Effects of Growth Stages on Leaf Nutrients, Metabolites, and Polyphenols of Three Varieties of Haskap
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Sample Collection
2.2. Chemicals
2.3. Soluble Sugar Determination
2.4. Metabolite Analysis
2.5. Polyphenol Analysis
2.6. Organic Acid Analysis
2.7. Statistical Analysis
3. Results
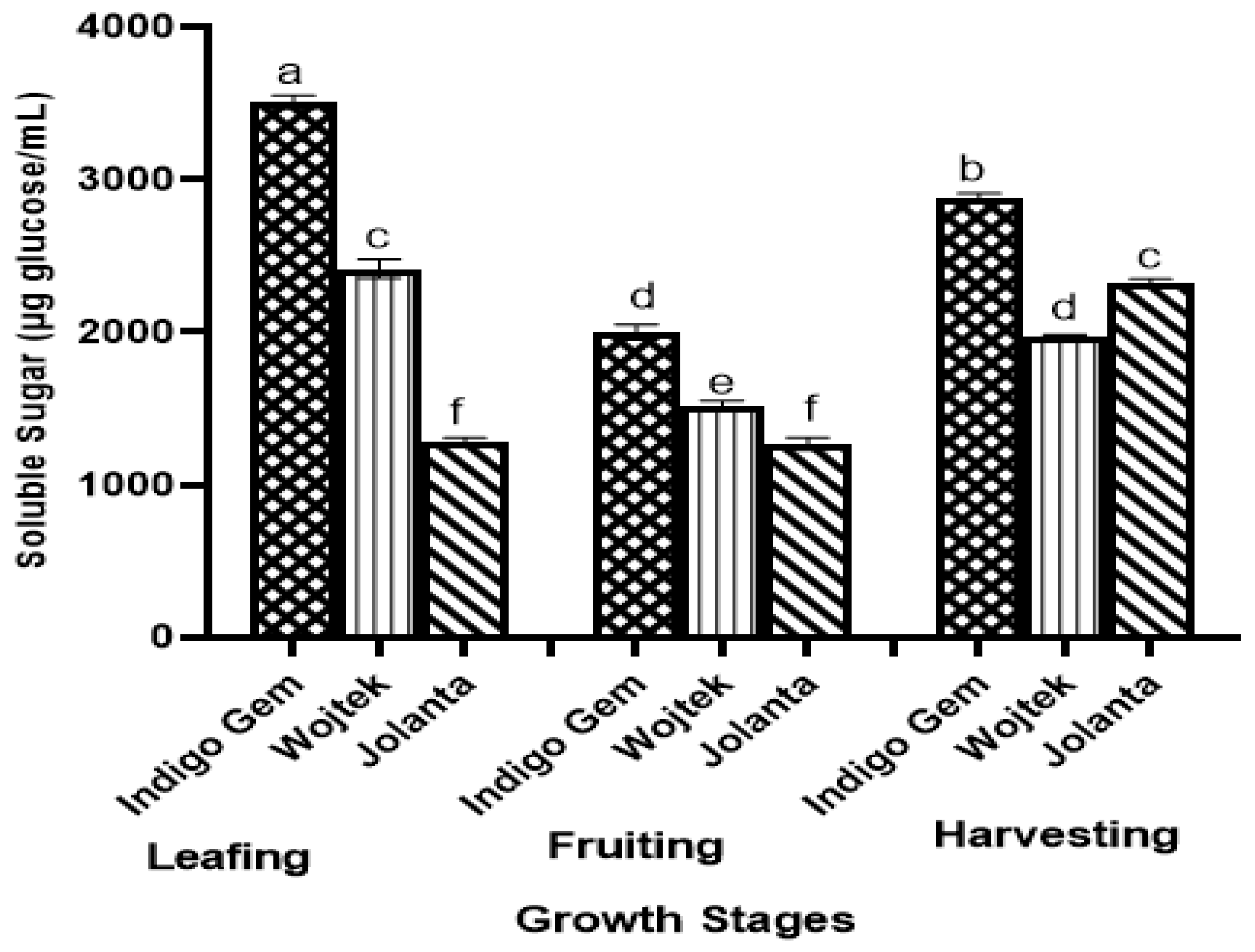
3.1. Soluble Sugar (SS) in Haskap Leaves
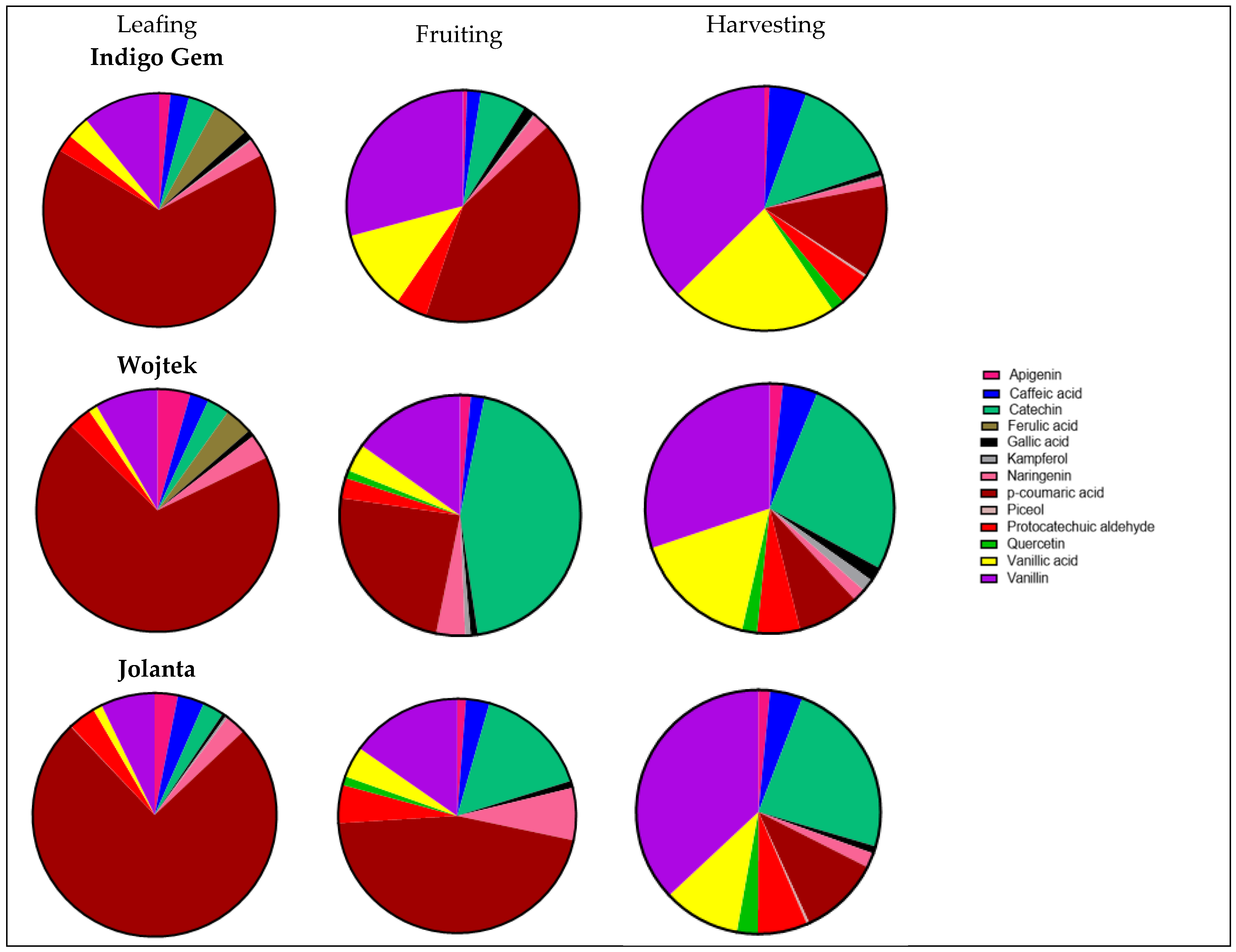
3.2. Polyphenols in Haskap Leaves
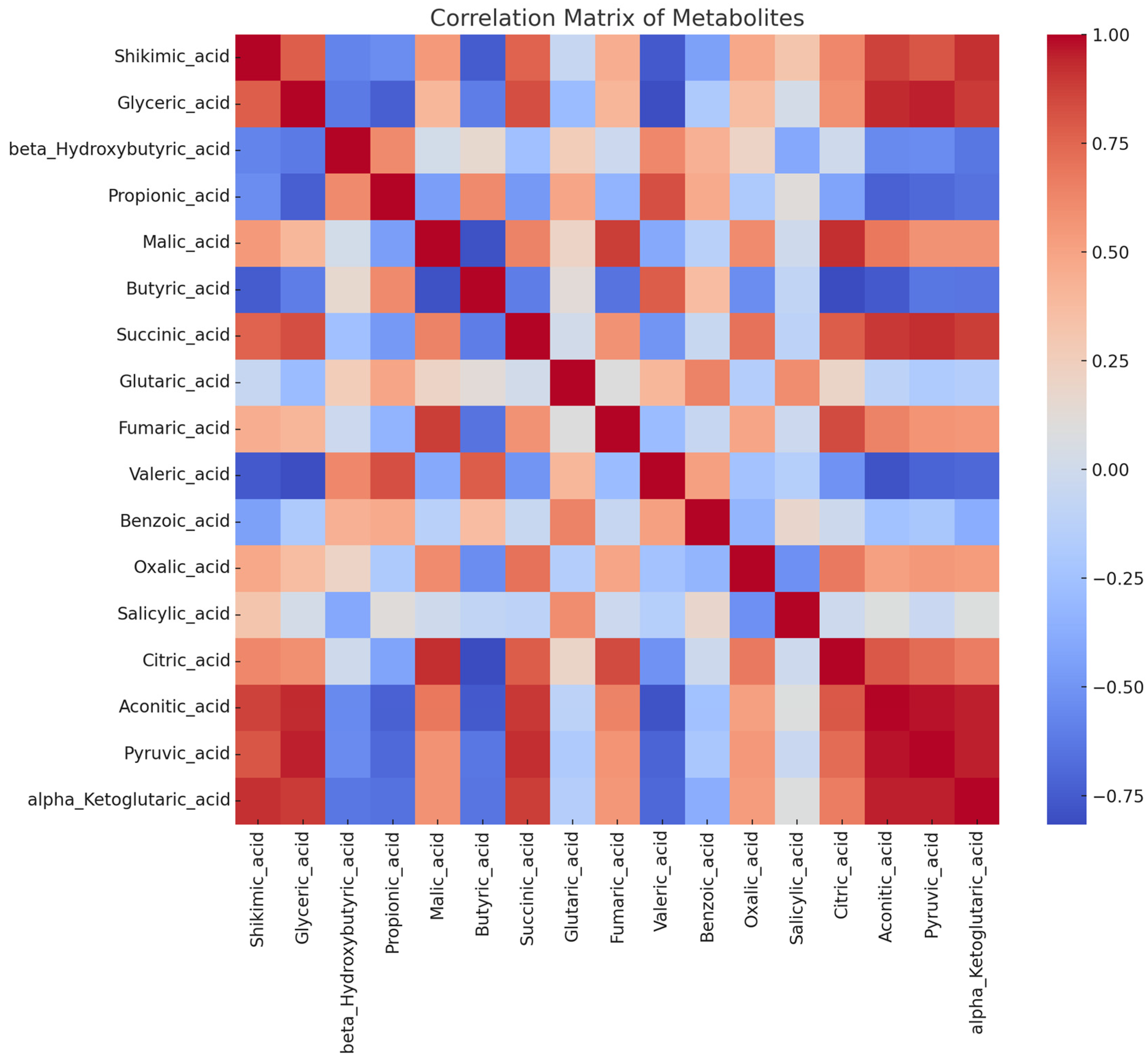
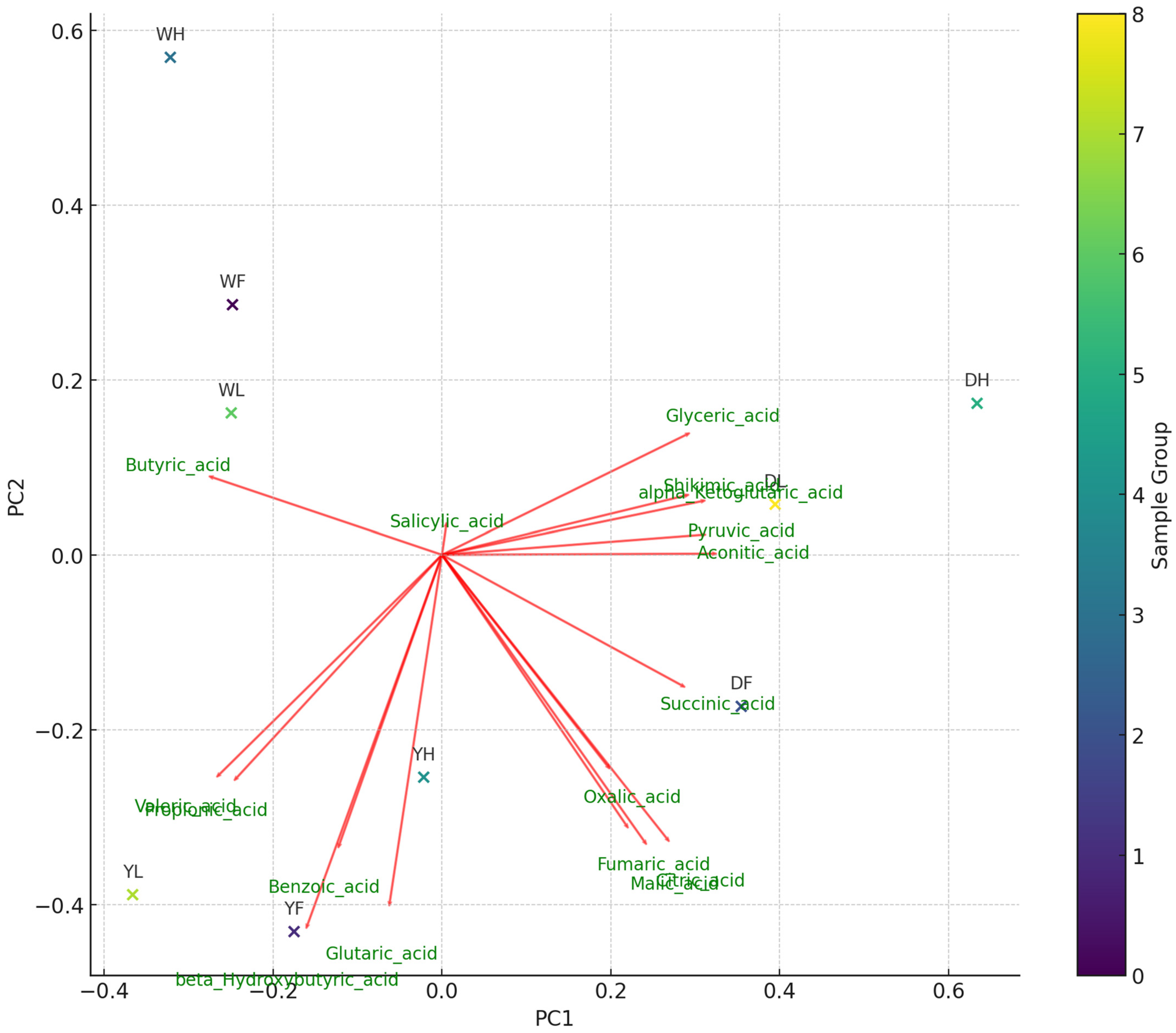
3.3. Organic Acids in Haskap Leaves
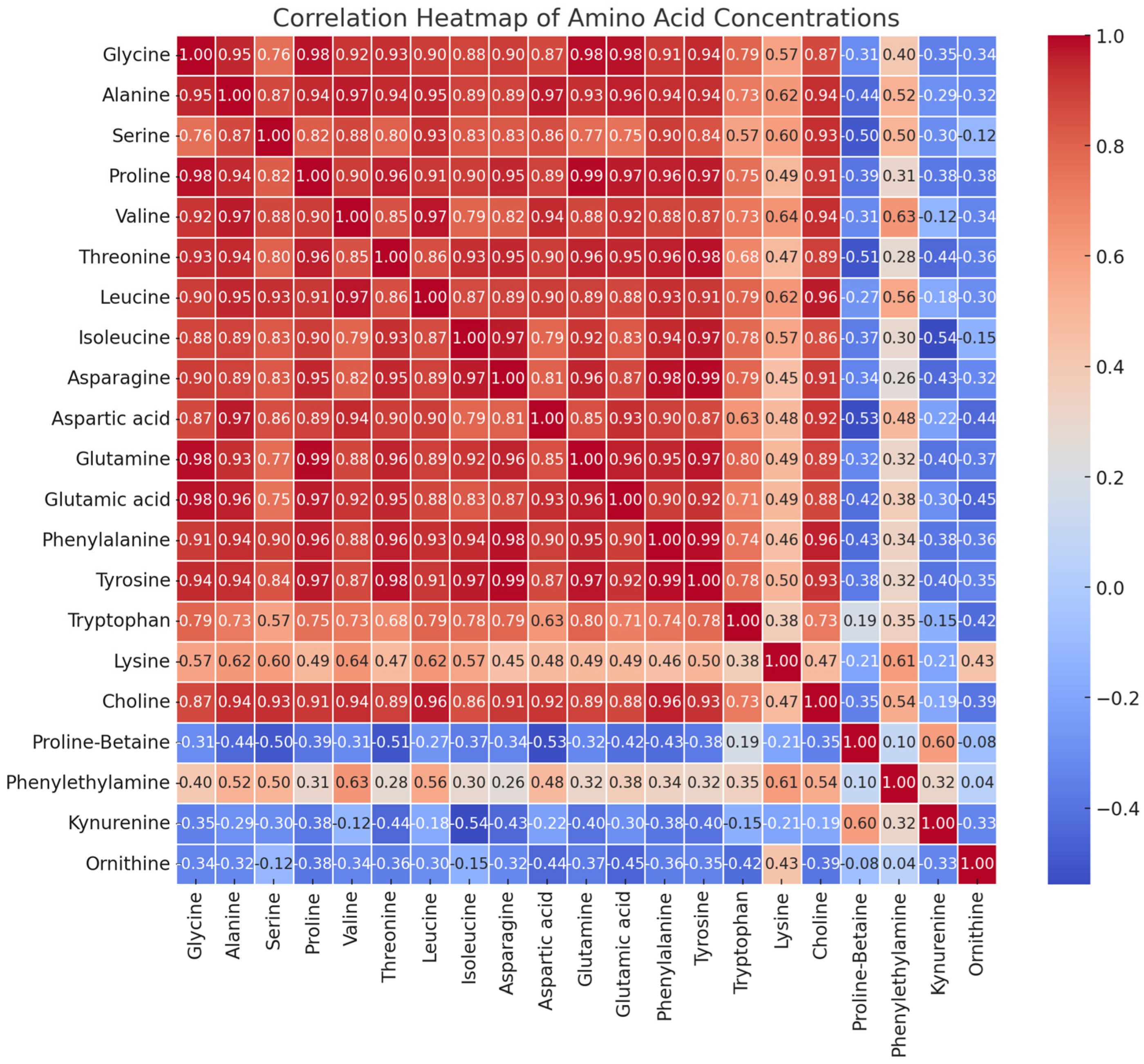
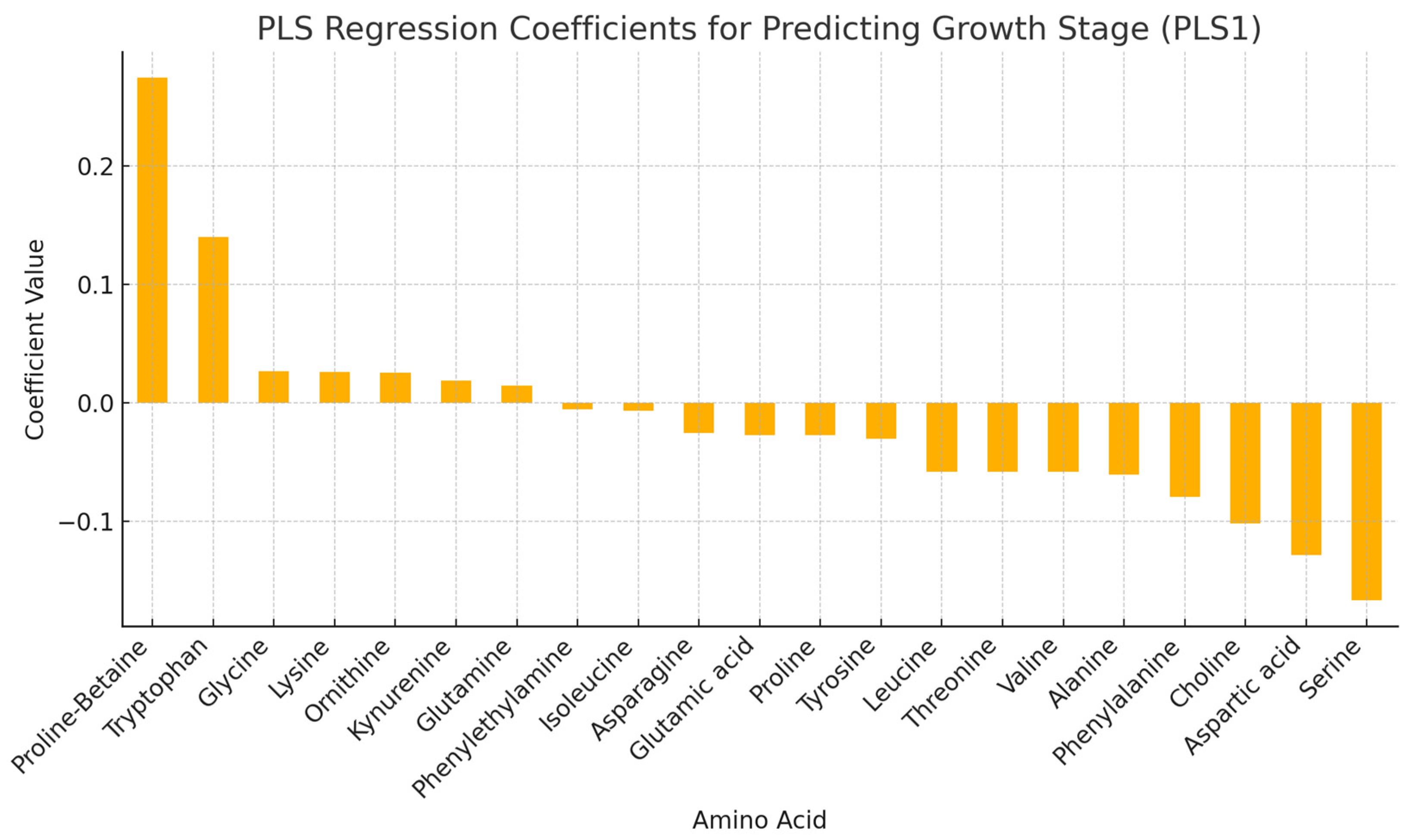
3.4. Amino Acids in Haskap Leaves
4. Discussion
4.1. Soluble Sugar (SS) Content of Haskap Leaves at Different Growth Stages
4.2. Polyphenol Concentrations of Haskap Leaves at Different Growth Stages
4.3. Organic Acid Concentrations of Haskap Leaves at Different Growth Stages
4.4. Amino Acid Concentrations of Haskap Leaves at Different Growth Stages
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, M.-M. Haskap arrives in North America. Pome. New. 2006, 31, 65–69. [Google Scholar]
- Liu, X.; Lv, Y.; Zheng, M.; Yin, L.; Wang, X.; Fu, Y.; Yu, B.; Li, J. Polyphenols from Blue Honeysuckle (Lonicera caerulea Var. edulis) Berry Inhibit Lipid Accumulation in Adipocytes by Suppressing Lipogenesis. J. Ethnopharmacol. 2021, 279, 114403. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.L. Haskap Berries (Lonicera caerulea L.)—A Critical Review of Antioxidant Capacity and Health-Related Studies for Potential Value-Added Products. Food Bio. Tech. 2014, 7, 1541–1554. