Sublethal Damage Caused by Cold Plasma on Bacillus cereus Cells: Impact on Cell Viability and Biofilm-Forming Capacity
Abstract
1. Introduction
- Surface functionalization and coating (avoiding subsequent bacterial adhesion) [5].
- Directly treating biofilms and their detaching procedures through sequential effects: First, it destroys the biofilm matrix through active species—mainly reactive oxygen species (ROS) and reactive nitrogen species (RNS)—breaking down bonds in hydrocarbon compounds, which collapses and destroys the EPS biofilm matrix and the protein structure and enzymatic activity of microorganisms in the biofilm’s architecture. Second, once the biofilm structure has been seriously affected, the microorganisms embedded in it are moved to a bulk solution, with some adopting a planktonic form. In parallel, active species, charge ions, and UV radiation affect the membrane integrity of the microorganisms, interfering with their cellular metabolic pathways, promoting intracellular material leakage, and finally killing the microorganisms in the biofilm. At the same time, the biofilm matrix separates from the biotic and abiotic surfaces, eliminating it on the solid substratum [6,7].
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Cold Plasma Equipment Used
2.3. Microbiological Enumeration
2.4. Sublethal Damage Evaluation
2.4.1. Sublethal Damage Quantification
2.4.2. Sublethal Morphological Damage Assessed by Scanning Electron Microscopy (SEM)
2.4.3. Cell Membrane Integrity and Intracellular Content Leakage
2.5. Impact of CP on Biofilm-Forming Capacity
2.5.1. Screening Biofilm-Forming Capacity in Bacillus cereus s.l. Group Cells
2.5.2. SEM Visualization of Biofilms Formed by Sublethally Damaged Cells after DBD-CP
2.5.3. SEM Visualization of Biofilms Directly Damaged by DBD-CP
