Lei, Y.-y.; Ye, Y.-h.; Liu, Y.; Xu, J.-l.; Zhang, C.-l.; Lyu, C.-m.; Feng, C.-g.; Jiang, Y.; Yang, Y.; Ke, Y. Achyranthes bidentata polysaccharides improve cyclophosphamide-induced adverse reactions by regulating the balance of cytokines in helper T cells. Int. J. Biol. Macromol. 2024, 265, 130736. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Ma, P.; Huang, J.; Bai, X.; Liu, P.; Zhu, L.; Min, X. Immunomodulatory effect of polysaccharides isolated from Lonicera japonica Thunb. in cyclophosphamide-treated BALB/c mice. Heliyon 2022, 8, e11876. [Google Scholar] [CrossRef]
- Kong, X.; Duan, W.; Li, D.; Tang, X.; Duan, Z. Effects of polysaccharides from Auricularia auricula on the immuno-stimulatory activity and gut microbiota in immunosuppressed mice induced by cyclophosphamide. Front. Immunol. 2020, 11, 595700. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.-C.; Kim, Y.-K. Microbiota-Gut-Brain axis in major depression: A new therapeutic approach. In Neuroinflammation, Gut-Brain Axis and Immunity in Neuropsychiatric Disorders; Springer: Singapore, 2023; pp. 209–224. [Google Scholar]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Barcik, W.; Boutin, R.C.; Sokolowska, M.; Finlay, B.B. The role of lung and gut microbiota in the pathology of asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef]
- Almeida, C.; Oliveira, R.; Baylina, P.; Fernandes, R.; Teixeira, F.G.; Barata, P. Current trends and challenges of fecal microbiota transplantation—An easy method that works for all? Biomedicines 2022, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, P.; Chen, J.; Li, C.; Tian, Y.; Xu, F. Prebiotic properties of the polysaccharide from Rosa roxburghii Tratt fruit and its protective effects in high-fat diet-induced intestinal barrier dysfunction: A fecal microbiota transplantation study. Food Res. Int. 2023, 164, 112400. [Google Scholar] [CrossRef] [PubMed]
- Zikou, E.; Koliaki, C.; Makrilakis, K. The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review. Biomedicines 2024, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Pandit, N.G.; Zaman, M.; Minkoff, N.Z.; Tanner-Smith, E.E.; Gomez-Duarte, O.G.; Acra, S.; Nicholson, M.R. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2023, 4, CD012774. [Google Scholar] [PubMed]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.-F.; et al. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe 2020, 27, 823–829.e3. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Xu, Z.; Mak, J.W.Y.; Yang, K.; Liu, Q.; Zuo, T.; Tang, W.; Lau, L.; Lui, R.N.; Wong, S.H.; et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: A 24-week, double-blind, randomised controlled trial. Gut 2022, 71, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan inhibits oxidative stress and alleviates LPS-induced inflammation and apoptosis of BMECs by activating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 222, 2375–2391. [Google Scholar] [CrossRef]
- Li, J.; Han, J.; Lv, J.; Wang, S.; Qu, L.; Jiang, Y. Saikosaponin A-Induced Gut Microbiota Changes Attenuate Severe Acute Pancreatitis through the Activation of Keap1/Nrf2-ARE Antioxidant Signaling. Oxidative Med. Cell. Longev. 2020, 2020, 9217219. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, J.; Xu, Y.; Liu, C.; Qian, W.; Bai, T.; Hou, X. Piezo1 regulates intestinal epithelial function by affecting the tight junction protein claudin-1 via the ROCK pathway. Life Sci. 2021, 275, 119254. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.-W.; Yu, K.-W.; Suh, H.-J. Polysaccharides fractionated from enzyme digests of Korean red ginseng water extracts enhance the immunostimulatory activity. Int. J. Biol. Macromol. 2019, 121, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hou, P.; Xin, H.; Zhang, Y.; Zhou, A.; Lai, C.; Xie, J. A glucogalactomanan polysaccharide isolated from Agaricus bisporus causes an inflammatory response via the ERK/MAPK and IκB/NFκB pathways in macrophages. Int. J. Biol. Macromol. 2020, 151, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, S.; Liu, Z.; Lv, H.; Cai, X.; Zhong, R.; Chen, L.; Zhang, H. Baicalin restore intestinal damage after early-life antibiotic therapy: The role of the MAPK signaling pathway. Pharmacol. Res. 2024, 204, 107194. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Pinto, S.; Šajbenová, D.; Benincà, E.; Nooij, S.; Terveer, E.M.; Keller, J.J.; van der Meulen–de Jong, A.E.; Bogaards, J.A.; Steyerberg, E.W. Dynamics of Gut Microbiota After Fecal Microbiota Transplantation in Ulcerative Colitis: Success Linked to Control of Prevotellaceae. J. Crohn’s Colitis 2024, jjae137. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Q.; Zhang, B.; Chen, Z.; Huang, Q.; Xu, H.; Ren, J.; Zhang, X. IDDF2019-ABS-0252 Effect of multidonor intensive fecal microbiota transplantation by capsules for active uncreative colitis: A prospective trial. In Proceedings of the International Digestive Disease Forum (IDDF) 2019, Hong Kong, China, 8–9 June 2019; BMJ Publishing Group: London, UK, 2019. [Google Scholar]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira-a candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, P.; Tian, Y.; Liu, B.; Huang, L.; Liu, Z.; Lin, N.; Xu, N.; Ruan, Y.; Zhang, Z. Gut microbiota serves a predictable outcome of short-term low-carbohydrate diet (LCD) intervention for patients with obesity. Microbiol. Spectr. 