Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Determination of Total Phenolic Content and Total Flavonoids
2.4. Screening Active Compounds by DPPH-UPLC-MS
2.5. Antioxidant Capacity of Polyphenol Compounds of Sugarcane Vinegar
2.5.1. Determination of DPPH Radical Scavenging Capacity
2.5.2. Determination of ABTS Radical Scavenging Capacity
2.5.3. Antioxidant Capacity of the Binary Interactions Among Phenolic Compounds
2.6. Network Pharmacology
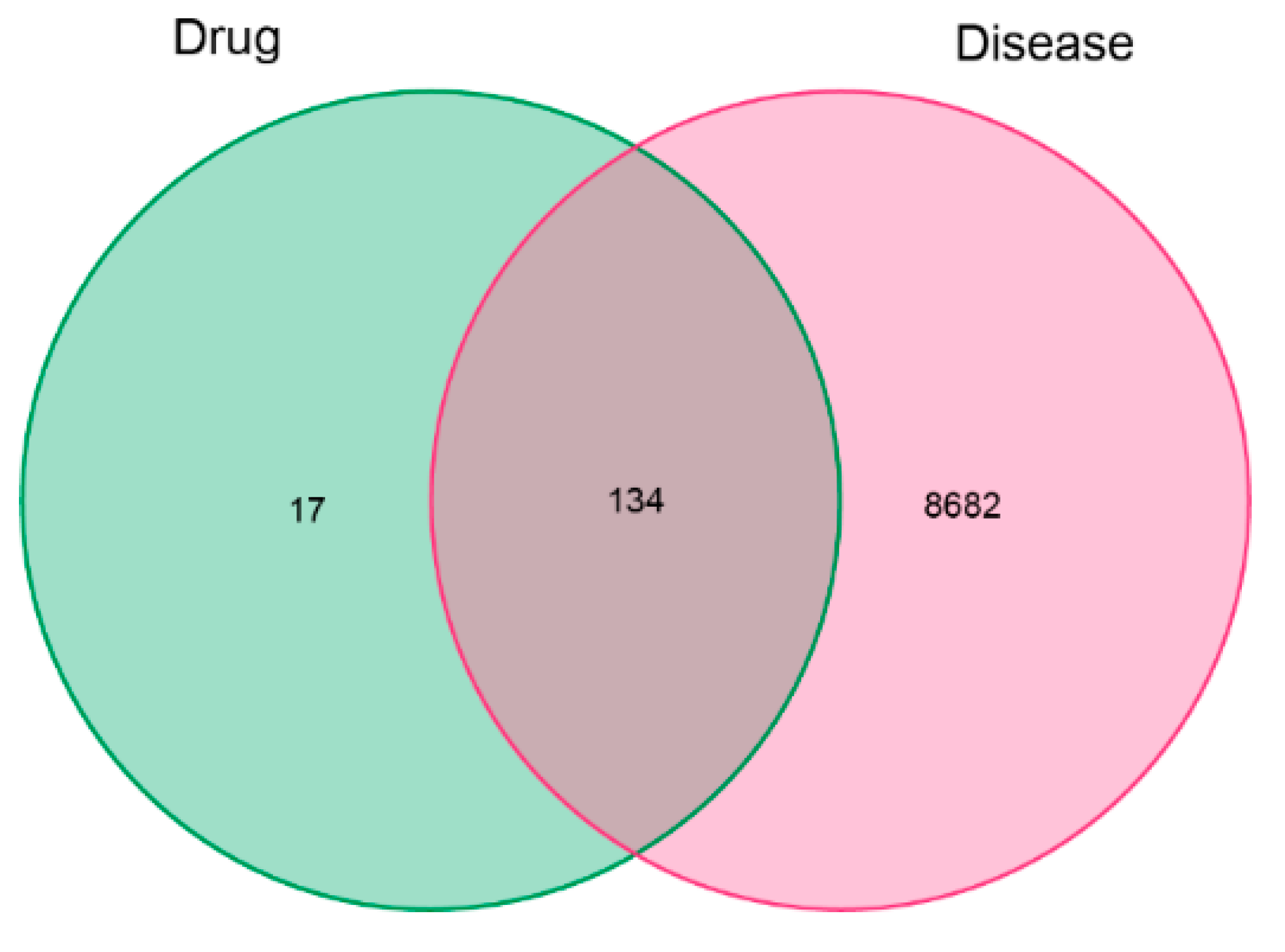
2.6.1. Screening Candidate Compounds and Potential Targets
2.6.2. Pathway Enrichment Analysis
2.6.3. Protein–Protein Interaction Network Construction and Screening of Core Targets
2.6.4. Molecular Docking Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. Correlation of TEAC, TPC, and TFC Values
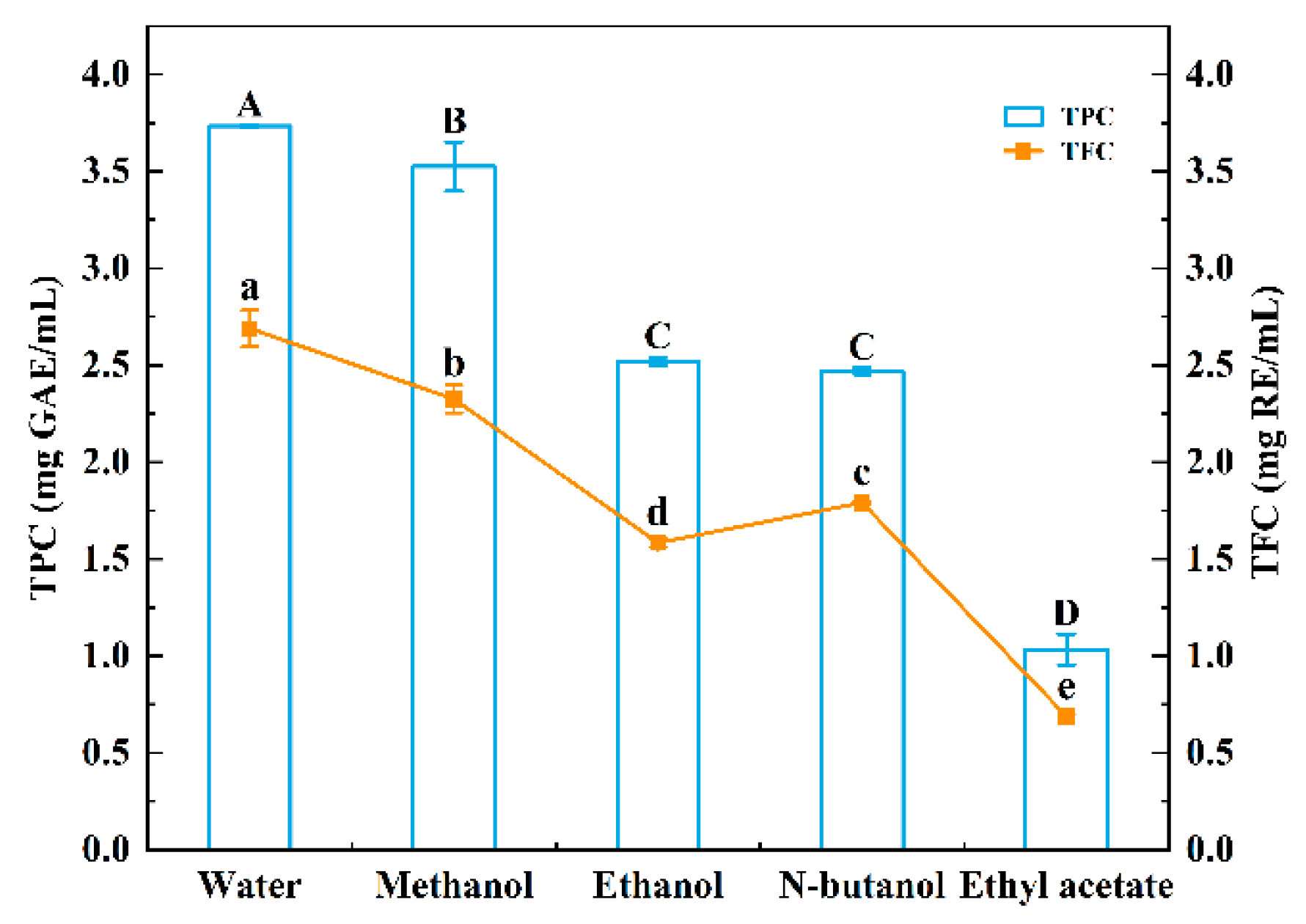
3.1.1. Effects of Extraction Solvents on TPC and TFC of Sugarcane Vinegar Extracts
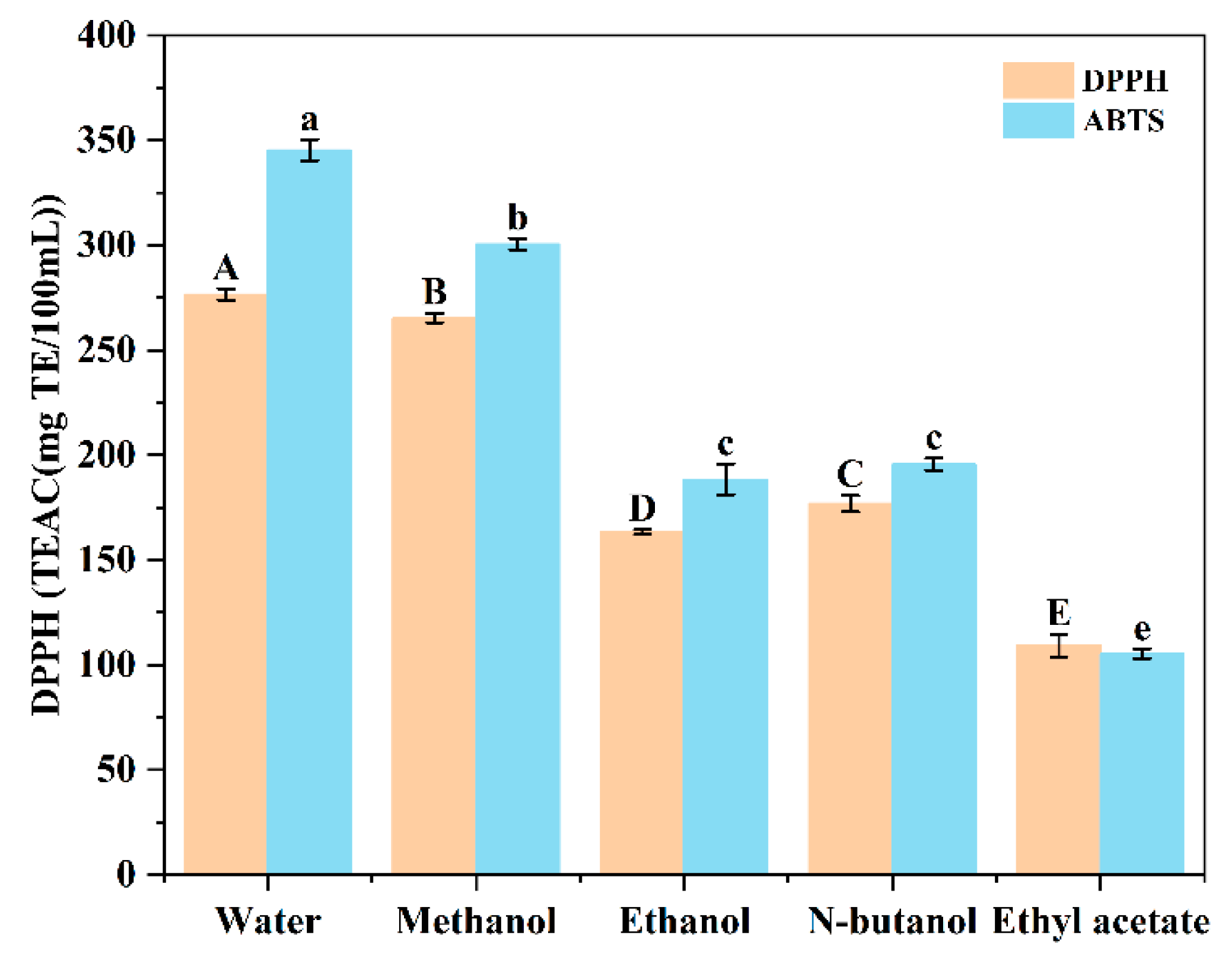
3.1.2. Effect of Extraction Solvents on Antioxidant Capacity of Sugarcane Vinegar Extracts
3.1.3. Correlation Analysis
3.2. Screening Active Compounds by DPPH-UPLC-MS
3.3. Antioxidant Capacity of Polyphenol Compounds of Sugarcane Vinegar
3.3.1. Effect of Solvent on Antioxidant Activity of Polyphenols from Sugarcane Vinegar
3.3.2. Interactions Between the Antioxidants in Sugarcane Vinegar
3.4. Network Pharmacology
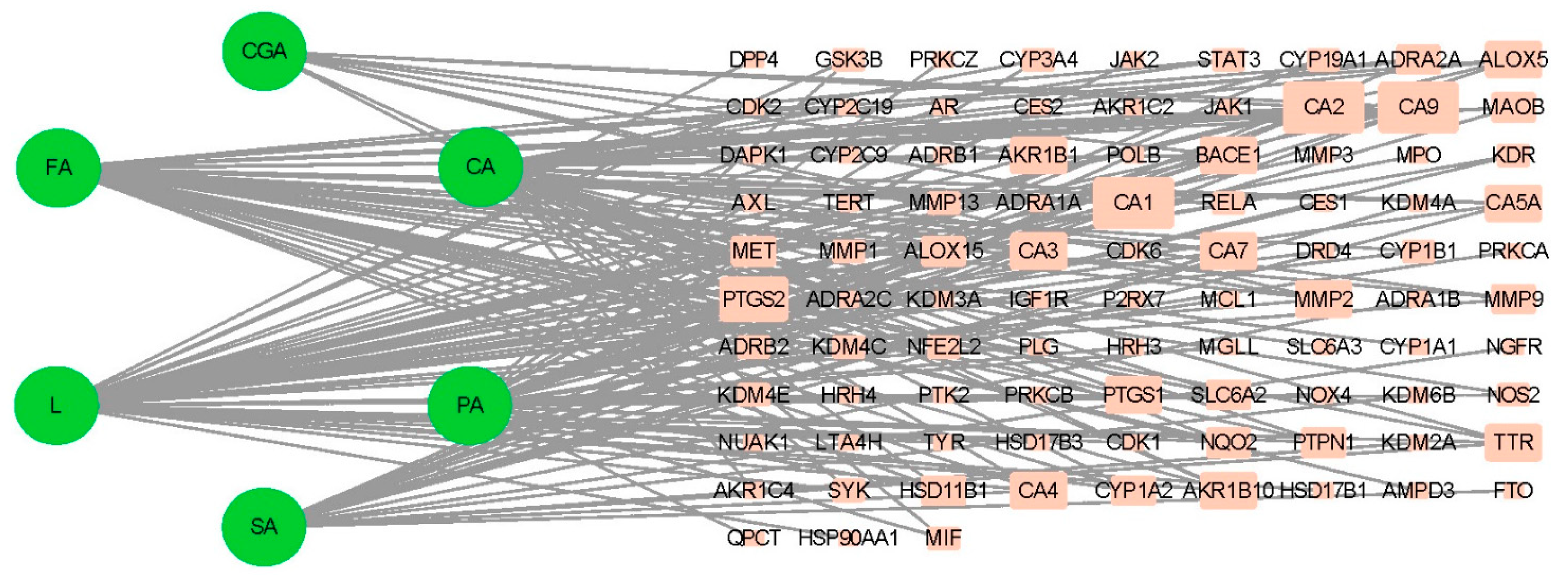
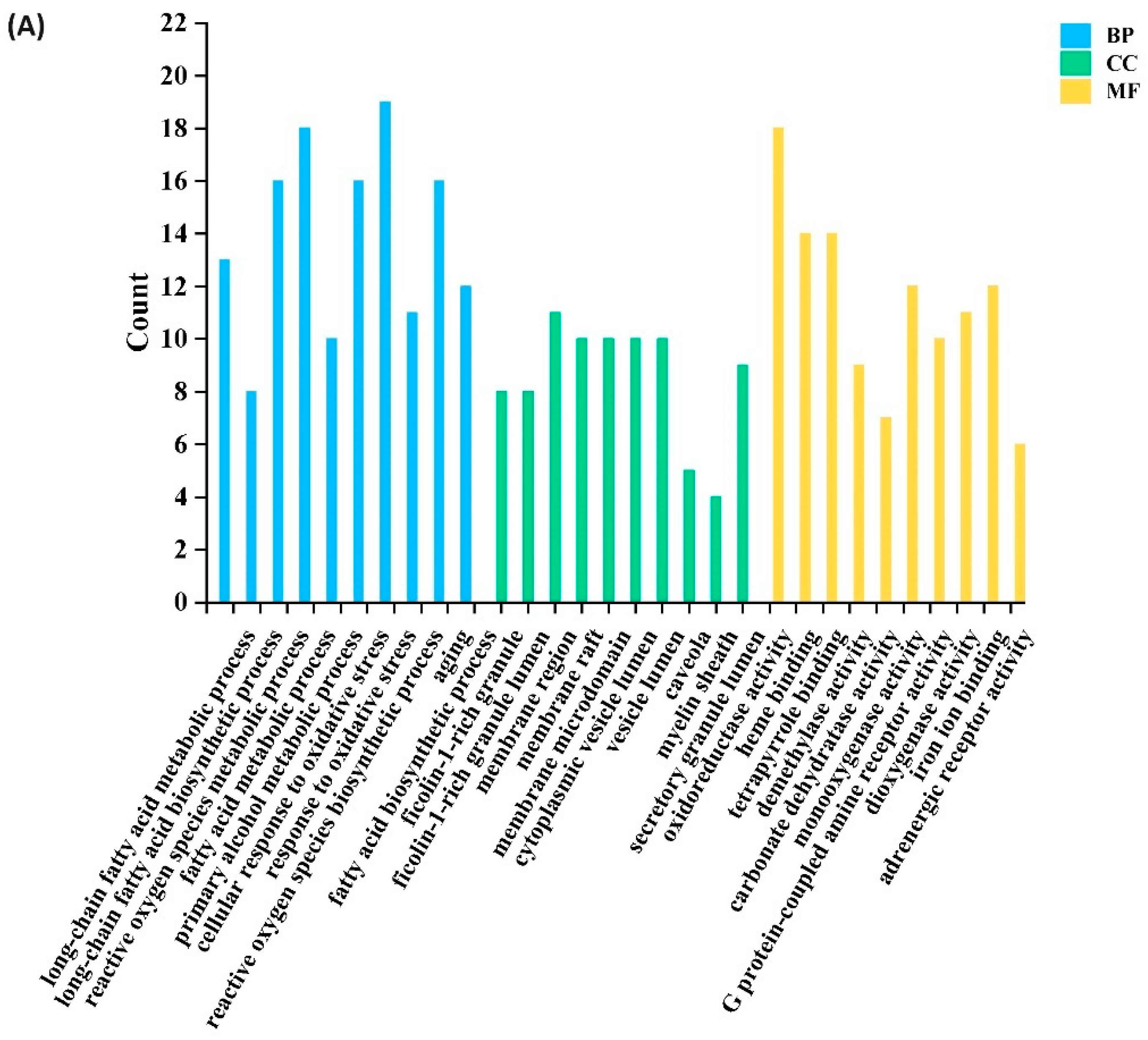
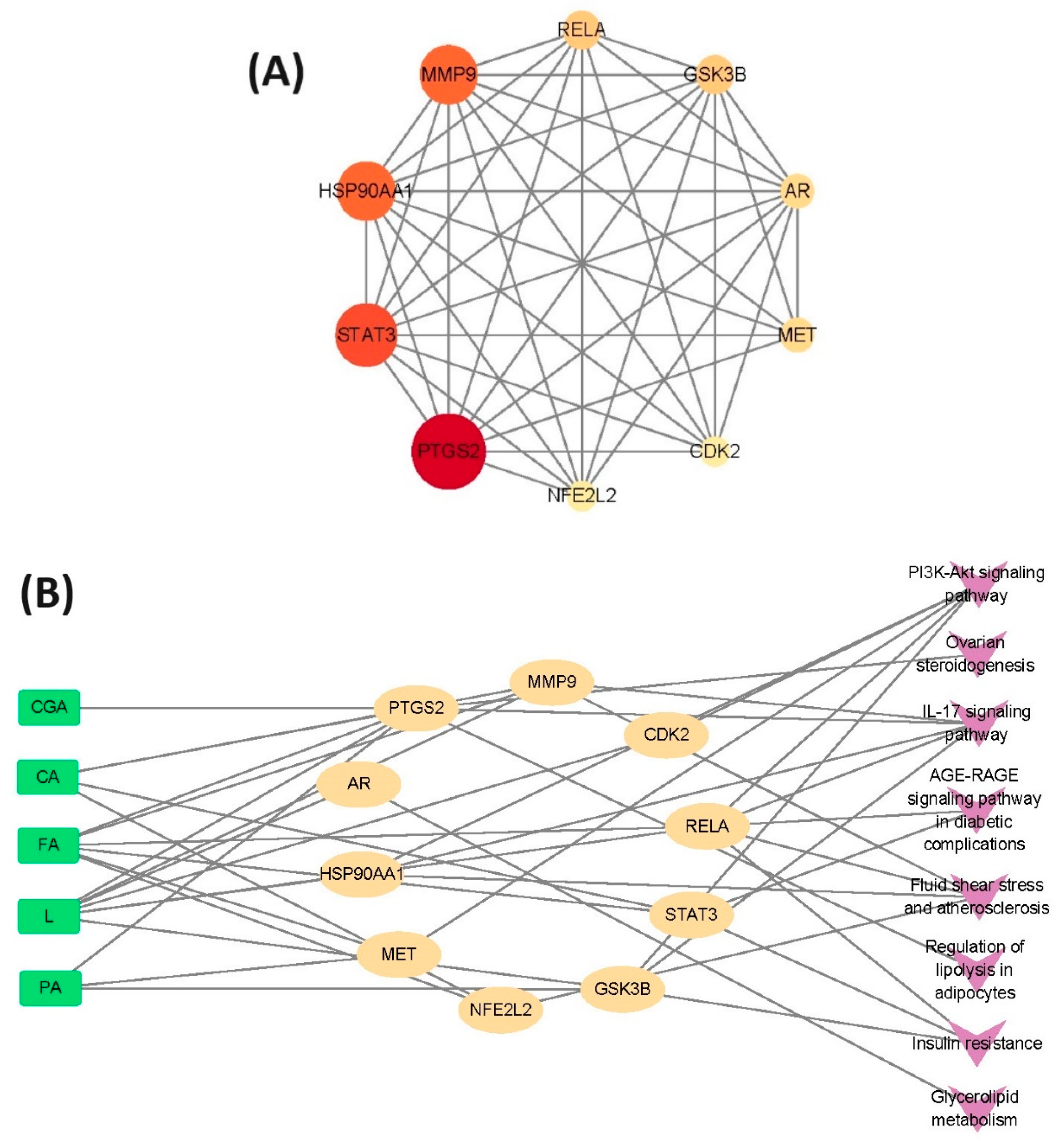
3.4.1. Potential Targets of Active Antioxidants and Compound Network Analysis
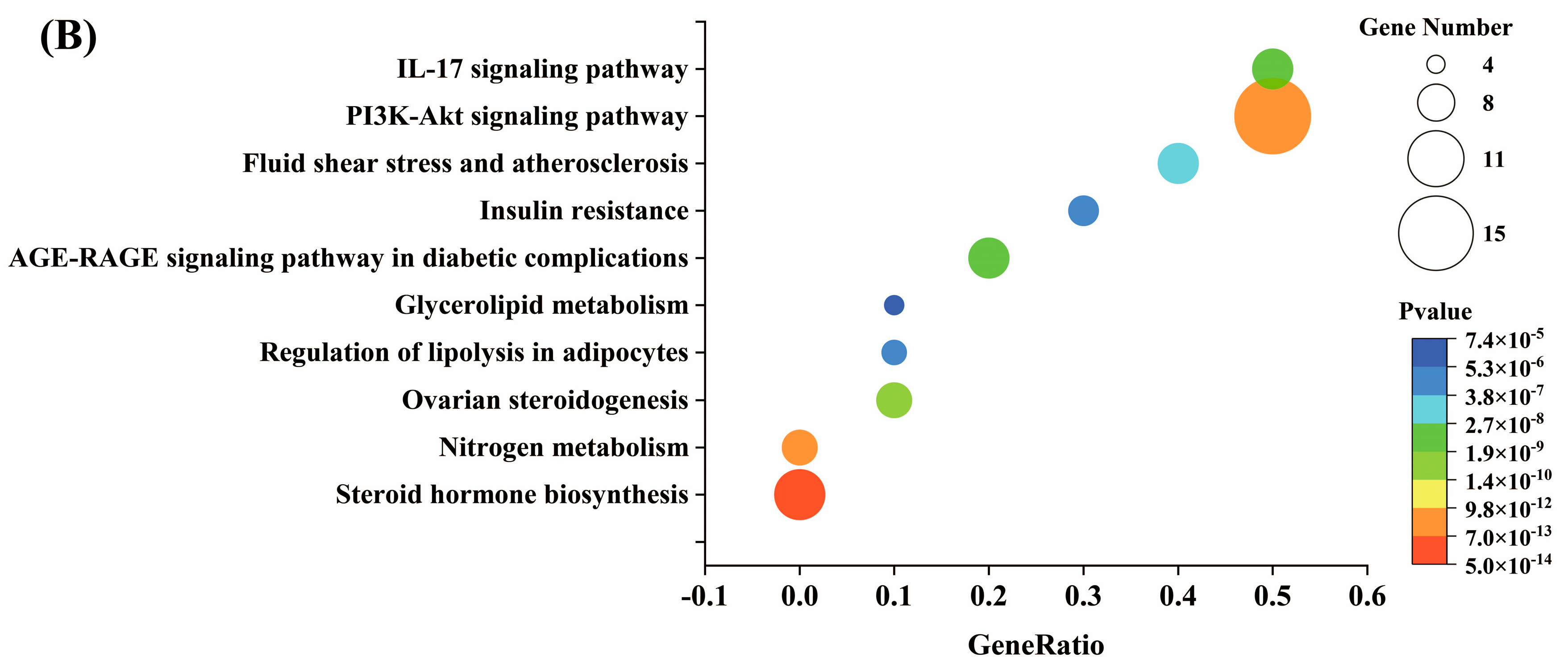
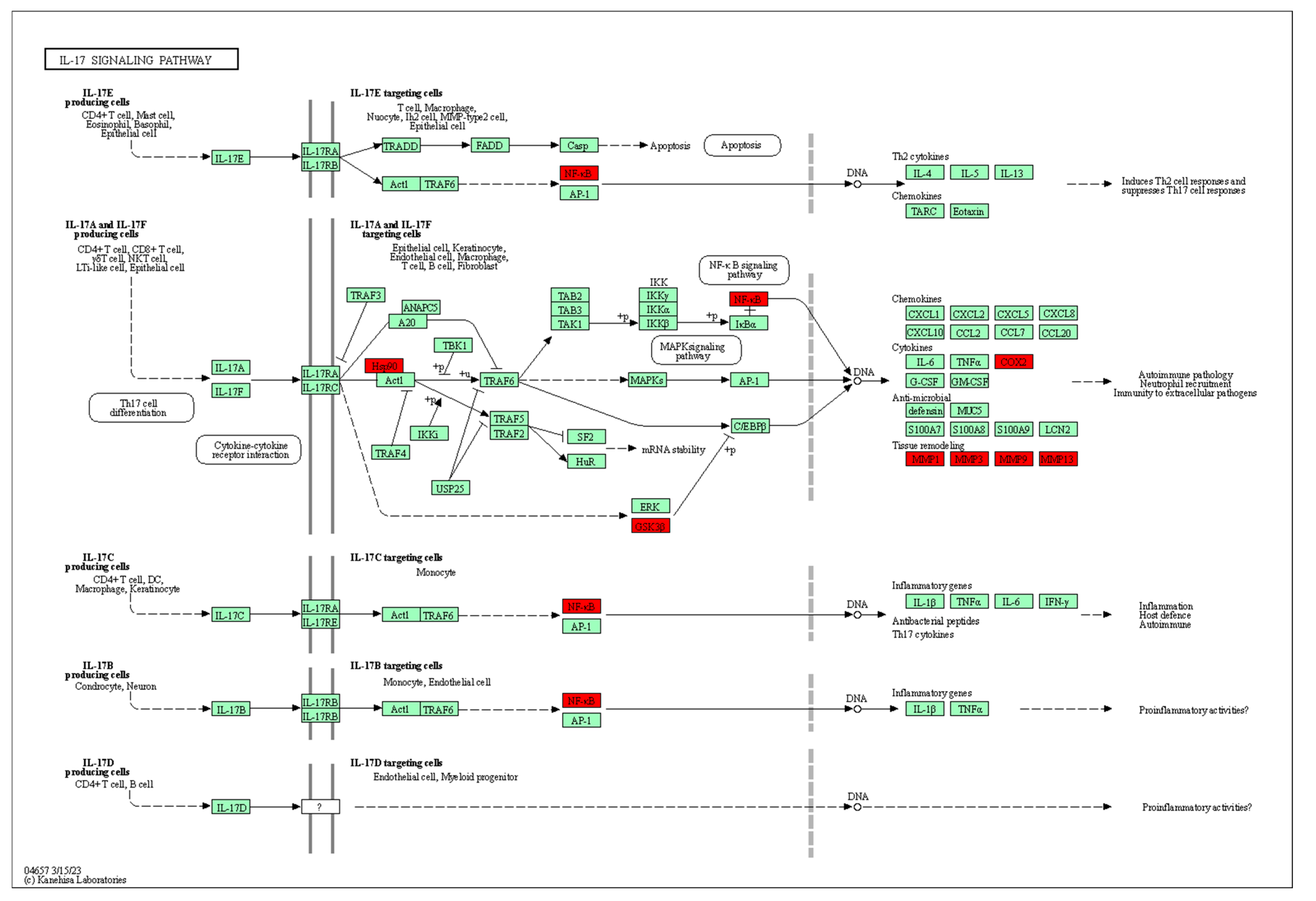
3.4.2. Characteristic Analysis of Antioxidant Target Protein Pathway Network
3.5. Molecular Docking of Active Antioxidants and Key Targets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.C.; Chen, G.L.; Zheng, F.J. Effects of Sugarcane Vinegar Supplementation on Oxidative Stress and Weight Reduction in Hyperlipidaemic Mice. Int. Food Res. J. 2020, 27, 1121–1131. [Google Scholar]
- Yun, J.H.; Kim, Y.J.; Koh, K.H. Investigation into Factors Influencing Antioxidant Capacity of Vinegars. Appl. Biol. Chem. 2016, 59, 495–509. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H. Bin Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.L.; Xia, T.; Qiang, X.; Zhao, Y.X.; Li, S.P.; Wang, Y.M.; Zheng, Y.; Yu, J.W.; Wang, J.X.; Wang, M. Nutrition, Bioactive Components, and Hepatoprotective Activity of Fruit Vinegar Produced from Ningxia Wolfberry. Molecules 2022, 27, 4422. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Park, B.-Y.; Kim, S.-H.; Jung, J.-H. Antioxidant, Anti-Obesity, and Anti-Aging Activities of Jeju Citrus Blended Vinegar. Foods 2021, 10, 1441. [Google Scholar] [CrossRef]