2.5.4. Viability of Cells Immersed in Biofilms
Statistical Analysis
3. Results and Discussion
3.1. Inactivation and Injured Cell Analysis after DBD-CP
3.2. Impact of DBD-CP on Integrity and Forming Capacity of Biofilm in B. cereus s.l. Group
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vignesh, A.; Cheeran Amal, T.; Sarvalingam, A.; Vasanth, K. A review on the influence of nutraceuticals and functional foods on health. Food Chem. Adv. 2024, 5, 100749. [Google Scholar] [CrossRef]
- Zhao, X.; Begyn, K.; Delongie, Y.; Rajkovic, A.; Uyttendaele, M. UV-C and wet heat resistance of Bacillus thuringiensis biopesticide endospores compared to foodborne Bacillus cereus endospores. Food Microbiol. 2023, 115, 104325. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public. Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cai, P.; Jing, X.; Niu, X.; Ji, D.; Ashry, N.M.; Gao, C.; Huang, Q. Soil biofilm formation enhances microbial community diversity and metabolic activity. Environ. Int. 2019, 132, 105116. [Google Scholar] [CrossRef] [PubMed]
- Hage, M.; Khelissa, S.; Akoum, H.; Chihib, N.E.; Jama, C. Cold plasma surface treatments to prevent biofilm formation in food industries and medical sectors. Appl. Microbiol. Biotechnol. 2022, 106, 81–100. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci. Technol. 2020, 99, 142–151. [Google Scholar] [CrossRef]
- Ziuzina, D.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Cold Plasma Inactivation of Bacterial Biofilms and Reduction of Quorum Sensing Regulated Virulence Factors. PLoS ONE 2015, 10, e0138209. [Google Scholar] [CrossRef]
- Nandula, S.R.; Kondeti, V.S.S.K.; Phan, C.; Wang, J.; Penningroth, M.R.; Granick, J.L.; Bruggeman, P.J.; Hunter, R.C. Plas-ma-induced inactivation of Staphylococcus aureus biofilms: The role of atomic oxygen and comparison with disinfectants and antibiotics. Plasma Process. Polym. 2022, 20, 2200147. [Google Scholar] [CrossRef]