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. An anthocyanin-rich extract from Kamchatka honeysuckle increases enzymatic activity within the gut and ameliorates abnormal lipid and glucose metabolism in rats. Nutrition 2013, 29, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.-P.-V.; Yu, L.-J.; Bhullar, K.-B.; Bors, B. Haskap (Lonicera caerulea): A new berry crop with high antioxidant capacity. Can. J. Plant Sci. 2012, 92, 1311–1317. [Google Scholar] [CrossRef]
- Li, B.; Cheng, Z.; Sun, X.; Si, X.; Gong, E.; Wang, Y.; Tian, J.; Shu, C.; Ma, F.; Li, D.; et al. Lonicera caerulea L. polyphenols alleviate oxidative stress-induced intestinal environment imbalance and lipopolysaccharide-induced liver injury in HFD-fed rats by regulating the Nrf2/HO-1/NQO1 and MAPK pathways. Mol. Nutr. Food Res. 2020, 64, 1901315. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Xu, R.; Zhang, X.; Zhang, W.; Du, M.; Liu, X.; Fan, L. Simultaneous Optimization of the Acidified Water Extraction for Total Anthocyanin Content, Total Phenolic Content, and Antioxidant Activity of Blue Honeysuckle Berries (Lonicera caerulea L.) Using Response Surface Methodology. Food Sci. Nutr. 2019, 7, 2968–2976. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Dong, Y.; Fang, Z.; Nisar, T.; Zhao, T.; Wang, Z.-C.; Guo, Y. Chemical Compositions and α-Glucosidase Inhibitory Effects of Anthocyanidins from Blueberry, Blackcurrant and Blue Honeysuckle Fruits. Food Chem. 2019, 299, 125102. [Google Scholar] [CrossRef]
- Senica, M.; Bavec, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue Honeysuckle (Lonicera caerulea Subsp. edulis (Turcz. Ex Herder) Hultén.) Berries and Changes in Their Ingredients across Different Locations. J. Sci. Food Agric. 2018, 98, 3333–3342. [Google Scholar] [CrossRef]