- He, J.; Lao, S.B.; Lin, B. Simultaneous Determination of 11 Phenolic Compounds in Apple Vinegar and Sugarcane Vinegar by HPLC—DAD. Sci. Technol. Food Ind. 2017, 38, 210–213. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Lv, J.M.; Gu, Y.; He, Y.; Chen, J.C.; Ye, X.Q.; Zhou, Z.Q. Mixed Fermentation of Chinese Bayberry Pomace Using Yeast, Lactic Acid Bacteria and Acetic Acid Bacteria: Effects on Color, Phenolics and Antioxidant Ingredients. LWT 2022, 163, 113503. [Google Scholar] [CrossRef]
- Guo, R.Z.; Liu, X.G.; Gao, W.; Dong, X.; Fanali, S.; Li, P.; Yang, H. A Strategy for Screening Antioxidants in Ginkgo Biloba Extract by Comprehensive Two-Dimensional Ultra High Performance Liquid Chromatography. J. Chromatogr. A. 2015, 1422, 147–154. [Google Scholar] [CrossRef]
- Guo, F.Q.; Yang, Y.C.; Duan, Y.; Li, C.; Gao, H.M.; Liu, H.Y.; Cui, Q.P.; Guo, Z.Y.; Liu, X.Q.; Wang, Z.M. Quality Marker Discovery and Quality Evaluation of Eucommia Ulmoides Pollen Using UPLC-QTOF-MS Combined with a DPPH-HPLC Antioxidant Activity Screening Method. Molecules 2023, 28, 5288. [Google Scholar] [CrossRef]
- Lam, S.C.; Luo, Z.; Wu, D.T.; Cheong, K.L.; Hu, D.J.; Xia, Z.M.; Zhao, J.; Li, S.P. Comparison and Characterization of Compounds with Antioxidant Activity in Lycium barbarum Using High-Performance Thin Layer Chromatography Coupled with DPPH Bioautography and Tandem Mass Spectrometry. J. Food Sci. 2016, 81, C1378–C1384. [Google Scholar] [CrossRef]
- Chen, G.L.; Zheng, F.J.; Lin, B.; Lao, S.B.; He, J.; Huang, Z.; Zeng, Y.; Sun, J.; Verma, K.K. Phenolic and Volatile Compounds in the Production of Sugarcane Vinegar. ACS Omega 2020, 5, 30587–30595. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Yu, W.X.; Ni, W.J.; Teng, C.Q.; Ye, W.L.; Yu, C.P.; Zeng, Y. Improvement of Obesity by Liupao Tea Is through the IRS-1/PI3K/AKT/GLUT4 Signaling Pathway According to Network Pharmacology and Experimental Verification. Phytomedicine 2023, 110, 154633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Wu, X.Y.; Wang, X.H.; Zeng, Y.; Liao, Y.X.; Zhang, R.Z.; Zhai, F.Q.; Zeng, Z.L. Grey Relational Analysis Combined With Network Pharmacology to Identify Antioxidant Components and Uncover Its Mechanism From Moutan Cortex. Front. Pharmacol. 2021, 12, 748501. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Ding, L.; Bao, T.T.; Li, Y.; Ma, J.; Li, Q.W.; Gao, Z.Z.; Song, S.Y.; Wang, J.; Zhao, J.C.; et al. Network Pharmacology and Experimental Assessment to Explore the Pharmacological Mechanism of Qimai Feiluoping Decoction Against Pulmonary Fibrosis. Front. Pharmacol. 