- Oliulla, H.; Mizan, F.R.; Ashrafudoulla, M.D.; Meghla, N.S.; Jie-won Ha, A.; Park, S.H.; Ha, S.D. The challenges and prospects of using cold plasma to prevent bacterial contamination and biofilm formation in the meat industry. Meat Sci. 2017, 217, 109596. [Google Scholar] [CrossRef]
- Patange, A.; Boehm, D.; Ziuzina, D.; Cullen, P.J.; Gilmore, B.; Bourke, P. High voltage atmospheric cold air plasma control of bacterial biofilms on fresh produce. Int. J. Food Microbiol. 2019, 293, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Bai, M.; Yuan, L.; Surendhiran, D.; Lin, L. Sequential effect of phages and cold nitrogen plasma against Escherichia coli O157:H7 biofilms on different vegetables. Int. J. Food Microbiol. 2018, 268, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Lv, X.; Pan, Y.; Sun, D.W. Foodborne bacterial stress responses to exogenous reactive oxygen species (ROS) induced by cold plasma treatments. Trends Food Sci. Technol. 2020, 103, 239–247. [Google Scholar] [CrossRef]
- Polito, J.; Kushner, M.J. A hierarchal model for bacterial cell inactivation in solution by direct and indirect treatment using cold atmospheric plasmas. J. Phys. D Appl. Phys. 2024, 57, 405207. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; O’Connell, D.; Welch, E.; Vann, R.; van der Woude, R.W. Spatial Dependence of DNA Damage in Bacteria due to Low-Temperature Plasma Application as Assessed at the Single Cell Level. Sci. Rep. 2016, 6, 35646. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, J.; Shen, J.; Lan, Y.; Ding, L.; Qian, S.; Xia, W.; Cheng, C.; Chu, P.K. Roles of membrane protein damage and intracellular protein damage in death of bacteria induced by atmospheric-pressure air discharge plasmas. RSC Adv. 2018, 8, 21139–21149. [Google Scholar] [CrossRef]