- Lauritzen, E.; Maughan, T.; Black, B. Haskap (Blue Honeysuckle) in the Garden; All Current Publications; Utah State University Extension: Logan, UT, USA, 2015; p. 752. Available online: https://digitalcommons.usu.edu/extension_curall/752 (accessed on 5 October 2024).
- Cheng, Z.; Bao, Y.; Li, Z.; Wang, J.; Wang, M.; Wang, S.; Wang, Y.; Wang, Y.; Li, B. Lonicera caerulea (Haskap berries): A review of development traceability, functional value, product development status, future opportunities, and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 8992–9016. [Google Scholar] [CrossRef]
- Sip, S.; Sip, A.; Szulc, P.; Cielecka-Piontek, J. Haskap Berry Leaves (Lonicera caerulea L.)—The Favorable Potential of Medical Use. Nutrition 2022, 14, 3898. [Google Scholar] [CrossRef] [PubMed]
- Studzίnska-Sroka, E.; Galanty, A.; Gósciniak, A.; Wieczorek, M.; Kłaput, M.; Dudek-Makuch, M.; Cielecka-Piontek, J. Herbal Infusions as a Valuable Functional Food. Nutrition 2021, 13, 4051. [Google Scholar] [CrossRef]
- Etheridge, C.-J.; Derbyshire, E. Herbal Infusions and Health: A Review of Findings from Human Studies, Mechanisms, and Future Research Directions. Nutr. Food Sci. 2020, 50, 969–985. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, Y.; Leng, F.; Wang, S.; Wang, F.; Zhuang, Y.; Liu, X.; Wang, X.; Ma, X. Establishment and application of a method for rapid determination of total sugar content based on colorimetric microplate. Sugar Tech. 2017, 19, 424–431. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Giuberti, G.; Bhumireddy, S.-R.; Mandal, R.; Trevisan, M.; Wishart, D.-S. Transformation of polyphenols found in pigmented gluten-free flours during in vitro large intestinal fermentation. Food chem. 2019, 298, 125068. [Google Scholar] [CrossRef]
- Zheng, J.; Johnson, M.; Mandal, R.; Wishart, D.-S. A comprehensive targeted metabolomics assay for crop plant sample analysis. Metabolites 2021, 11, 303. [Google Scholar] [CrossRef]