2021, 12, 770197. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Lu, M.X.; Zhang, Y.Q.; Liao, J.C.; Zou, J.D.; Li, W.L.; Shi, W.; Fang, Z.Y.; Li, C.Y. An Integrated Approach of High-performance Liquid Chromatography–Mass Spectrometry-based Chemical Profiling, Network Pharmacology, and Molecular Docking to Reveal the Potential Mechanisms of Qishen Gubiao Granules for Treating Coronavirus Disease 2019. J. Sep. Sci. 2023, 46, e2200953. [Google Scholar] [CrossRef]
- Özdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, I. Phytochemical Content, and Antioxidant Activity, and Volatile Compounds Associated with the Aromatic Property, of the Vinegar Produced from Rosehip Fruit (Rosa canina L.). LWT 2022, 154, 112716. [Google Scholar] [CrossRef]
- Hu, T.M.; Gavahian, M.; Pradhan, R.; Lu, S.Y.; Chu, Y.L. Functional, Antioxidant, and Sensory Properties of Mixed-fruit (Pitaya, Watermelon, and Mint) and Pitaya Wines. Food Sci. Nutr. 2023, 11, 3442–3449. [Google Scholar] [CrossRef]
- Bai, H.; Wang, S.; Wang, Z.-M.; Zhu, L.-L.; Yan, H.-B.; Wang, Y.-B.; Wang, X.-Y.; Peng, L.; Liu, J.-Z. Investigation of Bioactive Compounds and Their Correlation with the Antioxidant Capacity in Different Functional Vinegars. Food Res. Int. 2024, 184, 114262. [Google Scholar] [CrossRef]
- Duy, N.Q.; Thoai, H.A.; Lam, T.D.; Le, X.T. Effects of Different Extraction Solvent Systems on Total Phenolic, Total Flavonoid, Total Anthocyanin Contents and Antioxidant Activities of Roselle (Hibiscus sabdariffa L.) Extracts. Asian J. Chem. 2019, 31, 2517–2521. [Google Scholar] [CrossRef]
- Boasiako, T.A.; Xiong, Y.; Boateng, I.D.; Appiagyei, J.; Li, Y.; Clark, K.; Aregbe, A.Y.; Yaqoob, S.; Ma, Y. Innovative Bicultured Lactic–Acetic Acid Co-Fermentation Improves Jujube Puree’s Functionality and Volatile Compounds. Fermentation 2024, 10, 71. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.S.; Li, X.H.; Hu, L.M.; Wang, J.; Li, Y.; Zhao, T.Y.; Wang, H.; Wang, S.M. The Anti-Inflammatory Mechanism of the Medicinal Fungus Puffball Analysis Based on Network Pharmacology. Inform. Med. Unlocked 2021, 23, 100549. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus Densiflora Bark Extract. Biomed Res. Int. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Arzola-Rodríguez, S.I.; Muñoz-Castellanos, L.-N.; López-Camarillo, C.; Salas, E. Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review. Biomolecules 2022, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.