- Dolezalova, E.; Lukes, P. Membrane damage and active but nonculturable state in liquid cultures of Escherichia coli treated with an atmospheric pressure plasma jet. Bioelectrochemistry 2018, 103, 7–14. [Google Scholar] [CrossRef]
- Liao, X.; Xiang, Q.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Lethal and Sublethal Effect of a Dielectric Barrier Discharge Atmospheric Cold Plasma on Staphylococcus aureus. J. Food Prot. 2017, 80, 928–932. [Google Scholar] [CrossRef]
- Salinas, C.; Escobar, F.; Rodríguez, F.; Campuzano, A.; Almada, P.; Ortellado-Canese, J.; Basualdo, W.; Zárate, N.; Samudio, G.; Gómez, G.; et al. Evaluation of the biofilm-forming capacity of methicillinresistant strains of S. aureus that infected Paraguayan children. Pediatría 2017, 44, 233–238. [Google Scholar] [CrossRef][Green Version]
- Smet, C.; Baka, M.; Steen, L.; Fraeye, I.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J.F. Combined effect of cold atmospheric plasma, intrinsic and extrinsic factors on the microbial behavior in/on (food) model systems during storage. Innov. Food Sci. Emerg. Technol. 2019, 53, 3–17. [Google Scholar] [CrossRef]
- Lv, X.; Cheng, J.H. Evaluation of the Effects of Cold Plasma on Cell Membrane Lipids and Oxidative Injury of Salmonella typhimurium. Molecules 2022, 27, 640. [Google Scholar] [CrossRef] [PubMed]
- Schottroff, F.; Fröhling, A.; Zunabovic-Pichler, M.; Krottenthaler, A.; Schlüter, O.; Jagher, H. Sublethal Injury and Viable but Non-culturable (VBNC) State in Microorganisms During Preservation of Food and Biological Materials by Non-thermal Processes. Microbial Decontamination by Novel Technologies—Mechanisms and Application Concepts. Front. Microbiol. Sec. Food Microbiol. 