2021, 9, e00223-21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, D.; Sun, Z.; Chen, X. Effects of intestinal Desulfovibrio bacteria on host health and its potential regulatory strategies: A review. Microbiol. Res. 2024, 284, 127725. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 2021, 13, 1930874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Peng, X.; Zhou, Q.Y.; Huang, Y.Y.; Rao, X.; Tu, J.L.; Xiao, H.Y.; Liu, D.M. Bacillus coagulans 13002 and fructo-oligosaccharides improve the immunity of mice with immunosuppression induced by cyclophosphamide through modulating intestinal-derived and fecal microbiota. Food Res. Int. 2021, 140, 109793. [Google Scholar] [CrossRef] [PubMed]
- Worledge, C.S.; Kostelecky, R.E.; Zhou, L.; Bhagavatula, G.; Colgan, S.P.; Lee, J.S. Allopurinol Disrupts Purine Metabolism to Increase Damage in Experimental Colitis. Cells 2024, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, B.; Li, R.; Yang, P.; Leng, P.; Huang, Y. Exploration of the link between gut microbiota and purinergic signalling. Purinergic Signal. 2023, 19, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Clemente Plaza, N.; Reig García-Galbis, M.; Martínez-Espinosa, R.M. Effects of the Usage of l-Cysteine (l-Cys) on Human Health. Molecules 2018, 23, 575. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Li, Y.; Yun, H.; Zhang, T.; Huang, Y.; Zhou, J.; Yan, H.; Wei, J.; Liu, Y.; Zhang, Z.; et al. Lactobacillus maintains healthy gut mucosa by producing L-Ornithine. Commun. Biol. 2019, 2, 171. [Google Scholar] [CrossRef]
- Li, J.-Y.; Guo, Y.-C.; Zhou, H.-F.; Yue, T.-T.; Wang, F.-X.; Sun, F.; Wang, W.-Z. Arginine metabolism regulates the pathogenesis of inflammatory bowel disease. Nutr. Rev. 2022, 81, 578–586. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E.; Iglesias, A.A. Nucleotide-sugar metabolism in plants: The legacy of Luis F. Leloir. J. Exp. Bot. 2021, 72, 4053–4067. [Google Scholar] [CrossRef] [PubMed]
- Mosenden, R.; Taskén, K. Cyclic AMP-mediated immune regulation—Overview of mechanisms of action in T cells. Cell. Signal. 2011, 23, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Khamaisi, H.; Mahmoud, H.; Mahajna, J. 2-hydroxyestradiol overcomes mesenchymal stem cells-mediated platinum chemoresistance in ovarian cancer cells in an ERK-independent fashion. Molecules 2022, 27, 804. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, M.; Hwang, S.W. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J. Neuroinflamm. 2020, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, M.; Lundberg, I.E. The skeletal muscle arachidonic acid cascade in health and inflammatory disease. Nat. Rev. Rheumatol. 2014, 10, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut–brain axis. npj Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Ma, Q.; Zhang, B. Dietary gamma-aminobutyric acid ameliorates growth impairment and intestinal dysfunction in turbot (Scophthalmus maximus L.) fed a high soybean meal diet. Food Funct. 2022, 13, 290–303. [Google Scholar] [CrossRef]
- Das, B.; Bhadra, R.K. (p) ppGpp metabolism and antimicrobial resistance in bacterial pathogens. Front. Microbiol. 2020, 11, 563944. [Google Scholar] [CrossRef]
- Huang, Y.; Chan, S.; Chen, S.; Liu, X.; Li, M.; Zheng, L.; Dong, Z.; Yang, Z.; Liu, Z.; Zhou, D. Wnt/β-catenin signalling activates IMPDH2-mediated purine metabolism to facilitate oxaliplatin resistance by inhibiting caspase-dependent apoptosis in colorectal cancer. J. Transl. Med. 2024, 22, 133. [Google Scholar] [CrossRef]
- Junqueira, S.C.; dos Santos Coelho, I.; Lieberknecht, V.; Cunha, M.P.; Calixto, J.B.; Rodrigues, A.L.S.; Santos, A.R.S.; Dutra, R.C. Inosine, an endogenous purine nucleoside, suppresses immune responses and protects mice from experimental autoimmune encephalomyelitis: A role for A2A adenosine receptor. Mol. Neurobiol. 2017, 54, 3271–3285. [Google Scholar] [CrossRef]
- Jeyaraj, F.T.; Voruganti, V.S. Multifaceted role of inosine in complex diseases and human health. Nutr. Rev. 2024, nuae029. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Mueed, A.; Ma, Y.; Ibrahim, M.; Su, L.; Wang, Q. Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice. Foods 2024, 13, 3881. https://doi.org/10.3390/foods13233881
Ma H, Mueed A, Ma Y, Ibrahim M, Su L, Wang Q. Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice. Foods. 2024; 13(23):3881. https://doi.org/10.3390/foods13233881
Chicago/Turabian StyleMa, He, Abdul Mueed, Yanxu Ma, Muhammad Ibrahim, Ling Su, and Qi Wang. 2024. "Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice" Foods 13, no. 23: 3881. https://doi.org/10.3390/foods13233881
APA StyleMa, H., Mueed, A., Ma, Y., Ibrahim, M., Su, L., & Wang, Q. (2024). Fecal Microbiota Transplantation Activity of Floccularia luteovirens Polysaccharides and Their Protective Effect on Cyclophosphamide-Induced Immunosuppression and Intestinal Injury in Mice. Foods, 13(23), 3881. https://doi.org/10.3390/foods13233881