-D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant Properties and Efficacies of Synthesized Alkyl Caffeates, Ferulates, and Coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Gryko, K.; Kalinowska, M.; Ofman, P.; Choińska, R.; Świderski, G.; Świsłocka, R.; Lewandowski, W. Natural Cinnamic Acid Derivatives: A Comprehensive Study on Structural, Anti/Pro-Oxidant, and Environmental Impacts. Materials 2021, 14, 6098. [Google Scholar] [CrossRef]
- Shuab, R.; Lone, R.; Koul, K.K. Cinnamate and Cinnamate Derivatives in Plants. Acta Physiol. Plant 2016, 38, 64. [Google Scholar] [CrossRef]
- Katsuragi, H.; Shimoda, K.; Kubota, N.; Nakajima, N.; Hamada, H.; Hamada, H. Biotransformation of Cinnamic Acid, p-Coumaric Acid, Caffeic Acid, and Ferulic Acid by Plant Cell Cultures of Eucalyptus Perriniana. Biosci. Biotechnol. Biochem. 2010, 74, 1920–1924. [Google Scholar] [CrossRef]
- da Silva, A.P.G.; Sganzerla, W.G.; John, O.D.; Marchiosi, R. A Comprehensive Review of the Classification, Sources, Biosynthesis, and Biological Properties of Hydroxybenzoic and Hydroxycinnamic Acids. Phytochem. Rev. 2023, 1–30. [Google Scholar] [CrossRef]
- Pivetta, C.P.; Chitolina, S.F.; Dartora, N.; de Pelegrin, C.M.G.; dos Santos, M.V.; Cassol, F.; Batista, L.S. Copper Exposure Leads to Changes in Chlorophyll Content and Secondary Metabolite Profile in Lantana Fucata Leaves. Funct. Plant Biol. 2023, 50, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, L.; Reverón, I.; López de Felipe, F.; de las Rivas, B.; Muñoz, R. Unravelling the Reduction Pathway as an Alternative Metabolic Route to Hydroxycinnamate Decarboxylation in Lactobacillus Plantarum. Appl. Environ. Microbiol. 2018, 84, e01123-18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Xiao, H.Z.; Zheng, J.; Liang, G.Z. Structure-Thermodynamics-Antioxidant Activity Relationships of Selected Natural Phenolic Acids and Derivatives: An Experimental and Theoretical Evaluation. PLoS ONE 2015, 10, e0121276. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Jürgens, H.S. Effect of PH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Jiang, H.W.; Zhou, L.; Wang, Y.; Liu, G.X.; Peng, S.F.; Yu, W.Z.; Tian, Y.Q.; Liu, J.P.; Liu, W. Inhibition of Cinnamic Acid and Its Derivatives on Polyphenol Oxidase: Effect of Inhibitor Carboxyl Group and System PH. Int. J. Biol. Macromol. 2024, 259, 129285. [Google Scholar] [CrossRef]
- Chen, J.X.; Yang, J.; Ma, L.L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Çelik, E.E.; Rubio, J.M.A.; Andersen, M.L.; Gökmen, V. Interactions of Dietary Fiber Bound Antioxidants with Hydroxycinnamic and Hydroxybenzoic Acids in Aqueous and Liposome Media. Food Chem. 2019, 278, 294–304. [Google Scholar] [CrossRef]