2018, 9, 2773. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Akkermans, S.; Boehm, D.; Cullen, P.J.; Van Impe, J.; Bourke, P. Improving microbiological safety and quality characteristics of wheat and barley by high voltage atmospheric cold plasma closed processing. Food Res. Int. 2018, 106, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, T.; Zhang, Y.; Guo, C.; Shi, K.; Kurtovic, Y.; Yuan, Y.; Yue, T. Efficacy, kinetics, inactivation mechanism and application of cold plasma in inactivating Alicyclobacillus acidoterrestris spores. Int. J. Food Microbiol. 2024, 423, 110830. [Google Scholar] [CrossRef]
- Charoux, C.M.G.; Free, L.; Hinds, L.M.; Vijayaraghavan, R.K.; Daniels, S.; O’Donnell, C.P.; Tiwari, B.K. Effect of non-thermal plasma technology on microbial inactivation and total phenolic content of a model liquid food system and black pepper grains. LWT Food Sci. Technol. 2020, 118, 108716. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Han, Q. Mechanisms of bacterial inhibition and tolerance around cold atmospheric plasma. Appl. Microbiol. Biotechnol. 2023, 107, 5301–5316. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. NPJ Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Huang, M.; Zhuang, H.; Zhao, J.; Wang, J.; Yan, W.; Zhang, J. Differences in cellular damage induced by dielectric barrier discharge plasma between Salmonella typhimurium and Staphylococcus aureus. Bioelectrochemistry 2020, 132, 107445. [Google Scholar] [CrossRef]
- Ravash, N.; Hesari, J.; Feizollahi, E.; Dhaliwal, H.K.; Roopesh, M.S. Valorization of Cold Plasma Technologies for Eliminating Biological and Chemical Food Hazards. Food Eng. Rev. 2024, 16, 22–58. [Google Scholar] [CrossRef]