- Ortiz, A.; Echeverria, G.; Graell, J.; Lara, I. Overall quality of ‘Rich Lady’ peach fruit after air- or CA storage. The importance of volatile emission. LWT-Food Sci. Technol. 2019, 42, 1520–1529. [Google Scholar] [CrossRef]
- Gamble, L.; Pont, S.-D.; Allwood, J.-W.; Jarret, D.-A.; Hancock, R.-D. Comparative analysis of quality and nutritional traits from Lonicera caerulea (Honeyberry) cultivars and other berries grown in Scotland. Ann. Appl. Biol. 2023, 182, 171–182. [Google Scholar] [CrossRef]
- Dragovicuzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Debelo, H.; Li, M.; Ferruzzi, M.-G. Processing influences food polyphenol profiles and biological activity. Curr. Opin. Food Sci. 2020, 32, 90–102. [Google Scholar] [CrossRef]
- Archivio, D.; Filesi, M.; Di Benedetto, C.; Gargiulo, R.; Giovannini, R.; Masella, C.-R. Polyphenols, Dietary Sources and Bioavailability. Ann.-Ist. Super. Sanita 2007, 43, 348. [Google Scholar] [PubMed]
- Scalbert, A.; Johnson, I.-T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutri. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J.; Covas, M.-I.; Fitó, M.; Schröder, H.; Miró-Casas, E.; Gimeno, E.; López-Sabater, M.-C.; de la Torre, R.; Farré, M. Effects of Differing Phenolic Content in Dietary Olive Oils on Lipids and LDL Oxidation. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E.; Remesy, C. Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo? J. Cereal Sci. 2008, 48, 258–276. [Google Scholar] [CrossRef]