- Riethmüller, E.; Könczöl, Á.; Szakál, D.; Végh, K.; Balogh, G.T.; Kéry, Á. HPLC-DPPH Screening Method for Evaluation of Antioxidant Compounds in Corylus Species. Nat. Prod. Commun. 2016, 11, 1934578X1601100. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L.; Jóźwik-Dolęba, M. Are Mutual Interactions between Antioxidants the Only Factors Responsible for Antagonistic Antioxidant Effect of Their Mixtures? Additive and Antagonistic Antioxidant Effects in Mixtures of Gallic, Ferulic and Caffeic Acids. Eur. Food Res. Technol. 2019, 245, 1473–1485. [Google Scholar] [CrossRef]
- Xue, Y.S.; Zheng, Y.G.; An, L.; Dou, Y.Y.; Liu, Y. Density Functional Theory Study of the Structure–Antioxidant Activity of Polyphenolic Deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Çelik, E.E.; Gökmen, V. Interactions between Free and Bound Antioxidants under Different Conditions in Food Systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 5766–5782. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Guo, Y.; Li, Y.X.; Zhou, S.C.; Lu, C.J.; Cai, C.Q.; Yang, H.J.; Li, Y.Q.; Wang, W.C. Identification and Validation of PTGS2 Gene as an Oxidative Stress-Related Biomarker for Arteriovenous Fistula Failure. Antioxidants 2023, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Tian, R.M.; Yang, Y.Q.; Lu, Z.Y.; Han, X.D.; Liu, X.S.; Mao, W.; Xu, P.; Xu, H.; Liu, B. Triptriolide Antagonizes Triptolide-Induced Nephrocyte Apoptosis via Inhibiting Oxidative Stress in Vitro and in Vivo. Biomed. Pharmacother. 2019, 118, 109232. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, A.; Simayi, J.; Nuermaimaiti, M.; Han, M.; Hailati, S.; Talihati, Z.; Maihemuti, N.; Nuer, M.; Khan, N.; Abudurousuli, K.; et al. Quince Extract Resists Atherosclerosis in Rats by Down-Regulating the EGFR/PI3K/Akt/GSK-3β Pathway. Biomed. Pharmacother. 2023, 160, 114330. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Liu, P.; Feng, X.T.; Du, M.; Zhang, Y.F.; Wang, Y.R.; Wang, J.R. Mechanism of Tao Hong Decoction in the Treatment of Atherosclerosis Based on Network Pharmacology and Experimental Validation. Front. Cardiovasc. Med. 2023, 10, 1111475. [Google Scholar] [CrossRef]
- Nan, G.H.; Liu, L.S.; Wu, H.; Yin, S.Y.; Li, C.L.; Zhao, H.; Chen, H.; Wu, Q. Transcriptomic and Metabonomic Profiling Reveals the Antihyperlipidemic Effects of Tartary Buckwheat Sprouts in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2022, 70, 13302–13312. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ji, L.; Yang, D.W.; Wu, J.J.; Yang, F. Decoding Cardiovascular Risks: Analyzing Type 2 Diabetes Mellitus and ASCVD Gene Expression. Front. Endocrinol. 2024, 15, 1383772. [Google Scholar] [CrossRef]