- Than, H.A.Q.; Nguyen, T.T.; Do, N.K.; Tran, M.A.N.; Pham, T.H. Inactivation of Diutina catenulata Isolated from Longan Fruit Using Atmospheric Pressure Cold Plasma DBD in Argon, Air, and Argon-Air Mixture. Food Bioprocess. Technol. 2024. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljevic, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Takamatsu, T.; Kawano, H.; Sasaki, Y.; Uehara, K.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Azuma, T.; Okino, A. Imaging of the Staphylococcus aureus Inactivation Process Induced by a Multigas Plasma Jet. Curr. Microbiol. 2016, 73, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Matthes, R.; Jablonowski, L.; Pitchika, V.; Holtfreter, B.; Eberhard, C.; Seifert, L.; Gerling, T.; Vilardell Scholten, L.; Schlüter, R.; Kocher, T. Effciency of bioflm removal by combination of water jet and cold plasma: An in-vitro study. BMC Oral. Health 2022, 22, 157. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Graeffe, A.; Walsh, J.L.; Van Impe, J.F.M. Inactivation of L. monocytogenes and S. typhimurium biofilms by means of an air-based cold atmospheric plasma (CAP) system. Foods 2020, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cheng, J.H.; Lv, X.; Sun, D.W. Assessing the inactivation efficiency of Ar/O2 plasma treatment against Listeria monocytogenes cells: Sublethal injury and inactivation kinetics. LWT 2019, 111, 318–327. [Google Scholar] [CrossRef]
- He, M.; Duan, J.; Xu, J.; Ma, M.; Chai, B.; He, G.; Gan, L.; Zhang, S.; Duan, X.; Lu, X.; et al. Candida albicans biofilm inactivated by cold plasma treatment in vitro and in vivo. Plasma Process. Polym. 2019, 17, 1900068. [Google Scholar] [CrossRef]
- Lunder, M.; Dahle, S.; Fink, R. Cold atmospheric plasma for surface disinfection: A promising weapon against deleterious meticillin-resistant Staphylococcus aureus biofilms. J. Hosp. Infect. 2024, 143, 64–75. [Google Scholar] [CrossRef]