- Torres, T.-J.-L.; Rosazza, J.-P.-N. Microbial transformations of p-coumaric acid by Bacillus megaterium and Curvularia lunata. J. Nat. Prod. 2001, 64, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Abramovic, H.; Abram, V.; Ulrih, N.-P. Interactions of p-coumaric, caffeic and ferulic acids and their styrenes with model lipid membranes. Food Chem. 2011, 125, 1256–1261. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V.; Goel, N. Structural, thermal and quantum chemical studies of p-coumaric and caffeic acids. J. Mol. Struct. 2015, 1085, 242–248. [Google Scholar] [CrossRef]
- Ferguson, L.-R.; Zhu, S.-T.; Harris, P.-J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutri. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef]
- Garrait, G.; Jarrige, J.-F.; Blanquet, S.; Beyssac, E.; Cardot, J.-M.; Alric, M. Gastrointestinal absorption and urinary excretion of trans-cinnamic and p-coumaric acids in rats. J. Agri. Food Chem. 2006, 54, 2944–2950. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.-E.; Zakaria, Z.-A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.-E. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.-A.; Paiva, N.-L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.-J.; Narbad, A.; Parr, A.-J.; Parker, M.-L.; Walton, N.-J.; Mellon, F.-A.; Michael, A.-J. Rerouting the plant phenylpropanoid pathway by expression of a novel bacterial enoyl-CoA hydratase/lyase enzyme function. Plant Cell 2001, 13, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Siquet, C.-F.; Paiva-Martins, J.-L.; Lima, S.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy-and trihydroxyphenolic acids-A structure–activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef]
- Dawson, J. Concentration and Content of Secondary Metabolites. Doctoral Dissertation, University of Saskatchewan, Saskatoon, SK, Canada, 2017. [Google Scholar]