- Xing, N.; Wang, Y.; Wang, W.J.; Zhong, R.X.; Xia, T.Y.; Ding, Z.H.; Yang, Y.N.; Zhong, Y.M.; Shu, Z.P. Cardioprotective Effect Exerted by Timosaponin BII through the Regulation of Endoplasmic Stress-Induced Apoptosis. Phytomedicine 2020, 78, 153288. [Google Scholar] [CrossRef]
- Chai, S.; Yang, Y.; Wei, L.; Cao, Y.; Ma, J.; Zheng, X.; Teng, J.; Qin, N. Luteolin Rescues Postmenopausal Osteoporosis Elicited by OVX through Alleviating Osteoblast Pyroptosis via Activating PI3K-AKT Signaling. Phytomedicine 2024, 128, 155516. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Xu, P.; Yu, H.Y.; Shi, L. Luteolin Protects PC-12 Cells from H2O2-Induced Injury by up-Regulation of MicroRNA-21. Biomed. Pharmacother. 2019, 112, 108698. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Liu, Q.; Zhang, Z.; Yang, Y.; Zhou, Y.Z.; Yan, H.; Wang, X.; Li, X.R.; Zhao, J.; Hu, J.; et al. The Regulatory Role of PI3K in Ageing-Related Diseases. Ageing Res. Rev. 2023, 88, 101963. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, D.H.; Lee, E.K.; Kim, J.M.; Ha, Y.M.; Kim, N.D.; Jung, J.H.; Choi, J.S.; Yu, B.P.; Chung, H.Y. Attenuation of Age-Related Changes in FOXO3a Activity and the PI3K/Akt Pathway by Short-Term Feeding of Ferulate. Age 2012, 34, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, G.; Puig, L. Pathogenic Role of IL-17 and Therapeutic Targeting of IL-17F in Psoriatic Arthritis and Spondyloarthropathies. Int. J. Mol. Sci. 2023, 24, 10305. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Pan, F.Z.; Liu, J.; Zhang, J.C.; Zhang, F.; Wang, Y. Huayuwendan Decoction Ameliorates Inflammation via IL-17/NF-ΚB Signaling Pathway in Diabetic Rats. J. Ethnopharmacol. 2024, 319, 117328. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.; Liu, J.Q.; Aleksandrova, Y.; Klochkov, S.; Fan, R.T. Therapeutic Influence on Important Targets Associated with Chronic Inflammation and Oxidative Stress in Cancer Treatment. Cancers 2021, 13, 6062. [Google Scholar] [CrossRef]
- Liao, Y.L.; Ding, Y.L.; Yu, L.; Xiang, C.; Yang, M.Y. Exploring the Mechanism of Alisma Orientale for the Treatment of Pregnancy Induced Hypertension and Potential Hepato-Nephrotoxicity by Using Network Pharmacology, Network Toxicology, Molecular Docking and Molecular Dynamics Simulation. Front. Pharmacol. 2022, 13, 1027112. [Google Scholar] [CrossRef]