- Ebrahimi-Shaghaghi, F.; Noormohammadi, Z.; Atyabi, S.M.; Razzaghi-Abyaneh, M. Inhibitory effects of cold atmospheric plasma on the growth, virulence factors and HSP90 gene expression in Candida albicans. Arch. Biochem. Biophys. 2021, 700, 108772. [Google Scholar] [CrossRef]
- Guldimann, C.; Boor, K.J.; Wiedmann, M.; Guariglia-Oropeza, V. Resilience in the face of uncertainty: Sigma factor B fine-tunes gene expression to support homeostasis in gram-positive Bacteria. Appl. Environ. Microbiol. 2016, 82, 4456–4469. [Google Scholar] [CrossRef]
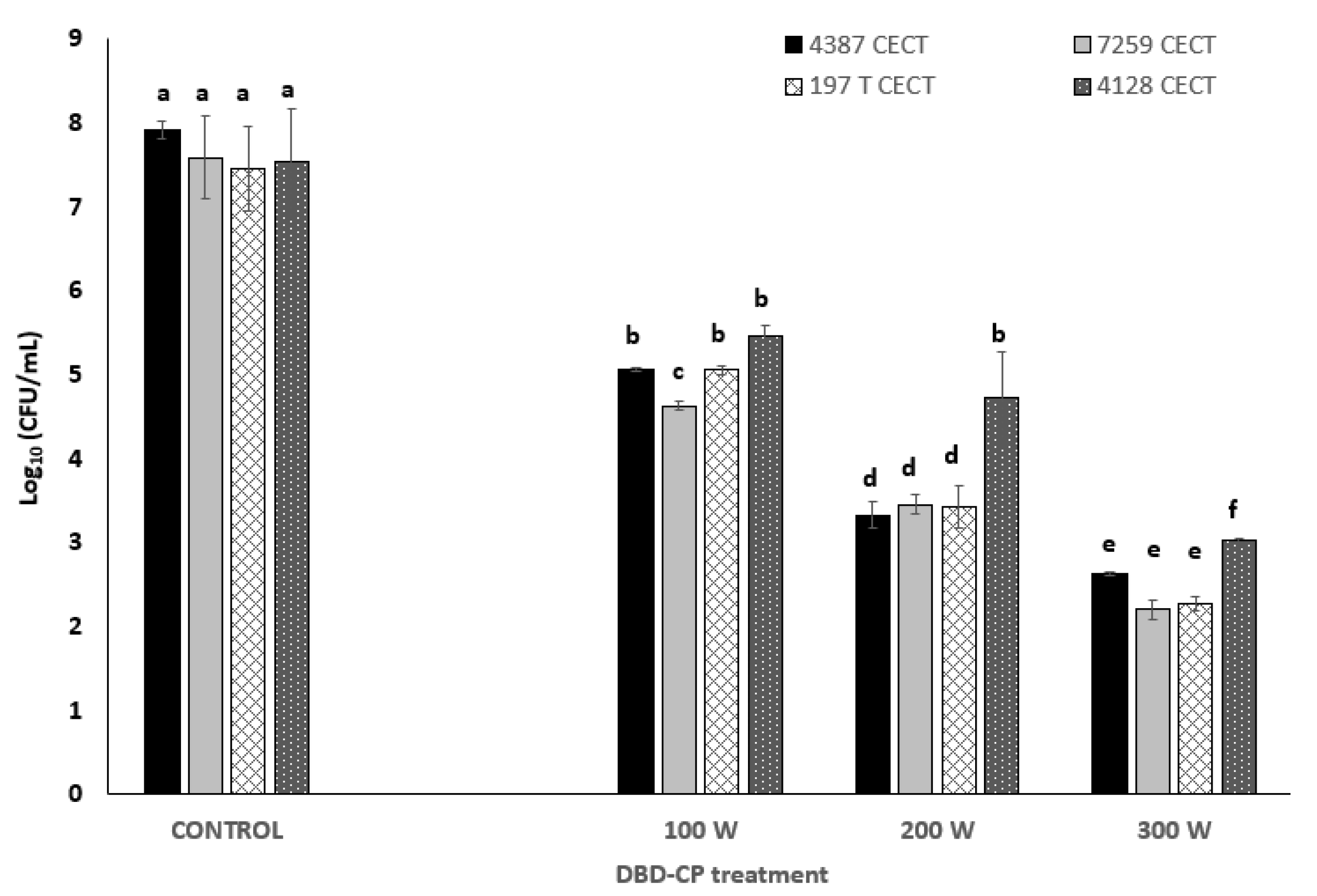

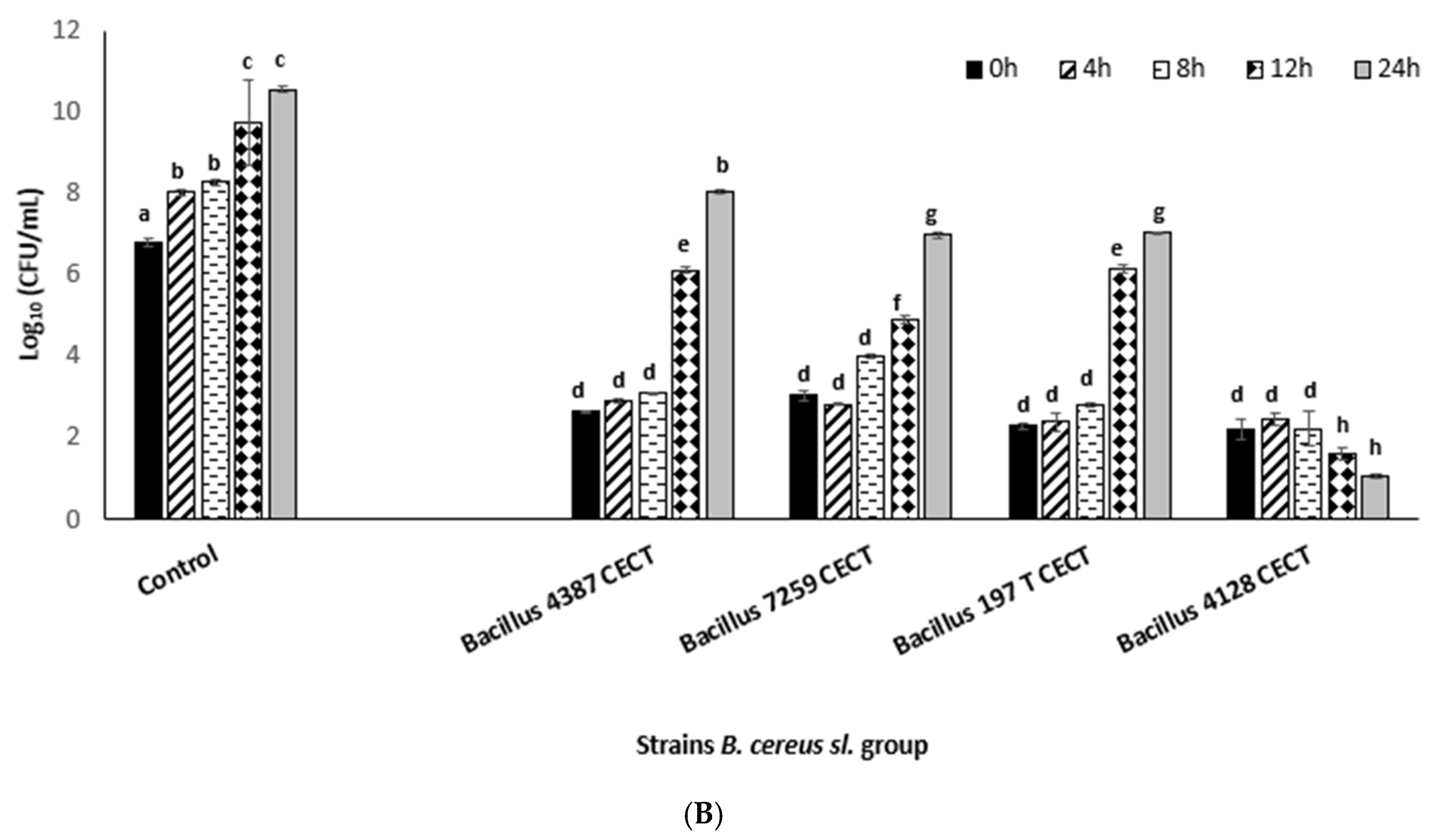
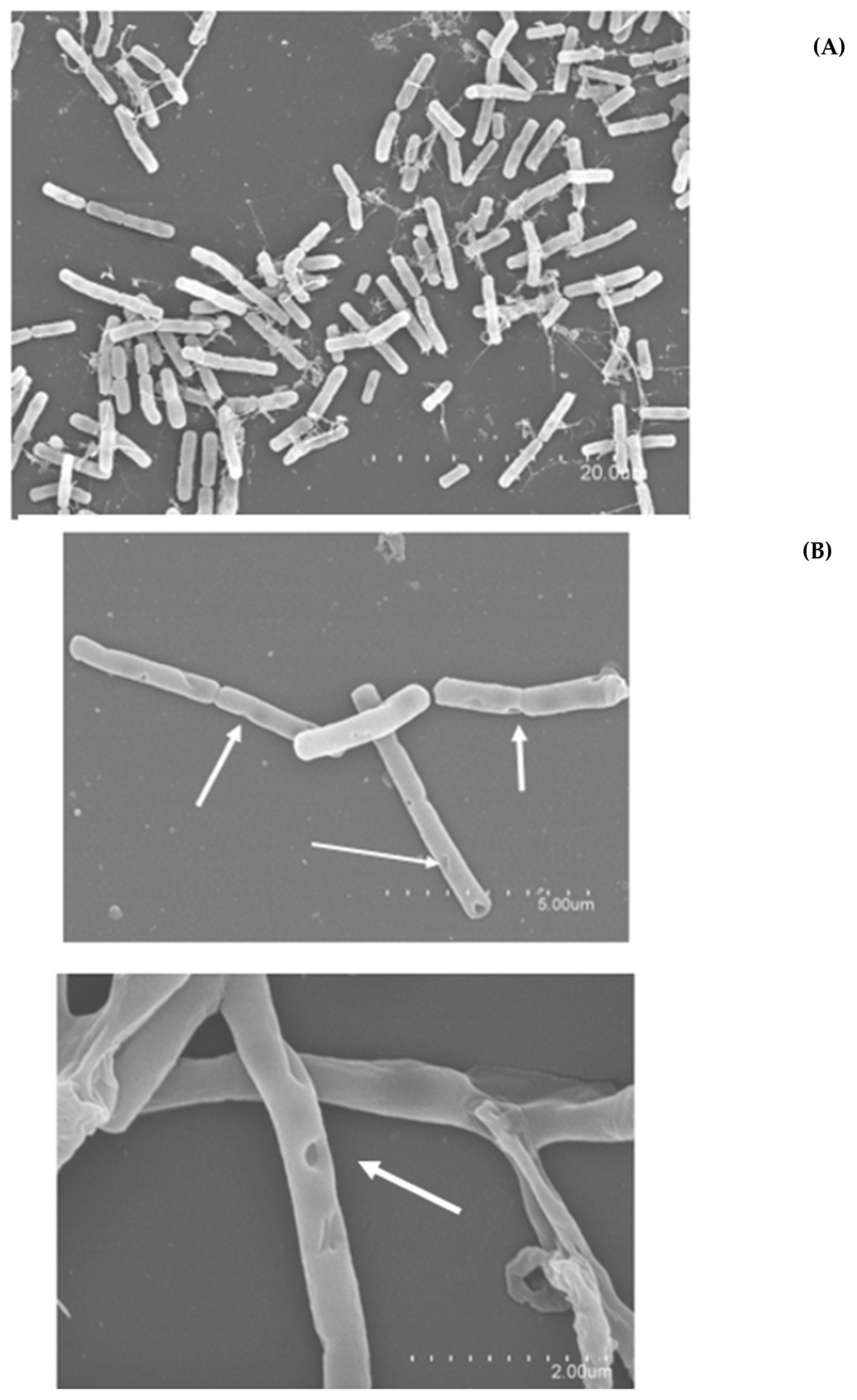



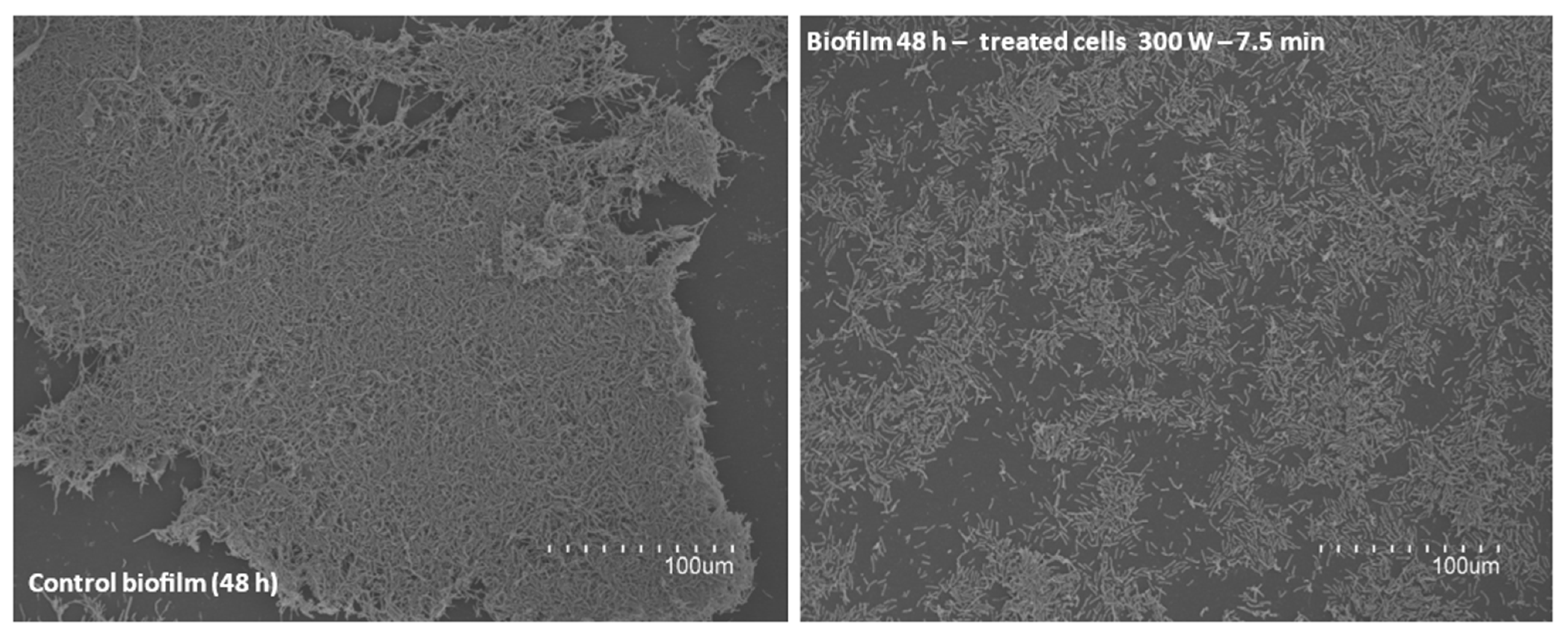
| DBD-CP Treatment Applied | Storage Time | B. cereus 4387 CECT | B. cereus 7259 CECT | B. thuringiensis 197 T CECT | B. mycoides 4128 CECT |
|---|---|---|---|---|---|
| 100 W–5 min | 0 h | 98.1 ± 8.6 a | 78.2 ± 2.5 a | 45.6 ± 4.3 a | 74.4 ± 7.6 a |
| 4 h | 38.4 ± 2.6 b | 76.7 ± 3.1 a | 24.3 ± 2.8 b | 57.9 ± 3.4 b | |
| 8 h | 35.3 ± 2.1 b | 43.1 ± 0.9 b | 26.1 ± 3.2 b | 1.73 ± 0.6 c | |
| 12 h | 22.3 ± 1.4 c | 16.3 ± 4.2 c | 21.1 ± 2.1 c | - | |
| 24 h | 26.7 ± 3.3 c | 24.3 ± 6.3 c | 13.1 ± 4.3 d | - | |
| 300 W–5 min | 0 h | 78.2 ± 7.2 d | 81.5 ± 7.3 a | 42.1 ± 4.8 a | 92.5 ± 2.3 d |
| 4 h | 34.6 ± 11.8 b | 62.3 ± 3.5 d | 17.6 ± 3.2 d | 99.1 ± 6.8 d | |
| 8 h | 39.4 ± 8.5 b | 7.4 ± 2.1 e | 21.3 ± 2.1 d | 92.4 ± 2.3 d | |
| 12 h | 24.9 ± 1.5 c | - | 18.5 ± 3.6 d | 65.1 ± 7.4 a | |
| 24 h | 23.8 ± 9.3 c | - | 16.2 ± 0.8 d | 31.7 ± 3.1 e |
| B. cereus 4387 CECT | 0 | 8 h | 24 h |
|---|---|---|---|
| Control biofilms (untreated cells) | 100 | 100 | 100 |
| 100 W–5 min | 45 ± 2.3 a | 95 ± 5.3 c | 97 ± 3.7 c |
| 200 W–5 min | 38 ± 3.5 a | 97 ± 2.4 c | 92 ± 1.2 c |