- Widy-Tyszkiewicz, E. Current evidence for disease prevention and treatment by protocatechuic acid (PCA) and its precursor protocatechuic aldehyde (PCAL) in Animals and Humans. In Plant Antioxidants and Health, 1st ed.; Springer: Cham, Switzerland, 2022; pp. 507–543. [Google Scholar] [CrossRef]
- Little, C.; Combet, E.; McMillan, D.; Horgan, P.; Roxburgh, C. The Role of Dietary Polyphenols in the Moderation of the Inflammatory Response in Early-Stage Colorectal Cancer. Crit. Rev. Food Sci. Nutr. 2015, 57, 2310–2320. [Google Scholar] [CrossRef]
- Boots, A.-W.; Haenen, G.-R.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Buchweitz, M.; Kroon, P.-A.; Rich, G.-T.; Wilde, P.-J. Quercetin Solubilisation in Bile Salts: A Comparison with Sodium Dodecyl Sulphate. Food Chem. 2016, 211, 356–364. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.; Liu, W.; McClements, D.-J. Potential of Excipient Emulsions for Improving Quercetin Bio-accessibility and Antioxidant Activity: An in Vitro Study. J. Agric. Food Chem. 2016, 64, 3653–3660. [Google Scholar] [CrossRef]
- Hisanaga, A.; Mukai, R.; Sakao, K.; Terao, J.; Hou, D.-X. Anti-Inflammatory Effects and Molecular Mechanisms of 8-Prenyl Quercetin. Mol. Nutr. Food Res. 2016, 60, 1020–1032. [Google Scholar] [CrossRef]
- Guillermo Gormaz, J.; Quintremil, S.; Rodrigo, R. Cardiovascular Disease: A Target for the Pharmacological Effects of Quercetin. Curr. Top. Med. Chem. 2015, 15, 1735–1742. [Google Scholar] [CrossRef]
- Clouth, A.; Schöfer, H. Treatment of Recalcitrant Facial Verrucae Vulgares with Sinecatechins (Green tea Catechins) Ointment. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.-V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Türközü, D.; Acar Tek, N. A Minireview of Effects of Green Tea on Energy Expenditure. Crit. Rev. Food Sci. Nutr. 2017, 57, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, P.