- Doss, H.M.; Dey, C.; Sudandiradoss, C.; Rasool, M.K. Targeting Inflammatory Mediators with Ferulic Acid, a Dietary Polyphenol, for the Suppression of Monosodium Urate Crystal-Induced Inflammation in Rats. Life Sci. 2016, 148, 201–210. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a Modulator of Skin Aging and Inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
| Range of Combination Index (CIwt) | Description | Assigned Symbols |
|---|---|---|
| 0.1–0.3 | Strong synergism | + + + + |
| 0.3–0.7 | Synergism | + + + |
| 0.7–0.85 | Moderate synergism | + + |
| 0.85–0.9 | Slight synergism | + |
| 0.9–1.10 | Nearly additive | ± |
| 1.10–1.20 | Slight antagonism | − |
| 1.20–1.45 | Moderate antagonism | − − |
| 1.45–3.3 | Antagonism | − − − |
| 3.3–10 | Strong antagonism | − − − − |
| Correlation Coefficient (R2) | TPC | TFC | TEAC | |
|---|---|---|---|---|
| DPPH | ABTS | |||
| TPC | 1 | |||
| TFC | 0.983 * | 1 | ||
| DPPH | 0.966 * | 0.968 * | 1 | |
| ABTS | 0.970 * | 0.978 * | 0.991 * | 1 |
| No. | Compound | TR (min) | Before Reaction (mg/L) | After Reaction (mg/L) | Limit of Quantification (mg/L) | Consumption Rate (%) | Antioxidant Activity |
|---|---|---|---|---|---|---|---|
| 1 | AG | 9.15 | 0.1 | 0.092 | 0.0004 | 8.00 | × |
| 2 | CA | 4.55 | 0.196 | 0.16 | 0.03 | 18.37 | √ |
| 3 | CMA | 8.97 | 0.052 | 0.056 | 0.01 | −7.69 | × |
| 4 | pCA | 5.99 | 7.414 | 7.682 | 0.03 | −3.61 | × |
| 5 | FA | 6.74 | 12.497 | 2.121 | 0.08 | 83.03 | √ |
| 6 | L | 8.61 | 0.088 | 0.006 | 0.004 | 93.18 | √ |
| 7 | PA | 2.83 | 6.302 | 1.594 | 0.05 | 74.71 | √ |
| 8 | SA | 4.75 | 9.467 | 2.555 | 0.07 | 73.01 | √ |
| 9 | CGA | 3.70 | 0.106 | - | 0.05 | >95.28 | √ |
| 10 | VA | 4.57 | 2.404 | 2.338 | 0.08 | 2.75 | × |
| No. | Compound | IC50 (µg/mL) | |
|---|---|---|---|
| Methanol | Sodium Acetate Buffer (pH 3.3) | ||
| 1 | CGA | 25.59 | 10.22 |
| 2 | CA | 11.18 | 5.59 |
| 3 | FA | 23.29 | 25.56 |
| 4 | L | 9.84 | 7.28 |
| 5 | SA | 15.17 | 16.6 |
| 6 | PA | 11.26 | 5.95 |
| No. | Combinations | Methanol | Sodium Acetate Buffer (pH 3.3) | ||
|---|---|---|---|---|---|
| CIwt | Assigned Symbol | CIwt | Assigned Symbol | ||
| 1 | CGA + CA | 2.1 | − − − | 1.16 | − |
| 2 | CGA + FA | 1.73 | − − − | 0.43 | + + + |
| 3 | CGA + L | 2.46 | − − − | 0.42 | + + + |
| 4 | CGA + PA | 6.23 | − − − − | 0.55 | + + + |
| 5 | CGA + SA | 2.99 | − − − | 0.49 | + + + |
| 6 | CA + FA | 2.62 | − − − | 1.04 | ± |
| 7 | CA + L | 2.36 | − − − | 1.49 | − − − |
| 8 | CA + PA | 2.18 | − − − | 1.69 | − − − |
| 9 | CA + SA | 1.85 | − − − | 2.79 | − − − |
| 10 | FA + L | 1.29 | − − | 0.19 | + + + + |
| 11 | FA +PA | 0.74 | + + | 0.31 | + + + |