| 300 W–5 min | 22 ± 1.8 b | 52 ± 3.3 d | 43 ± 4.8 d |
| B. mycoides 4128 CECT | 0 | 8 h | 24 h |
| Control biofilms (untreated cells) | 100 | 100 | 100 |
| 100 W–5 min | 65 ± 2.5 a | 87 ± 6.1 d | 98 ± 3.4 d |
| 200 W–5 min | 58 ± 1.3 b | 91 ± 3.5 d | 95 ± 2.8 d |
| 300 W–5 min | 36 ± 2.6 c | 45 ± 1.8 e | 52 ± 1.8 e |
| B. thuringiensis 193T CECT | 0 | 8 h | 24 h |
| Control biofilms (untreated cells) | 100 | 100 | 100 |
| 100 W–5 min | 43 ± 1.3 a | 84 ± 2.8 d | 90 ± 1.5 e |
| 200 W–5 min | 32 ± 2.1 b | 81 ± 3.1 d | 95 ± 2.5 e |
| 300 W–5 min | 22 ± 0.9 c | 27 ± 1.1 c | 38 ± 1.3 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eced-Rodríguez, L.; Beyrer, M.; Rodrigo, D.; Rivas, A.; Esteve, C.; Pina-Pérez, M.C. Sublethal Damage Caused by Cold Plasma on Bacillus cereus Cells: Impact on Cell Viability and Biofilm-Forming Capacity. Foods 2024, 13, 3251. https://doi.org/10.3390/foods13203251
Eced-Rodríguez L, Beyrer M, Rodrigo D, Rivas A, Esteve C, Pina-Pérez MC. Sublethal Damage Caused by Cold Plasma on Bacillus cereus Cells: Impact on Cell Viability and Biofilm-Forming Capacity. Foods. 2024; 13(20):3251. https://doi.org/10.3390/foods13203251
Chicago/Turabian StyleEced-Rodríguez, Laura, Michael Beyrer, Dolores Rodrigo, Alejandro Rivas, Consuelo Esteve, and Maria Consuelo Pina-Pérez. 2024. "Sublethal Damage Caused by Cold Plasma on Bacillus cereus Cells: Impact on Cell Viability and Biofilm-Forming Capacity" Foods 13, no. 20: 3251. https://doi.org/10.3390/foods13203251
APA StyleEced-Rodríguez, L., Beyrer, M., Rodrigo, D., Rivas, A., Esteve, C., & Pina-Pérez, M. C. (2024). Sublethal Damage Caused by Cold Plasma on Bacillus cereus Cells: Impact on Cell Viability and Biofilm-Forming Capacity. Foods, 13(20), 3251. https://doi.org/10.3390/foods13203251