-V.; Balaraman, M. Catechins: Sources, Extraction, and Encapsulation: A Review. Food Bioprod. Proc. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Arts, I.-C.; Hollman, P.-C.; Feskens, E.-J.; De Mesquita, H.-B.-B.; Kromhout, D. Catechin Intake Might Explain the Inverse Relation between Tea Consumption and Ischemic Heart Disease: The Zutphen Elderly Study. Am. J. Clin. Nutr. 2001, 74, 227–232. [Google Scholar] [CrossRef]
- Kivits, G.; Weststrate, J.; Tijburg, L. Bioavailability of Catechins from Tea: The Effect of Milk. Eur. J. Clin. Nutr. 1998, 52, 356–359. [Google Scholar] [CrossRef]
- Burri, J.; Graf, M.; Lambelet, P.; Loliger, J. Vanillin: More than a flavouring agent—A potent antioxidant. J. Sci. Food Agric. 1989, 48, 49–56. [Google Scholar] [CrossRef]
- Davidson, P.-M.; Naidu, A.-S. Phyto-phenols. In Natural Food Antimicrobial Systems; Naidu, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 265–294. [Google Scholar]
- Chidi, B.; Rossouw, D.; Buica, A.; Bauer, F. Determining the impact of industrial wine yeast strains on organic acid production under white and red wine-like fermentation conditions. S. Afr. J. Enol. 2015, 36, 316–327. [Google Scholar] [CrossRef]
- Rupasinghe, H.-P.-V.; Boehm, M.-A.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A.-R. Anti-inflammatory activity of haskap cultivars is polyphenols-dependent. Biomolecules 2015, 5, 1079–1098. [Google Scholar] [CrossRef]
- Česonien, L.; Labokas, J.; Jasutienė, I.; Šarkinas, A.; Kaškonienė, V.; Kaškonas, P.; Kazernavičiūtė, R.; Pažereckaitė, A.; Daubaras, R. Bioactive compounds, antioxidant, and antibacterial properties of Lonicera caerulea berries: Evaluation of 11 cultivars. Plants 2021, 10, 624. [Google Scholar] [CrossRef]