| 12 | FA + SA | 0.31 | + + + | 0.23 | + + + + |
| 13 | L + PA | 1.52 | − − − | 0.31 | + + + |
| 14 | L + SA | 0.49 | + + + | 0.34 | + + + |
| 15 | PA + SA | 0.53 | + + + | 0.45 | + + + |
| No. | Molecule Name | Target | Residues Involved in H Bonding | H-Bond Length (Å) | Docking Energy (kcal/mol) |
|---|---|---|---|---|---|
| 1 | CGA | PTGS2 | TYR373, PHE-371, SER-126, GLN-372 | 2.2, 2.1, 2.1, 2.9 | −8.9 |
| 2 | CA | NFE2L2 | ILE-33, VAL-37 | 2.5, 2.5 | −5.7 |
| 3 | CA | STAT3 | LYS-370 | 1.9 | −5.9 |
| 4 | CA | MMP9 | HIS-91, GLN-126, TYR-92, TYR-98, VAL-172 | 2.2, 2.6, 2.0, 2.4, 2.0 | −6.9 |
| 5 | CA | PTGS2 | GLN-374, TYR-373, ASN-537, VAL-228 | 2.5, 2.5, 2.2, 2.6 | −6.9 |
| 6 | FA | MET | MET-1160 | 2.4 | −6.9 |
| 7 | FA | NFE2L2 | PHE-35 | 2.8 | −5.5 |
| 8 | FA | STAT3 | ASP-570, GLN-511 | 2.3, 2.7 | −5.8 |
| 9 | FA | RELA | ARG-208, ARG-358, ASP-309 | 2.4, 1.9, 2.4 | −5.8 |
| 10 | FA | MMP9 | ARG-97, ARG-162, ALA-164 | 2.5, 2.7, 2.8 | −6 |
| 11 | FA | PTGS2 | ARG-44, ARG-469, GLY-45, GYS-47 | 2.4, 2.2, 2.2, 2.1 | −6.6 |
| 12 | L | AR | GLN-711, ARG-752 | 2.5, 2.1 | −9.6 |
| 13 | L | HSP90AA1 | PHE-138, GLY-135, GLY-137 | 2.3, 2.4, 2.8 | −8.1 |
| 14 | L | GSK3B | ARG-209, VAL-208, GLU-211, ASN-213 | 2.4, 2.7, 2.9, 0.8 | −8.3 |
| 15 | L | RELA | ARG-208, GLU-359, ARG-358, ASP-309 | 2.7, 2.0, 2.6, 2.1 | −7.7 |
| 16 | L | MMP9 | ARG-97, ARG-162, ASP-103, ALA-164, GLY-195 | 2.7, 2.8, 2.3, 2.1, 2.7 | −8.5 |
| 17 | L | CDK2 | HIS-84, ASP-86, LYS-89 | 2.5, 2.4, 2.4 | −8.9 |
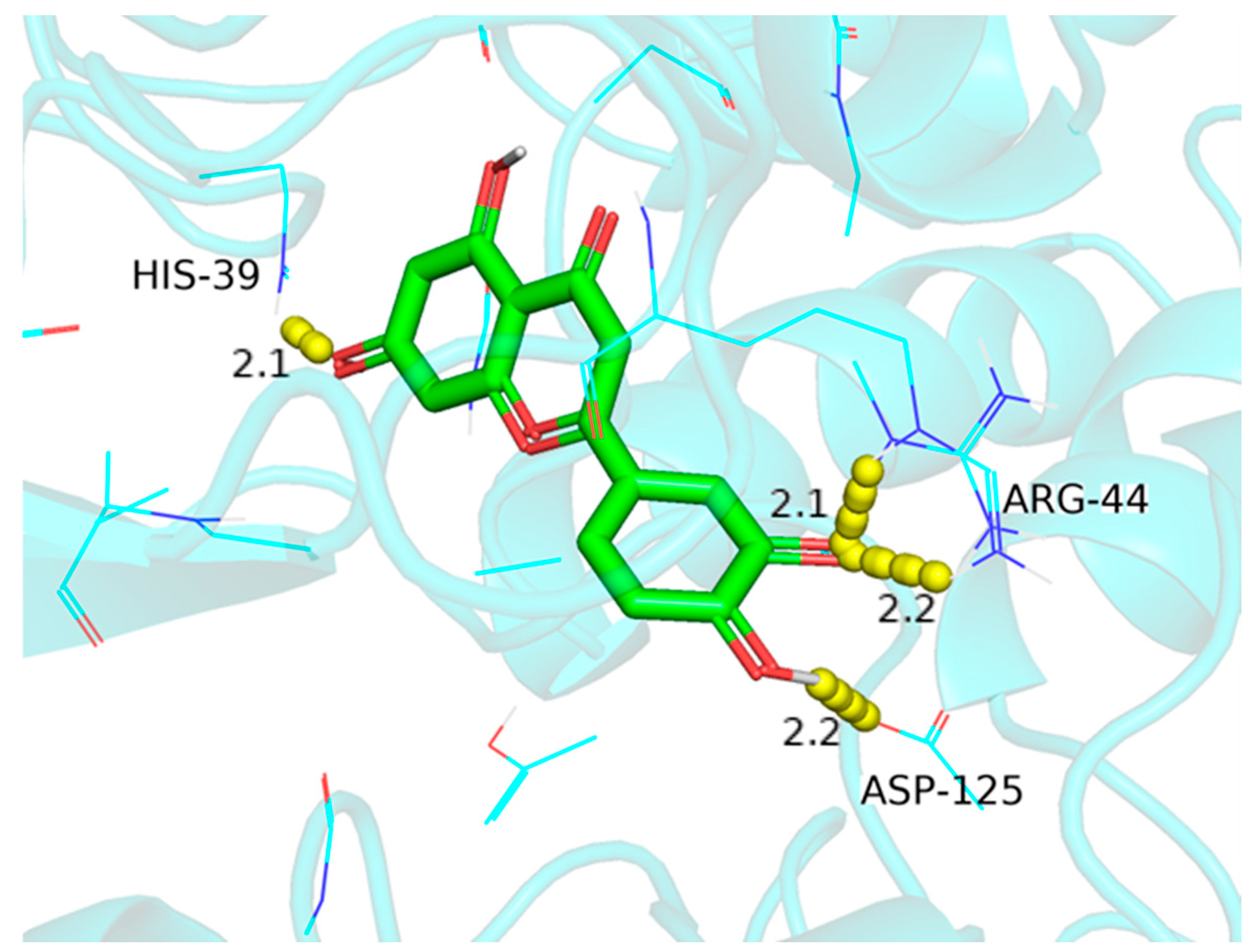
| 18 | L | PTGS2 | ARG-44, ASP-125, HIS-39 | 2.2, 2.2, 2.1 | −10 |
| 19 | PA | MET | ASP-1222 | 2.6 | −5.5 |
| 20 | PA | GSK3B | THR-289 | 2.3 | −5.1 |
| 21 | PA | PTGS2 | GLY-45 | 1.9 | −5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Lin, B.; Chen, J.; Zheng, F.; Yang, Y.; Rasheed, U.; Chen, G. Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking. Foods 2024, 13, 3379. https://doi.org/10.3390/foods13213379
Wu F, Lin B, Chen J, Zheng F, Yang Y, Rasheed U, Chen G. Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking. Foods. 2024; 13(21):3379. https://doi.org/10.3390/foods13213379
Chicago/Turabian StyleWu, Feifei, Bo Lin, Jing Chen, Fengjin Zheng, Yuxia Yang, Usman Rasheed, and Ganlin Chen. 2024. "Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking" Foods 13, no. 21: 3379. https://doi.org/10.3390/foods13213379
APA StyleWu, F., Lin, B., Chen, J., Zheng, F., Yang, Y., Rasheed, U., & Chen, G. (2024). Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking. Foods, 13(21), 3379. https://doi.org/10.3390/foods13213379