- Sochor, J.; Jurikova, T.; Pohanka, M.; Skutkova, H.; Baron, M.; Tomaskova, L.; Balla, S.; Klejdus, B.; Pokluda, R.; Mlcek, J.; et al. Evaluation of antioxidant activity, polyphenolic compounds, amino acids and mineral elements of representative genotypes of Lonicera edulis. Molecules 2014, 19, 6504–6523. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.-H.; Da Costa, K.-A.; Franklin, P.-D.; Alexander, E.-A.; Lamont, J.-T.; Sheard, N.-F.; Beiser, A.-L. Choline, an essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.-H.; Smith, R.-A.; Williams, C.-L. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav. Neurosci. 1989, 103, 1234. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.-H.; Williams, C.-L. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 1997, 8, 2831–2835. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emenike, C.; Azeez, S.; English, M.; Udenigwe, C.; Abbey, L. Effects of Growth Stages on Leaf Nutrients, Metabolites, and Polyphenols of Three Varieties of Haskap. Foods 2024, 13, 3762. https://doi.org/10.3390/foods13233762
Emenike C, Azeez S, English M, Udenigwe C, Abbey L. Effects of Growth Stages on Leaf Nutrients, Metabolites, and Polyphenols of Three Varieties of Haskap. Foods. 2024; 13(23):3762. https://doi.org/10.3390/foods13233762
Chicago/Turabian StyleEmenike, Chijioke, Shakirah Azeez, Marcia English, Chibuike Udenigwe, and Lord Abbey. 2024. "Effects of Growth Stages on Leaf Nutrients, Metabolites, and Polyphenols of Three Varieties of Haskap" Foods 13, no. 23: 3762. https://doi.org/10.3390/foods13233762
APA StyleEmenike, C., Azeez, S., English, M., Udenigwe, C., & Abbey, L. (2024). Effects of Growth Stages on Leaf Nutrients, Metabolites, and Polyphenols of Three Varieties of Haskap. Foods, 13(23), 3762. https://doi.org/10.3390/foods13233762







